This study investigated the coagulation potential of Moringa oleifera seed powder (SP) or its seed aqueous extract (SAE) on pH, electrical conductivity (EC) and heavy metals of sewage water after 1, 3 and 6 h contact time. After optimizing, 2 g L−1 moringa SP, 40 mL L−1 SAE and 20 mg L−1 of alum alone or in combination were evaluated. Moringa SP was more effective than SAE and its combination to decrease EC and maintenance of pH of treated or untreated sewage water. Moringa SAE was more effective than SP or their combination to decrease Lead (Pb) and Chromium (Cr) load from sewage water. Naturally occurring amino acids in moringa seeds might increased the metal binding and decreased heavy metals load with maximum desorption on SP. Nonetheless, use of Moringa oleifera SP or its SAE can be inexpensive and alternative coagulant of sewage water treatment.
With rapid increase in population, industrial development and water usage, faecal pollution of drinking water is creating enormous public health hazards like water born diseases due to careless disposal of sewage water [1]. Drainage of sewage water without treatment and its use for irrigation purpose are possible ways of heavy metals accumulation in plants food chain [1]; [2]. Several conventional methods are used for waste water treatment including coagulation-flocculation followed by sedimentation, filtration, precipitation, ion exchange resins and reverse osmosis before its distribution to consumers [3]; [4]. Many inorganic coagulants, synthetic inorganic polymer and naturally occurring coagulants are also widely used in conventional water treatment processes [4]. Nonetheless, use of ploy ammonium chloride, poly alumnium silico sulphate and inorganic alum salt is most common [5]; [6]; [7]. And use of aluminium containing coagulants is expensive and synthetic organic polymers have strong carcinogenic properties [8]; [9]. Further, aluminium is causative agent of neurological disorder presenile dementia and alum itself may induce Alzheimer’s disease [10] and have low coagulation efficiency when react with natural alkalinity in water leading to pH reduction [11]. Moringa oleifera Lam. is widely adapted in tropic and subtropics and cultivated in developing world. Its seeds are used as organic natural polymer containing polypeptide with cationic polyelectrolyte properties good for softening hard water by adsorption [12]. According to Ndabigengesere et al [13], aqueous extract of Moringa oleifera Lam. seeds contain stable and water soluble dimeric proteins active in coagulation. Many studies highlight the efficiency of Moringa oleifera seed extract as coagulant [14] used in coagulation of model turbid water [12], quality of treated water, as primary coagulant and in conjunction with alum for coagulation of river water [11]. Moringa is native to Indo-Pak and its use as multipurpose tree is rapidly increasing [15]. Further, with increasing urbanization, industrialization and depletion of ground water resources, sewage wastewater treatment is widely propagating from small to industrial scale for domestic purposes [2]. Very few studies are available on usage of Moringa oleifera seed powder or its aqueous extract as alternative coagulant or its conjunctive use with alum in wastewater treatment. The present study therefore investigated the potential of Moringa oleifera seed powder or its aqueous extract on pH, electrical conductivity (EC) to minimize heavy metals load of sewage waste water.
2Materials and Methods2.1Preparation of Moringa oleifera Seed Powder (SP)Seeds of Moringa oleifera Lam. were collected from a tree located at Agronomic Research Area, University of Agriculture, Faisalabad during May-June, 2010. Seeds were given washings with distilled water to remove impurities if any and dried at 65°C for 24 h. The seeds shell was removed and kernel was ground to fine powder using blender. The fine powder was then put into cheese cloth sack till further use [16].
2.2Preparation of Moringa oleifera Seed Aqueous Extract (SAE)For preparation of aqueous extracts, fine moringa seed powder (2 g) was added into 100 mL distilled water. The suspension was given vigorous shaking for 30 min using magnetic stirrer to promote water extraction of the coagulant proteins and filtered using Whatman No.1. Before use, filtrate was shaken again [13].
2.3Optimization of Moringa SP or SAESewage water was collected from entrance and exit point of local municipal wastewater treatment plant. The concentrations of alum (5, 10, 15, 20, 25 mg L−1), moringa SP (0.50, 1.0, 1.5, 2.0, 2.5 g L−1) and SAE were optimized based on pH and EC of sewage water. Later, optimized concentrations i.e. SP (2 g L−1), SAE (40 mL L−1) and alum (20 mg L−1) alone or in combination with alum was used for further experimental procedures.
2.4pH and EC MeasurementsFor pH and EC measurement, a batch test with five beakers of 1000 mL capacity each with optimized concentration and three replications was accommodated with five spindle steel paddles. After homogenous mixing, pH and EC was measured by using calibrated conductivity (Model, Horiba, Germany) and pH meter (Hinna, Germany) after 1, 3 and 6 h respectively according to the method prescribed by [17].
2.5Heavy Metals AnalysisHeavy metals were determined as described by [18]. 50 mL suspension of each coagulant was filtered after 1, 3 and 6 h into a 100 mL beaker and then 20 mL conc. HNO3 was added. The sample was strongly heated till half of the volume obtained. After cooling, 5 mL H2O2 was added to decolorize the sample. The solution was recovered in a 50 mL volumetric flask and made volume up to mark using distilled water. For metal ion determination, atomic absorption spectrometry using a Perkin Elmer Analyst 300 Atomic Absorption Spectrophotometer with air/acetylene flame and different hollow cathode lamps for specific cations was used. The calibration was done manually by aspirating the prepared working standards of Pb and Cr one by one into the flame. For atomization, the samples were also aspirated manually into the flame.
2.6Statistical AnalysisSignificant difference among treatments and hours was determined using standard errors. Microsoft Excel program was used to present data graphically to establish a relationship between various traits.
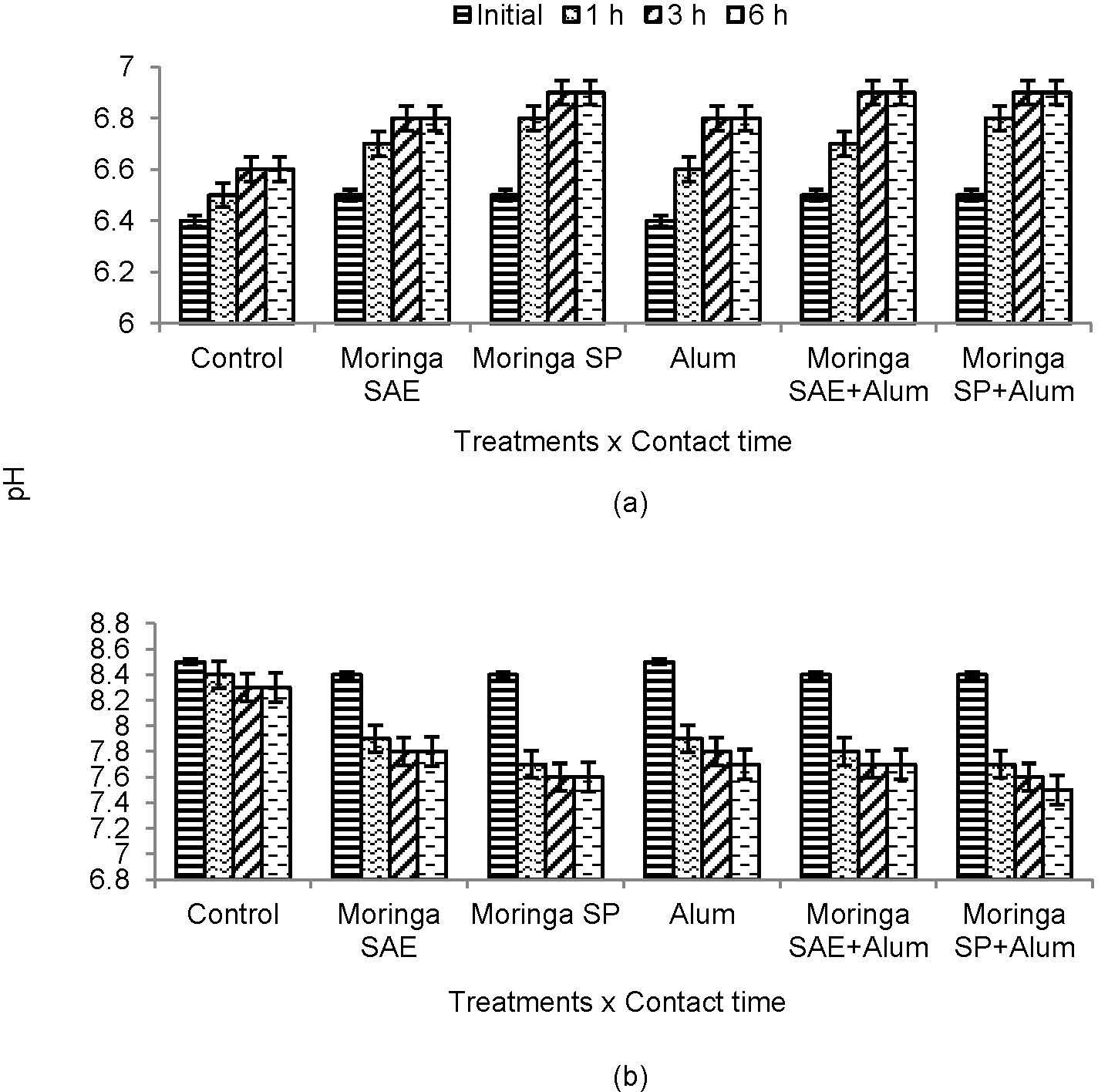
3Results and Discussion3.1Effect on pHSignificant difference was found among the treatments for pH of untreated sewage water after different contact times with addition of coagulants (Figure 1). Initial pH of untreated sewage water was low and thereafter increased. This increase in pH was maximum after 1 h contact time when moringa seed powder (SP) alone or used in combination with alum as compared to control or alum alone. However, increase in pH was constant after 3 or 6 h contact time for moringa SP or with combined use of moringa SAE and SP with alum than control in case of untreated sewage water (Figure 1). While opposite response was observed for treated sewage water with decrease in pH after different contact time (Figure 1). This rapid decrease in pH of treated sewage water was found for moringa SP after 1 h and effect was similar when SP and alum were used in combination. Nonetheless, individual effect of moringa SP or alum to decrease pH of treated sewage water was also similar. In fact, Moringa oleifera seeds contain water soluble proteins which aid in coagulation and keeps adsorption power between pH of 5-8 (Vieira et al 2010) [19]. The amino acids present in moringa seed proteins accept protons from water and release hydroxyl group ions causing the solution pH to basic with natural buffering capacity (Ndabigengesere et al 1995; Ndabigengesere and Narasiah 1998) [13; 11]. Decreased pH of treated sewage water due to moringa seed powder in our study might be due to presence of low molecular weight proteins. These proteins carries positive charge and attracts negatively charged particles such as silt, clay and other toxic particles causing the pH to decrease (Amagloh and Benang 2009; Salam et al 2007) [20; 21]. Alum is also used to maintain pH of sewage water and alum decreased pH of treated sewage water had been also reported by Yongbai (2005) [22] and Imran et al [30].
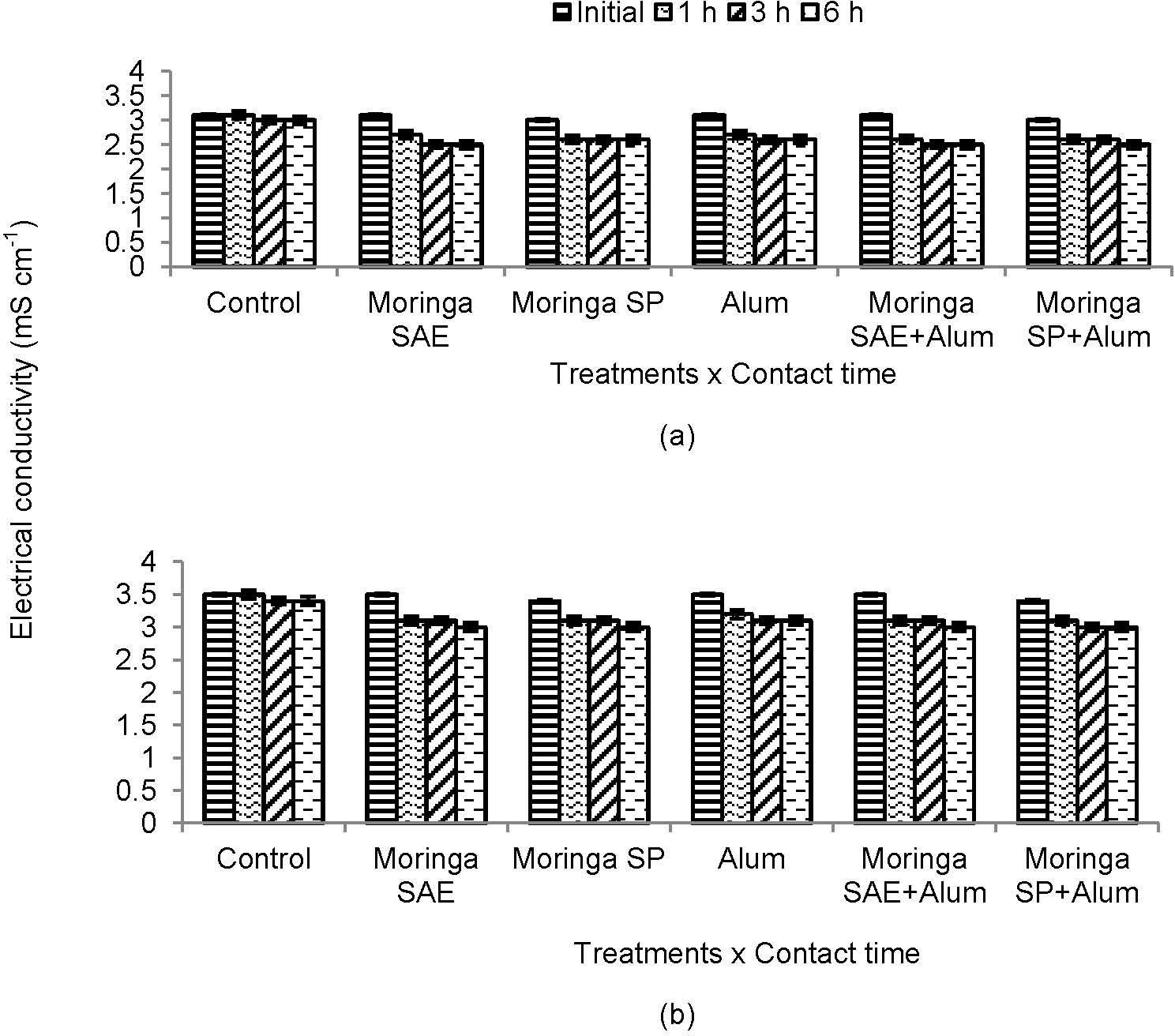
3.2Effect on Electric ConductivityInitial EC of treated and untreated sewage water was high and more decrease in EC was observed in untreated sewage water. Among the coagulants, moringa SAE decreased EC more and followed by moringa SAE and SP when used in combination with alum after 6 h in untreated sewage water. Nonetheless, individual effect of moringa SP and SAE was more pronounced to decrease EC of treated sewage water than alum after 6 h contact time and statistically was similar when used in combination with alum (Figure 2).
The EC of untreated sewage water was lower than treated water due to solubility of minerals. More decrease in EC of sewage water with Moringa oleifera seeds was due to the presence of lower molecular weight water soluble proteins which carry positive charge (Eman et al 2010; Agrawal et al 2007) [23; 24]. Addition of optimized concentrations of moringa seeds or other coagulants into untreated and treated sewage water, the seed proteins produce positive charges attracting negatively charged particles removing anions in gaseous form (Amagloh and Benang 2009) [20]. Moringa oleifera seeds had coagulating and softening properties in addition to pH reductant and can handle groundwater with moderate to high alkalinity (Muyibi 1994) [25]. Therefore, treated sewage water takes more time for settlement of particles and removal of anions in gaseous form as observed for present study.
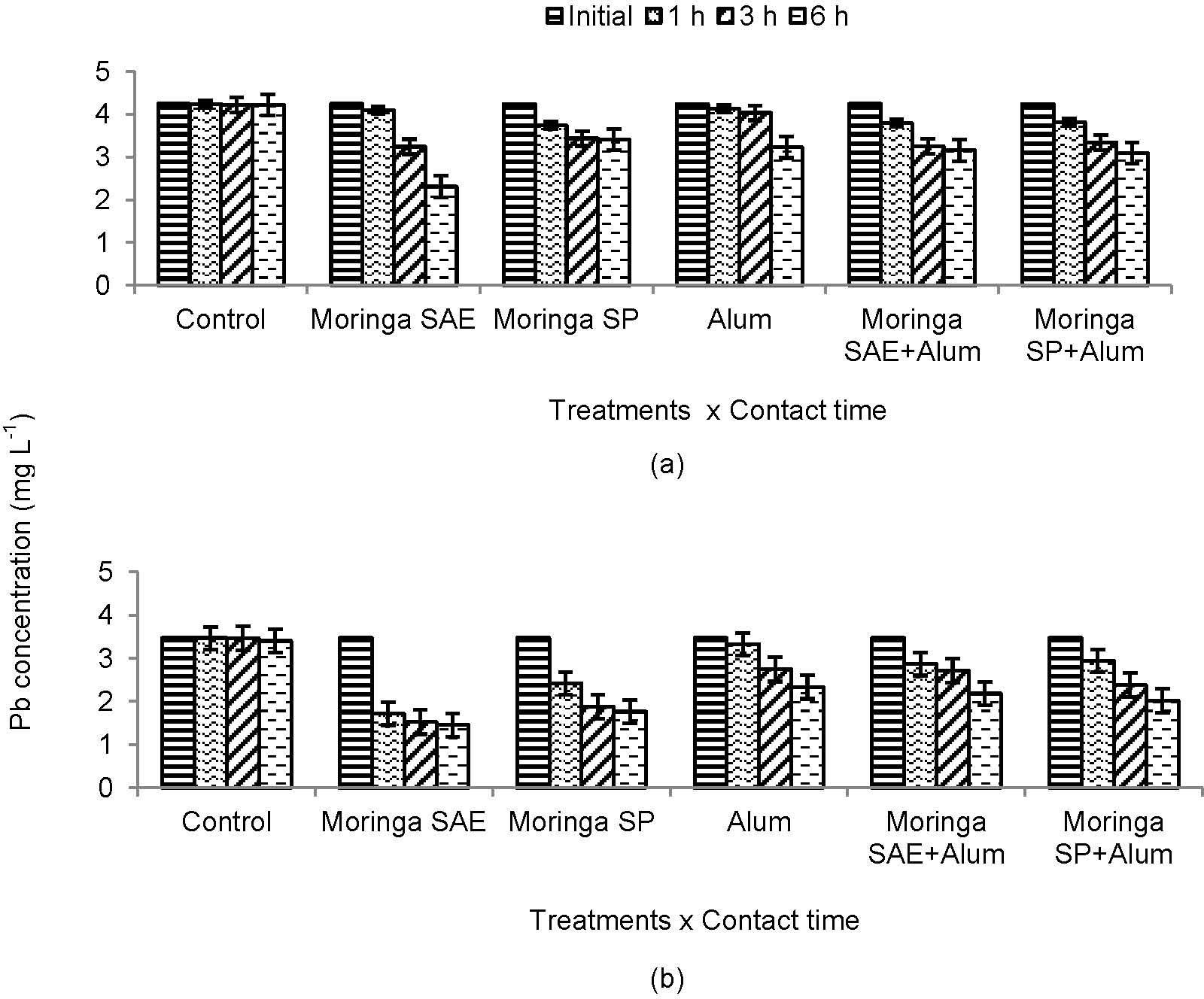
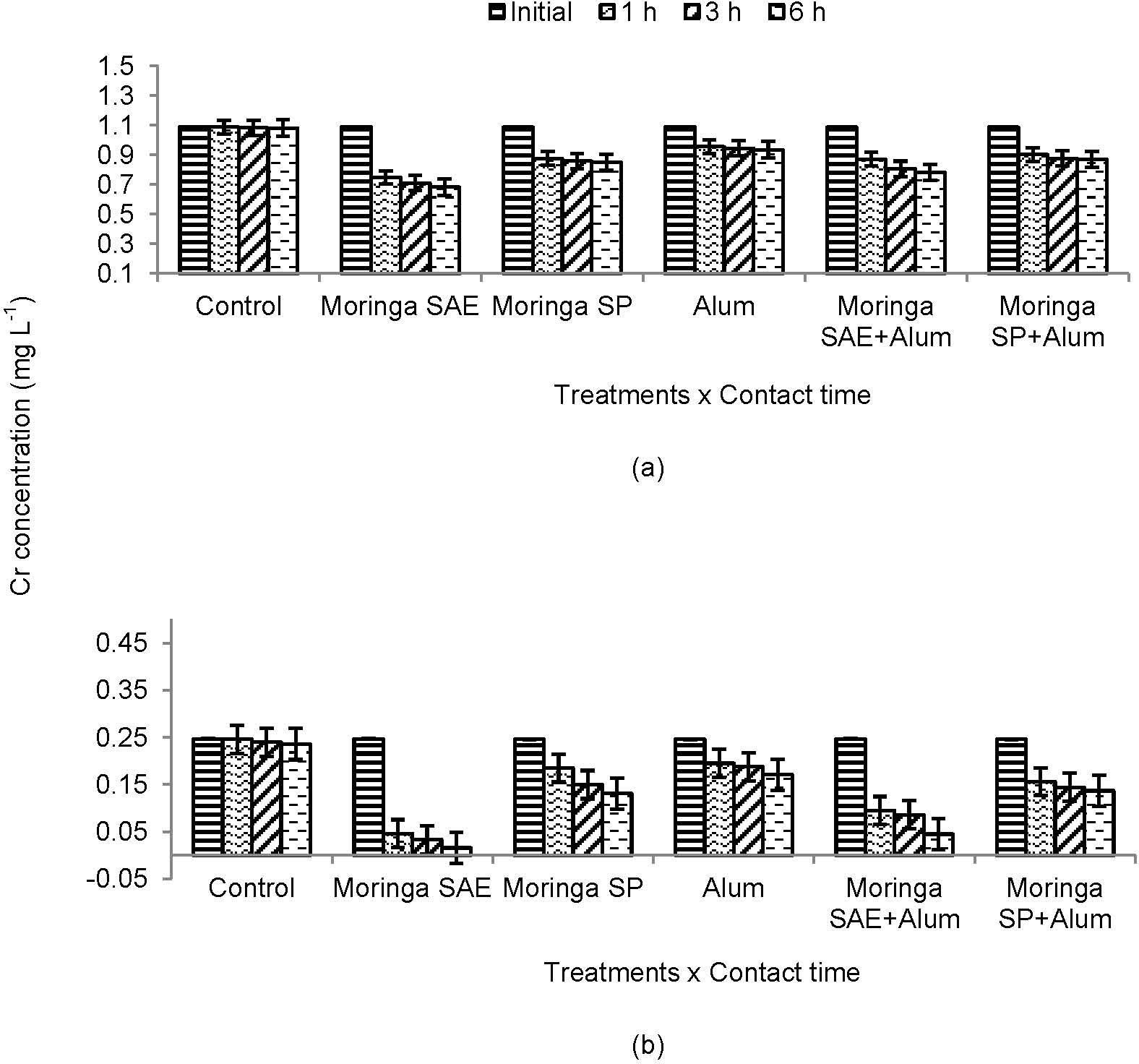
3.3.The Changes in Concentration of Heavy Metals with Contact TimeUse of moringa SP and SAE alone or in combination with alum reduced heavy metals concentration significantly after different time contacts (Figure 3 and 4). The removal of metal ions increased gradually with contact time and individual effect of moringa SAE and SP to adsorb and remove lead (Pb) and chromium (Cr) after 1, 3 and 6 h was more significant in treated sewage water than untreated. More decrease in metals load was observed for moringa SP alone and in combination with alum after different time contacts. However, maximum decrease in metal concentration and adsorption was recorded for Cr in both treated and untreated sewage water with rapidly decrease in t reated water for moringa SAE and its combination with alum (Figure 3).
Due to heterogeneous properties, the aqueous solution of moringa seeds contains low molecular weight amino acids. These amino acids contain physiologically active group of binding agents which at low concentration interact with metals to increase the sorption of metal ions (Brostlap and Schuurmans 1988) [26]. The rapid decrease in Pb or Cr concentration with contact time due to moringa SP or its combination with alum in present study depicts amino acid interaction with metal ions. Similar effects found for moringa SAE might be the result of naturally occurring amino acids for bisorption of Pb or Cr from sewage water (Figure 3 and 4; Sharma et al 2006; 2007) [16; 27]. In addition, these proteinous amino acids have pH dependant properties which generate negative charged environment playing important role in binding of heavy metals (Costa et al 1997; Muyibi, Leong, & Loon, 2002) [28; 29].
4ConclusionThe study concludes that moringa oleifera SP is more effective than SAE or their combination with alum to reduce the EC and maintenance of pH of sewage water. Similarly moringa SAE and its combination with alum offers natural tool for removal of heavy metals from sewage water. Therefore considering the potential of moringa SP and SAE, their use as alternative coagulant should be promoted. Amino-acid and metal binding interactions seems to be responsible for the metal binding using moringa SAE which warrants further studies.