To analyze surgical safety through postoperative COVID-19 incidence and mortality at the urology department of a tertiary hospital located in Madrid (Spain).
MethodsObservational, prospective study including all patients undergoing urological surgery from 1st March 2020 to 28th February 2021. According to the hospital organization and local epidemiological situation we delimitate three epidemic waves. A set of screening and protective measures was applied from 4th May onwards. Demographic, baseline, surgical and perioperative variables, as well as postoperative outcomes, were collected. Telephone follow-up was performed at least 3 weeks after hospital discharge.
Results940 urological surgeries were performed, 12 of them had to be rescheduled due to active or recent SARS-CoV-2 infection identified by the screening protocol. Thirty-one patients developed COVID-19 (3.3% incidence) and 7 died (22.6% mortality). The average time to onset of symptoms was 62.6 days after discharge, being 25 cases attributable to community transmission. The remaining 6 cases, due to in-hospital transmission, had worse outcomes. Five of them were identified during the first wave, especially when no preoperative PCR was obtained. In contrast, during the second and third waves, fewer and milder cases were diagnosed, with just 1 in-hospital transmission among 857 urological patients.
ConclusionsAfter implementing complete protective measures, postoperative in-hospital COVID-19 cases almost disappeared, even during the second and third waves. Most of the cases were due to community transmission and thus driven by the general epidemiological situation. While hospitals follow recommendations to avoid COVID-19 infection, urological surgery remains safe and can be maintained.
Analizar la seguridad de la cirugía, en términos de incidencia y mortalidad de los casos de COVID-19 postoperatorio, en el Servicio de Urología de un hospital terciario de Madrid (España).
MétodosEstudio prospectivo que incluye todos los pacientes sometidos a cirugía urológica, desde el 1 de marzo de 2020 al 28 de febrero de 2021. Según la organización hospitalaria y la situación epidemiológica local, distinguimos tres ondas epidémicas. Un conjunto de medidas de cribado y protección se aplicó desde el 4 de mayo en adelante. Se recogieron variables demográficas, basales, quirúrgicas, peri y postoperatorias. Se realizó seguimiento telefónico al menos 3 semanas tras el alta hospitalaria.
ResultadosSe realizaron 940 cirugías urológicas, teniendo que posponer 12 de ellas debido a infección activa o reciente por SARS-CoV-2 (identificadas por el protocolo de cribado). Treinta y un pacientes desarrollaron COVID-19 (incidencia del 3.3%) y 7 murieron (mortalidad del 22.6%). La media hasta el desarrollo de los síntomas fue de 62.6 días tras el alta, siendo 25 de los casos atribuibles a contagio en la comunidad. Los 6 casos restantes, por transmisión intrahospitalaria, fueron más severos. Cinco de ellos ocurrieron en la primera ola, sobre todo cuando aún no se realizaba PCR preoperatoria. Por el contrario, durante la segunda y tercera olas se diagnosticaron menos casos y fueron más leves, con solo una transmisión intrahospitalaria entre 857 pacientes.
ConclusionesTras implementar un protocolo de medidas preventivas, los casos de COVID-19 postoperatorio casi desaparecieron, incluso durante los repuntes de segunda y tercera ola. La mayoría de casos se debieron a transmisión comunitaria, influidos por la situación epidemiológica local. Mientras los hospitales sigan las recomendaciones para evitar la COVID-19, la cirugía urológica es segura y puede continuar.
The SARS-CoV-2 pandemic has made us change urological practice in several ways.1,2 During the beginning of the pandemic, the collapse of health systems and the scarce data regarding safety of surgical interventions, lead to a temporary suspension of elective surgery in many urology departments worldwide. Over 115 million operations were cancelled globally (almost 1% urological, estimations for 190 countries).3 As the epidemiological situation got better and the evidence grew, elective surgery was restarted again, following the recommendations from numerous urological associations.4–7
With the ease of the lockdowns and the most restrictive measures to stop the spread of the virus, new pandemic waves occurred during autumn and winter 2020, which had greater incidences in many countries.8–10 The impact of those peaks on surgical safety is not well studied.
Our objective was to describe patient outcomes in the urology department of a tertiary hospital located in Madrid, in terms of postoperative COVID-19 cases and their features, comparing the second and third waves with the first one, and considering pandemic behavior in the general population.
Material and methodsA prospective observational study was performed including every patient who underwent urological surgery at our institution during the first year of the pandemic at Spain (March 2020 to February 2021).
Study variablesWe collected the preoperative characteristics (sex, age, Charlson Comorbidity Index, risk factors for COVID-19,11 preoperative status of the disease and vaccination), surgical variables (anesthetic risk, procedure and its related postoperative complications by Clavien–Dindo classification) and follow-up (at least 3 weeks after discharge to exceed the incubation period). That follow-up was made by and structured telephonic interview and updated during the in person postoperative consultations, and searched for COVID-19 symptoms, treatment or hospitalizations needed.
Definition of study periodThe country's epidemiological situation and the organization and policies of our center changed across the stages of the pandemic.12 The first wave lasted from 1st March to 3rd May 2020. Within the first 2 weeks there were not significant changes on elective surgery, but during the following 7 weeks the hospital had an almost exclusive dedication to the disease and consequently, only emergency surgeries were allowed after nasopharyngeal swab polymerase chain reaction (PCR) testing.
Since May 4th elective surgery was resumed following a multidisciplinary protocol13 and the prioritization scheme provided by the EAU Guidelines.4 The protocol included screening measures before hospital admission (telephone consultation 1 week before surgery asking for symptoms or close contact with COVID-19 patients; PCR testing 2 days before income, plus serology, blood analysis or chest computed tomography – CT – if needed) and in-hospital measures (separated surgical pathways, department organization, operating room equipment and instructions for all health workers).
The number of daily cases increased again on August 13th, giving rise to the second wave. Fewer patients required hospitalization due to COVID-19 and elective surgeries were not cancelled, even so they were reduced transiently. This wave lasted until 15th November. After a short break of 6 weeks with a better epidemiological situation (November 16th to December 27th), we had a third wave which lasted until 28th February 2021. Thus, the study period comprises 1 year with 3 epidemiological waves.
To analyze COVID-19 patients features, we compared their characteristics with those who did not develop the infection. Regarding the impact of the pandemic in the general population, we used the information provided by the Madrid Regional Government14 in the districts whose population belongs to our hospital (Arganzuela, Villaverde, Usera, Carabanchel).
Ethical considerationsThe study was approved by our Institutional Review Board. Due to the contact restrictions, informed consent for data management was given orally by the patient and written on their electronic medical record.
Statistical analysisThe Stata/IC 16.1 software was used for data analysis (Stata-Corp., College Station, Texas). Mean and standard deviation were used to characterize the quantitative variables, and frequency expressed in percentages was used for the qualitative variables. The comparison of independent continuous quantitative variables was carried out using non-parametric tests, Kruskal–Wallis or Mann–Whitney U tests. Chi-squared test with Yates correction or Fisher's exact test was used for frequencies.
ResultsDuring the twelve months of study duration, 940 patients underwent surgery in our urology department. The distribution throughout the epidemic stages is shown in Table 1, as well as the main baseline, operative and follow-up variables.
Baseline characteristics, operative and follow-up variables across the different periods.
| First wave (n=83) | Inter-mediate I (n=355) | Second wave (n=186) | Inter-mediate II (n=150) | Third wave (n=166) | Total (n=940) | |
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Age (mean±SD) | 61.5±17.3 | 64.4±15.1 | 62.9±17.3 | 64.4±15.9 | 63.3±16.6 | 63.6±16.1 |
| Sex – women n (%) | 25 (30.1) | 88 (24.8) | 53 (28.5) | 44 (29.3) | 42 (25.3) | 252 (26.8) |
| CCI≥2 n (%) | 3.3±2.2 | 4.4±3.1 | 4.0±2.8 | 3.9±2.6 | 5.0±2.9 | 4.3±2.9 |
| Comorbidities – n (%) | ||||||
| HT | 40 (48.2) | 185 (52.1) | 94 (50.5) | 60 (40.0) | 88 (53.0) | 467 (49.7) |
| Diabetes | 21 (25.3) | 79 (22.3) | 38 (20.4) | 32 (21.3) | 36 (21.7) | 206 (21.9) |
| Obesity | 21 (25.3) | 55 (15.5) | 20 (10.8) | 11 (7.3) | 9 (5.4) | 116 (12.3) |
| AMI | 7 (8.4) | 32 (9.0) | 14 (7.5) | 15 (10.0) | 9 (5.4) | 77 (8.2) |
| Pulm | 11 (13.3) | 47 (13.2) | 13 (7.0) | 9 (6.0) | 11 (6.6) | 91 (9.7) |
| Immuno | 12 (14.5) | 49 (13.8) | 35 (18.8) | 10 (6.7) | 32 (19.3) | 138 (14.7) |
| KT | 7 (8.4) | 23 (6.5) | 16 (8.6) | 6 (4.0) | 22 (13.3) | 74 (7.9) |
| Neoplasia | 29 (34.9) | 156 (43.9) | 88 (47.3) | 74 (49.3) | 82 (49.4) | 429 (45.6) |
| ASA – n (%) | ||||||
| I–II | 49 (59.0) | 192 (54.1) | 87 (46.8) | 84 (56.0) | 86 (51.8) | 498 (53.0) |
| III–V | 34 (41.0) | 163 (45.9) | 99 (53.2) | 66 (44.0) | 80 (48.2) | 442 (47.0) |
| Approach - n (%) | ||||||
| Open | 22 (26.5) | 57 (16.1) | 28 (15.1) | 15 (10.0) | 34 (20.5) | 155 (16.5) |
| Endoscopic | 52 (62.7) | 226 (63.7) | 130 (69.9) | 100 (66.6) | 103 (62.0) | 612 (65.1) |
| Laparoscopic | 9 (10.8) | 72 (20.3) | 28 (15.1) | 35 (23.3) | 29 (17.5) | 173 (18.4) |
| Type – n (%) | ||||||
| Scheduled | 41 (49.4) | 278 (78.3) | 114 (61.3) | 114 (76.0) | 119 (71.7) | 666 (70.9) |
| Urgent | 42 (50.6) | 77 (21.7) | 72 (38.7) | 36 (24.0) | 47 (28.3) | 274 (29.1) |
| Complications and all-cause mortality – n (%) | ||||||
| Total complications | 9 (10.8) | 52 (14.6) | 33 (17.7) | 16 (10.7) | 30 (18.1) | 140 (14.9) |
| Clavien I–II | 6 (66.7) | 37 (71.2) | 22 (66.7) | 9 (56.3) | 18 (60.0) | 92 (65.7) |
| Clavien III–V | 3 (33.3) | 15 (28.8) | 11 (33.3) | 7 (43.7) | 12 (40.0) | 48 (34.3) |
| In-hospital mortality | 3 (3.6) | 1 (0.3) | 2 (1.1) | - | 1 (0.6) | 7 (0.7) |
| 90-day mortality | 4 (4.8) | 11 (3.1) | 6 (3.2) | 5 (3.3) | 3 (1.8) | 29 (3.1) |
| COVID-19 – n (%) | ||||||
| Total incidence | 10 (12.0) | 15 (4.2) | 2 (1.1) | 1 (0.7) | 3 (1.8) | 31 (3.3) |
| In-hospital incidence | 5 (6.0) | 0 | 0 | 0 | 1 (0.6) | 6 (0.6) |
| Disease mortality | 3 (30.0) | 3 (20.0) | 0 | 0 | 1 (33.3) | 7 (22.6) |
CCI: Charlson comorbidity index. HT: hypertension. AMI: acute myocardial infarction. Pulm: pulmonary pathology (asthma or chronic obstructive pulmonary disease). Immuno: immunosuppression. KT: kidney transplantation. ASA: American Society of Anaesthesiologists classification. In-hospital COVID-19 patients are those diagnosed during admission or up to 14 days after discharge.
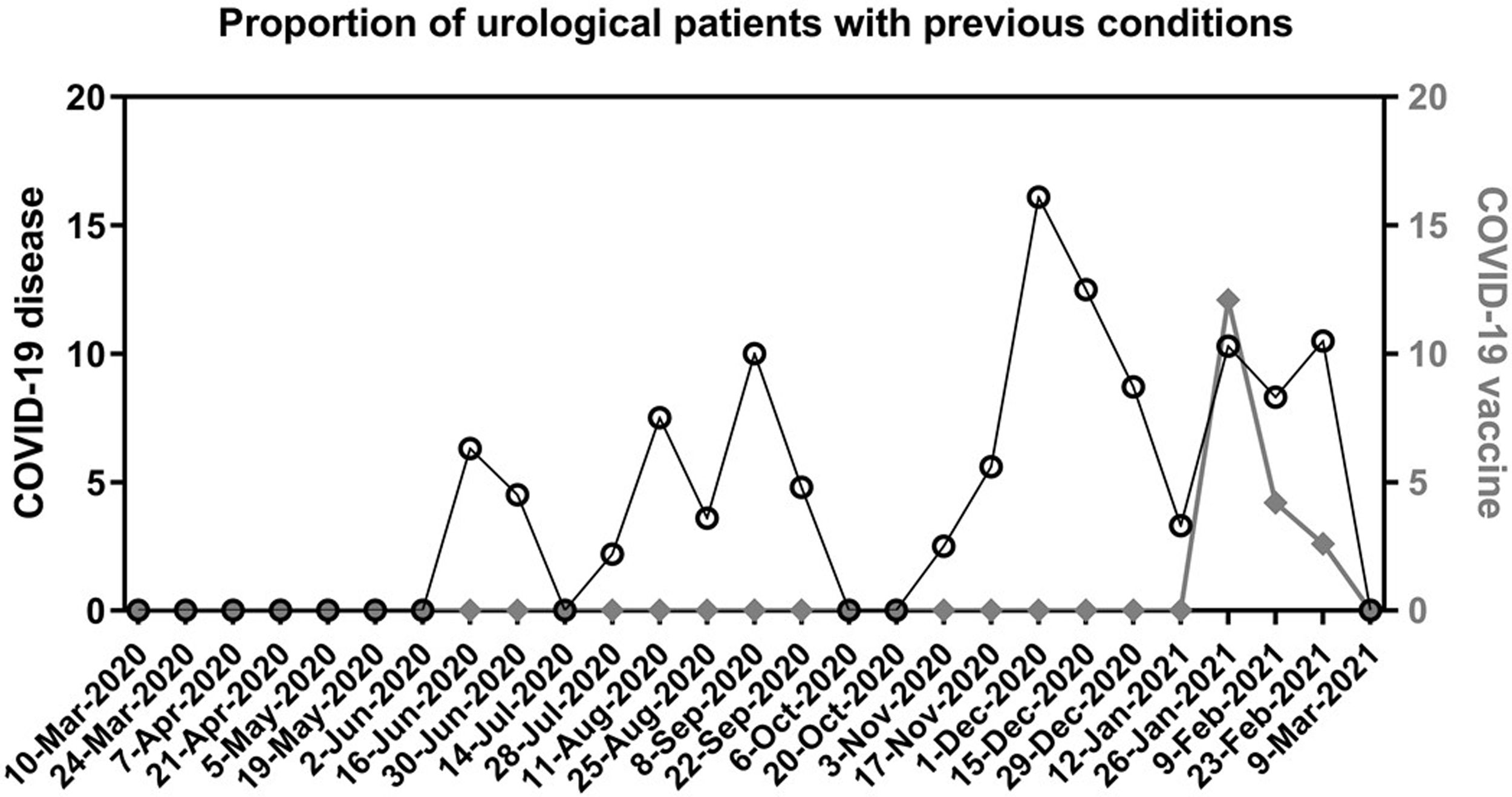
The preoperative screening measures identified twelve COVID-19 cases the week before surgery (7 asymptomatic patients with positive PCR, 1 asymptomatic with negative PCR but abnormal findings in both blood test and chest CT, 4 in isolation due to recent diagnosis). These patients were all rescheduled at least 4 weeks after the positive PCR result or beginning of symptoms. None of them had postoperative complications. Thirty-nine additional patients reported having had the disease 1 to 5 months before surgery, and 9 reported having received the vaccine. The distribution of all these patients along the study period, is represented in Fig. 1.
Proportion of urological patients with a previous condition.
Each date on X axis represents a cut-off point to calculate the proportion (%) of patients who underwent urological surgery during the previous 14 days and reported during the preoperative screening: having passed the disease (left Y axis, black circles) or having received a complete vaccination regimen (right Y axis, diamonds).
The mean follow-up was 95.0 days (standard deviation 45.1 days), identifying 31 patients diagnosed of COVID-19 following a mean of 62.6 days after discharge (range 8–276). As the incubation period of the disease is estimated to be up to 14 days for 99% of patients,15 we make the distinction between those happening during admission and up to 14 days after discharge (in-hospital transmission, 6 patients) vs >14 days after discharge (community transmission, 25 patients). During the first wave there was a 12% incidence (10 out of 83 patients) including 5 nosocomial infections. During the second wave the incidence was 1.1% (2 out of 186 patients) and during the third wave a 1.8% (3 out of 166 patients, one of them with in-hospital transmission).
A comparison of in-hospital, community and non-COVID-19 patients is shown in Table 2. An additional comparison was made on first vs second vs third wave COVID-19 cases. Although higher rates of comorbidities, ASA III–IV and mortality were found on the first wave patients, none of the variables reached statistical significance. To note, just 3 patients of the whole cohort developed a pulmonary complication (acute respiratory distress syndrome), all of them belonging to the first wave, with intensive care unit admission but 100% fatal outcome.
In-hospital (≤14 days from surgery or discharge), community (>14 days after discharge) and non-COVID-19 patients’ comparison.
| In-hospital COVID-19 (n=6) | Community COVID-19 (n=25) | Non-COVID-19 patients (n=909) | p-value* | |
|---|---|---|---|---|
| Distribution | ||||
| First wave | 5 (83.3) | 5 (20.0) | 73 (8.0) | <0.001 |
| Intermediate I | 0 | 15 (60.0) | 340 (37.4) | |
| Second wave | 0 | 2 (8.0) | 184 (20.2) | |
| Intermediate II | 0 | 1 (4.0) | 149 (16.4) | |
| Third wave | 1 (16.7) | 2 (8.0) | 163 (17.9) | |
| Baseline characteristics | ||||
| Age (mean±SD) | 77.8±8.8 | 66.2±13.4 | 63.5±16.2 | 0.060 |
| Sex – women n (%) | 2 (33.3) | 10 (40.0) | 240 (26.4) | 0.298 |
| CCI>=2 n (%) | 6 (100.0) | 20 (80.0) | 736 (81.0) | 0.490 |
| Comorbidities – n (%) | ||||
| Hypertension | 4 (66.7) | 15 (60.0) | 448 (49.3) | 0.404 |
| Diabetes | 3 (50.0) | 10 (40.0) | 193 (21.2) | 0.020 |
| Obesity | 2 (33.3) | 7 (28.0) | 107 (11.8) | 0.015 |
| AMI | 2 (33.3) | 1 (4.0) | 74 (8.1) | 0.060 |
| Pulm | 1 (16.7) | 4 (16.0) | 86 (9.5) | 0.466 |
| Immuno | 2 (33.3) | 6 (24.0) | 130 (14.3) | 0.173 |
| KT | 1 (16.7) | 4 (16.0) | 69 (7.6) | 0.221 |
| Neoplasia | 2 (33.3) | 11 (44.0) | 416 (45.8) | 0.819 |
| ASA - n (%) | ||||
| I–II | 1 (16.7) | 10 (40.0) | 487 (53.6) | 0.082 |
| III–V | 5 (83.3) | 15 (60.0) | 422 (46.4) | |
| Approach – n (%) | ||||
| Open | 2 (33.3) | 6 (24.0) | 147 (16.2) | 0.491 |
| Endoscopic | 4 (66.7) | 14 (56.0) | 594 (65.4) | |
| Laparoscopic | 0 | 2 (20.0) | 168 (18.5) | |
| Type – n (%) | ||||
| Scheduled | 2 (33.3) | 17 (68.0) | 663 (72.9) | 0.084 |
| Urgent | 4 (66.7) | 8 (32.0) | 246 (27.1) | |
| Complications and mortality – n (%) | ||||
| Clavien I–II | 3 (75.0) | 1 (33.3) | 82 (65.6) | 0.468 |
| Clavien III–V | 1 (25.0) | 2 (66.7) | 43 (34.4) | |
| In-hospital mortality | 3 (50.0) | 0 | 4 (0.44) | <0.001 |
| 90-day mortality | 3 (50.0) | 3 (12.0) | 23 (2.5) | <0.001 |
CCI: Charlson comorbidity index. AMI: acute myocardial infarction. Pulm: pulmonary pathology (asthma or chronic obstructive pulmonary disease). Immuno: immunosuppression. KT: kidney transplantation. ASA: American Society of Anaesthesiologists classification.
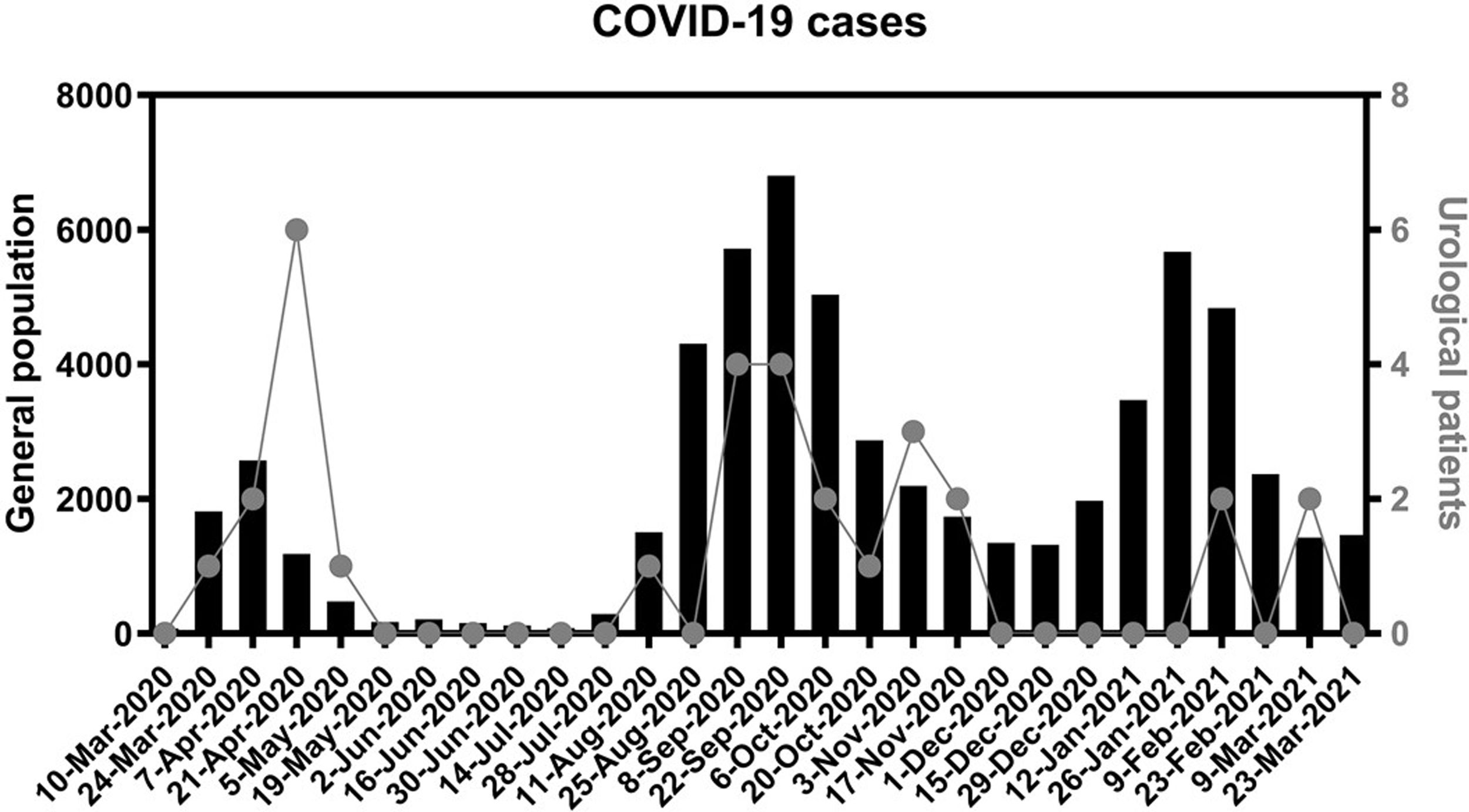
In Fig. 2 we represent the 14-day cumulative incidence of cases in the population attended by our hospital. We also represent the postoperative COVID-19 patients, according to the date in which the symptoms began or the positive PCR was documented.
COVID-19 cases in the general and urological populations.
Each date on X axis represents a cut-off point to calculate the cumulative incidence (sum of diagnosis) during the previous 14 days. Black columns and left Y axis represent this incidence on the general population covered by our hospital (4 districts located in the south of Madrid). Dots and right axis represent this incidence on urological patients that underwent surgery from March 1st 2020 to February 28th 2021.
The first data available about surgical safety during SARS-CoV-2 pandemic were not encouraging. A study from Wuhan reported a 44.1% intensive care unit admission and 20.5% mortality on patients undergoing surgery during the incubation phase of the virus.16 The limited evidence about how to fight the disease and the shortage of resources, led to a complete cessation of elective surgery in many countries.17
Many groups worked quickly on recommendations in order to minimize these risks.4–7 The key points to control the pandemic focused on an overall hospital response for COVID-19 management, healthcare supplies, staff training and multidisciplinary collaboration.18 The importance of logistics to maintain COVID-free pathways (dedicated areas, personnel and operating theatres) and patient selection was marked in many articles.6,19,20 Given that different surgical series achieved good outcomes and the fact that disease mortality was falling, experienced groups recommended to re-start elective surgery.3,21
As many other hospitals located in high incidence areas of SARS-CoV-2 transmission, our institution changed its organization and policies across the epidemiological stages, according to the growing evidence. This study includes every urological surgical patient during all those periods thanks to a prospective design, which allows the analysis of certain aspects that may be helpful to other departments with a similar situation. As observed in Table 1, the main baseline and surgical variables did not change throughout the periods, with the exception of the first wave, when only emergency surgeries were performed for 7 weeks (mainly double J catheter insertions). This fact may influence the lower proportion of comorbid patients (Charlson=2) and postoperative complications.
We found in our series a 0.74% prevalence (7 out of 940) of asymptomatic patients testing positive for coronavirus on preoperative PCR. This prevalence was 0.12% in a study from Victoria22 and 0.86% in another series from North Carolina.23 Authors suggest these rates depend on the coronavirus incidence in the community. For instance, in the first study they considered a suburb to be a “hotspot” if daily incidence was >20 per 100000 inhabitants while at our hospital population it was >50 during both the second and third waves.14
Data from the COVIDSurg studies reveal that patients with SARS-CoV-2 infection diagnosed within 7 days before and 30 days after surgery, have 51.2% pulmonary complications and 38% disease mortality.24 Those who had their surgery delayed at least 4 weeks after the infection had no complications, so it is suggested to wait that period.25 We followed this recommendation, rescheduling 12 patients, without symptoms or findings suggestive of reinfection due to SARS-CoV-2 during follow-up.
A pooled analysis of confirmed COVID-19 cases estimated the median incubation period to be 5.1 days; just 1% of patients will develop symptoms after 14 days.15 Taking this cut-off point, we elaborated the Table 2. Six COVID-19 patients (19.4% of diagnosis) developed symptoms during hospitalization or up to 10 days after discharge, thus being suspicious of in-hospital contagion. Most of them (5 out of 6) happened during the first wave: three when no measures were applied, two when just preoperative PCR testing was performed. Age, comorbidities, anesthetic risk and prognosis was worse for them, being diabetes, obesity, and mortality higher with statistical significance. Thus, avoiding transmission during hospitalization is of paramount importance. This objective was achieved by the complete set of protective measures, as after their implementation, just 1 out of 857 patients contracted this kind of disease.
On the other hand, 25 patients (80.6%) developed symptoms from 15 to 276 days after discharge, hence due to community contagion, with less marked differences compared to non-COVID patients. Furthermore, as we can notice in Fig. 2, all of these COVID-19 patients were diagnosed coinciding within pandemic waves, supporting the idea of community transmission. Among those 25 patients, 16 could be managed with outpatient care, 8 required new admission for treatment (3 died) and 1 died in a nursing home. These kinds of patients are often underrepresented on surgical series, due to their short follow-up. In the Dublin series with 101 urological surgeries, they report a 3% incidence and 33.3% mortality due to COVID-19, being the latest diagnosis 20 days after surgery.26 In a multicenter cohort study from Paris with 552 patients, they found a 2.9% incidence and 18.75% mortality rate, with a median of 37 days follow-up.27 In our study, the mean follow-up is substantially longer (95 days), and that influences our higher COVID-19 incidence (3.3%), while mortality was within the expected range (22.6%). With 37 days of follow-up, we would have missed 14 cases and 3 deaths (45.2% and 42.9% of them, respectively).
No statistical differences were found on the direct comparison of first vs second vs third wave COVID-19 patients, probably due to the low number of cases within the second and third waves (5 patients in total). It is important to note that during those wave peaks, just 1 case was due to in-hospital transmission, which supports the idea that surgery remained safe at our institution after adopting complete protective measures.
We consider that the impact of the vaccination is still not seen in our analysis, as it started in Madrid on January 2021 and very few patients received it before surgery (Fig. 1). It may play a role in the following months and could be the topic of future studies.
Finally, there is additional evidence about specific subgroups of patients undergoing surgery during the pandemic. Regarding cancer patients, given their immunocompromised status (caused both by the neoplasia and its treatments) a greater susceptibility is expected.28 A multicenter study showed that those operated within COVID-free surgical pathway hospitals, had better outcomes: 3.9% SARS-CoV-2 infection, 2.3% pulmonary complications and 0.8% mortality due to COVID.19 All those rates were lower at our institution: 3% incidence (13 out of 429 cancer patients), 0.7% pulmonary complications and mortality (3 out of 429).
Another susceptible group, due to immunosuppressive therapy, major surgery and long admission time, are the kidney transplant recipients.29 Compared to the same period on the previous 2 years, an excess of deaths on kidney transplant candidates and recipients was noted in France between 1 March and 1 June 2020.30 COVID-19 incidence was 1.42% among recipients and 2.95% among candidates (probably due to the difficulties in achieving social distancing while being on chronic hemodialysis), leading to the conclusion that renal transplantation should continue during future waves. Our data support the continuation of renal transplantation as, after the implantation of the preventive measures, just 1 case of COVID-19 was registered 140 days after discharge. This makes an incidence of 1.5% (1 out of 67 transplants) compared to 57.1% (4 out of 7) on the 1st wave.
The main limitations of the study are those inherent to every single-center (single country) series with a modest sample size and low number of events (COVID-19 cases). The main strengths are its real-life setting and wide range of study period (12 months). We believe it may reflect how lessons learnt from the 1st wave via multidisciplinary protocol allowed to better fight the infection during the 2nd and 3rd waves and thus be representative of the experience in many hospitals within the most affected areas around the world. Given the high number of delayed surgeries worldwide, neither the society nor the scientific community would understand another stop on surgical activity during future pandemic waves. As the Spanish Association of Surgeons state, we should continue our fight against the virus without stopping other activities in order to avoid even worse consequences than those generated by the virus itself.17
ConclusionsAfter the implementation of protective and screening measures, surgical safety remained intact even during the pandemic peaks of SARS-CoV-2 pandemic at Madrid, one of the most affected areas in Europe. The cases detected during follow-up were mainly caused by community transmission and were managed with outpatient care, in contrast with the first wave, when there were no specific measures to avoid the infection and many in-hospital cases happened, leading to a high disease mortality. While hospitals follow recommendations to avoid COVID-19 infection, urological surgery is safe and can be maintained.
FundingNothing to declare.
ContributionsState of the art research: Alejandro González-Díaz.
Conception and design: Alejandro González-Díaz, Carolina Varela-Rodríguez, Ángel Tejido-Sánchez.
Acquisition of data: Alejandro González-Díaz, Rocío Santos-Pérez de la Blanca, Javier Gil-Moradillo, Cristina Calzas-Montalvo, María del Prado Caro-González, Silvia Juste Álvarez, Ana de la Calle-Moreno, Julio Téigell-Tobar, Pablo Abad-López, Natalia Rocío Miranda-Utrera.
Statistical analysis: Nicolás Rosillo-Ramírez, Andrés Mauricio Brandini-Romersi.
Interpretation of data: Alejandro González-Díaz, Carolina Varela-Rodríguez, Ángel Tejido-Sánchez.
Drafting of the manuscript: Alejandro González-Díaz.
Critical revision of the manuscript: Javier Gil-Moradillo, Carolina Varela-Rodríguez, Alfredo Rodríguez-Antolín, Ángel Tejido-Sánchez.
Informed consentInformed consent was obtained from all individual participants included in the study.
Conflicts of interestNothing to declare.
We kindly appreciate the contributions of Rocío Santos-Pérez de la Blanca, Cristina Calzas-Montalvo, María del Prado Caro-González, Silvia Juste-Álvarez, Ana de la Calle-Moreno, Julio Téigell-Tobar, Pablo Abad-López, Natalia Rocío Miranda-Utrera and Andrés Mauricio Brandini-Romersi to data acquisition and management.