Currently, there is no treatment approved for COVID-19. Numerous drugs are being used in an empirical manner according to experience and availability. Studies demonstrating their efficacy and safety are still to be published. Thus, it is of vital importance for healthcare workers to be well informed and updated regarding possible immunological and non-immunological adverse effects regarding such treatments. In this narrative revision, the rationale use of these treatments in the SARS-CoV-2 infection is emphasized as well as their most frequently described adverse drug reactions.
Drugs that are being essayed to counteract both clinical phases that are thought to take place in the severe stage of this disease are included; an initial phase where a viral infection prevails and a second phase where an inflammatory response takes over. Adverse reactions registered in the Pharmacovigilance Program of our hospital before the onset of this pandemic have also been included.
Actualmente no hay ningún fármaco aprobado para el tratamiento de la COVID-19. Se emplean fármacos de manera empírica según experiencia y disponibilidad, pero no existen estudios controlados que demuestren su eficacia y seguridad. En este contexto, es importante que los médicos dispongan de información de los posibles efectos adversos tanto inmunológicos como no inmunológicos de estos medicamentos. En esta revisión se repasa el fundamento para su uso en la infección por SARS-Cov-2, así como las reacciones adversas más frecuentes; no se trata de una revisión sistemática sino narrativa.
Se han incluido aquellos fármacos que se utilizan con el fin de abordar adecuadamente las dos fases clínicas que parece tener la enfermedad en su manifestación más grave: una primera fase con predominio de infección viral y una segunda fase con predominio de una respuesta inflamatoria. También se han repasado los casos de reacciones a dichos fármacos recogidas en el Programa de Farmacovigilancia del hospital antes del inicio de la pandemia.
The disease caused by the new Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), Coronavirus Disease 2019 (COVID-19), which started in Wuhan (China) in December 2019, has spread in the form of a global pandemic. As of 15th June 2020, the World Health Organization (WHO) had confirmed 7,823,289 cases and 431,541 deaths worldwide.1 For the medical community, this new disease represents a challenge when it comes to identifying effective therapeutic options for its treatment and prevention. At the moment drugs are used empirically according to experience and availability, so there is still no drug approved for the treatment of COVID-19.
Currently there are multiple randomized clinical trials underway and in clinical practice very diverse drugs are being administered in order to adequately address the two clinical phases that the disease seems to have in its most severe manifestation: a first phase with a predominance of viral infection and a second phase with a predominance of an inflammatory response that can produce an acute respiratory distress syndrome in adults.2
It is foreseeable that, even if the contagion curve flattens, transmission will continue to occur in the coming months, so the number of patients exposed to all this therapeutic arsenal will continue to increase and, with it, the number of potential adverse drug reactions (ADRs) related to these treatments will also increase.
From the Unitat d'Al.lergologia and the Servei de Farmacologia Clínica of the Hospital Universitari de Bellvitge we decided to review the main adverse reactions, both immunological and non-immunological, reported with the drugs used for the specific treatment of COVID-19, in order to identify them early and address their management in a comprehensive manner. Other drugs used to treat possible comorbidities derived from this disease (such as antithrombotic agents to treat coagulopathy) have not been included. To carry out this review we used a selection of the literature identified through the PubMed-Medline databases and search engines, which includes literature references from 1966 to the present, SIETES (www.sietes.org), an information system on developments in clinical and therapeutic pharmacology, the clinical resource UptoDate (https://www.uptodate.com), and the Medinteract drug interaction database (https://www.medinteract.net/). Cases or case series, observational studies, or clinical trials, as well as systematic or narrative reviews with some of the evaluated drugs were included. Information was also collected from the SmPC of each of the products. The result of this review is the object of this study.
The main drugs proposed to treat COVID-19 are reviewed below. The drugs with antiviral and anti-inflammatory activity most widely used in the standard of care (established protocol) of the different hospitals have been considered.
Lopinavir/ritonavirBoth are antiretrovirals of the Human Immunodeficiency Virus (HIV) family of protease inhibitors that are metabolized through the CYP3A isoform of cytochrome P450. This inhibition prevents cleavage of the polyprotein gag-pol, leading to the production of a non-infectious immature virus. Lopinavir provides antiviral activity while ritonavir acts as a pharmacokinetic enhancer, increasing the plasma half-life of the former. Inhibitory activity against SARS-CoV, the virus that causes Severe Acute Respiratory Syndrome (SARS) in humans3 has been demonstrated for lopinavir in vitro. Although some studies with patients hospitalized for severe COVID-19 pneumonia have not been able to demonstrate significant clinical improvement with this drug,4 combining this with other antiviral agents, as it has been done for SARS,3 could open new therapeutic horizons.
Gastrointestinal adverse reactions have been reported in up to 28% of treated patients (diarrhoea, nausea, and vomiting), dyslipidaemia and liver (between 2% and 20%) and pancreatic abnormalities.4 A prolonged QT segment has been reported in the electrocardiogram (ECG), although less frequently. On the other hand, its activity on cytochrome CYP3A carries a high risk of interactions with drugs that also use this metabolic pathway. A review of the concomitant medication and the risks associated with such interactions is recommended.
Cases of hypersensitivity to drug excipients5 and to the antiretroviral drug under consideration are well known. It should be noted that the majority are HIV-infected patients (more likely than the general population to suffer drug-related rashes) and that hypersensitivity reactions due to protease inhibitors are anecdotal, occurring much more commonly in other families within antiretrovirals, such as reverse transcriptase inhibitors.6 Mild skin rashes, in the form of a maculopapular rash, as well as severe skin reactions have been reported.
RemdesivirIt is an unauthorized nucleotide analogue that interferes with the polymerization of the virus RNA. It was initially developed as a treatment for Ebola virus disease but is also active in vitro against other viruses, including the coronavirus. Some studies show greater antiviral in vitro activity combining it with interferon-® versus the lopinavir/ritonavir combination.7 An open study has been published with the first critically ill patients who received compassionate use treatment, showing a clinical improvement of 68%.8 Recently, however, other studies have not confirmed this improvement but, rather, a tendency to shorten the time to reach a clinical response.9 Remdesivir is being evaluated in various clinical trials.10
It is a drug with a safety profile yet to be characterized. The main adverse effect is hypotension secondary to the infusion. Other possible adverse reactions compromise the gastrointestinal tract (nausea, vomiting, diarrhoea, constipation, abdominal pain, etc.).11
HydroxychloroquineIt is a 4-aminoquinoline similar to chloroquine. It has antimalarial and immunosuppressant action. As an immunosuppressant, it inhibits eosinophil chemotaxis, neutrophil migration, and reduces complement-dependent antigen-antibody reactions. Initially used as an antimalarial, this drug has subsequently been used for autoimmune diseases. Several studies have demonstrated the in vitro efficacy of the 4-aminoquinolines against viruses, attributed to different mechanisms, including the cessation of viral replication by increasing endosomal pH, which inactivates the enzymes that are essential for this process.12 Among them, the angiotensin-2 converting enzyme, used by SARS-CoV-2 to enter the cell, stands out. However, these effects have been highly variable12,13 due, in part, to difficult extrapolation of culture concentrations to human doses.14
The Spanish Agency of Medicines and Medical Devices (AEMPS)11,15,16 alerts to the possibility that hydroxychloroquine may prolong the QT interval. Patients with congenital long QT syndrome, uncorrected fluid and electrolyte imbalance, heart disease, or simultaneous treatment with medications (such as azithromycin) that can lengthen this interval are considered risk groups. This risk increases at high doses. During treatment, gastrointestinal disorders (nausea, vomiting and diarrhoea) are common. Hypoglycaemia, blood dyscrasias, headache, deafness, tinnitus, impaired liver function, and photosensitivity have also been reported. Treatment could exacerbate porphyria, psoriasis, and myasthenia gravis.
There are cases of mild reactions in the form of a measles-like rash,17 severe skin reactions such as acute generalized exanthematous pustulosis and systemic drug-induced hypersensitivity syndrome or DRESS syndrome (Drug Rash with Eosinophilia and Systemic Symptoms)18,19 and anaphylaxis.20
IvermectinIt is an avermectin, a type of broad-spectrum semisynthetic antiparasitic derived from Streptomyces avermitilis fermentation. Avermectins bind to chlorine channels of nerve and muscle cells (in invertebrate microorganisms) causing paralysis and death of the parasite.
Originally identified as an inhibitor of the interaction between HIV-1 integrase protein (IN) and importin heterodimer (IMP) 〈/®1 responsible for the nuclear import of IN, antiviral activity has also been found in vitro against various viruses.21 SARS-CoV studies reveal the potential role of IMP〈/®1 during infection. In short, they suggest that the inhibitory activity on nuclear transport of ivermectin may be effective against SARS-CoV-2, demonstrating in vitro loss of viral material in 48 h with a single dose.21 These results have not yet been translated into clinical practice.
The most common adverse reactions are fever, myalgia, headache, itching, and skin rash, which usually occur during the first days of treatment. Ocular adverse reactions have also been reported (anterior uveitis, eyelid oedema, conjunctivitis, or keratitis), gastrointestinal symptoms, arthralgias, drowsiness, or transient increases in transaminases.22 Less common, orthostatic hypotension, ECG abnormalities, tachycardia, etc.
Cases of toxic epidermal necrolysis23 and Stevens-Johnson syndrome24 associated with oral ivermectin have only been rarely reported.
AzithromycinIt is an azalide, a subclass of macrolide antibiotics. It works by inhibiting the synthesis of RNA-dependent bacterial proteins (in sensitive organisms) by binding to the 50 s subunit of the ribosome and inhibiting the translocation of peptides. Macrolides have known antibacterial activity but also have immunomodulatory effects, including anti-inflammatory potential. Recently, its antiviral power has gained great interest.25 Regarding COVID-19, different studies evaluate the use of azithromycin alone or in combination with hydroxychloroquine.26,27
Azithromycin may cause gastrointestinal adverse reactions (with Clostridioides difficile colitis being the most severe) and hepatotoxicity in the form of cholestatic jaundice. Due to its association with a prolonged QT interval, it is recommended to monitor and avoid the simultaneous use of other drugs that can prolong said interval. Other adverse reactions reported are headaches, hearing and balance disorders, psychiatric disorders (agitation and anxiety), arthralgia, interstitial nephritis, and skin rashes.11,16
Allergic reactions are rare with macrolides. Allergy cases occur between 0.4 and 3%.28 However, there are cases of both immediate hypersensitivity (hives, angioedema, and anaphylaxis), and delayed hypersensitivity, with the occurrence of skin symptoms of variable severity.29,30
TocilizumabIt is a humanized monoclonal antibody targeting the interleukin 6 (IL-6) receptor. IL-6 is a pro-inflammatory cytokine that participates in various physiological processes such as the activation of T lymphocytes, the induction of immunoglobulin secretion, the induction of acute phase hepatic protein synthesis, and the stimulation of hemopoiesis. It also participates in the pathogenesis of inflammatory diseases, osteoporosis, and neoplasms. Tocilizumab specifically binds to IL-6 receptors, both soluble and membrane-bound forms (IL-6Rs and IL-6Rm), inhibiting their activation.
Studies conducted in patients who died from SARS and Middle East Respiratory Syndrome (MERS) suggest that mortality is associated with an aberrant response of the immune system, favouring a cytokine storm.31 Fu et al.32 showed an increase in pathogenic Th1 cells, together with inflammatory monocytes with overexpression of IL-6 in patients with severe COVID-19 pneumonia. Thus, in addition to the proposed standard treatment of lopinavir, methylprednisolone, oxygen therapy and symptomatic treatments, they treated critically ill patients with tocilizumab, observing a reduction in mortality.
Upper respiratory tract infections, headache, hypertension, neutropenia, thrombocytopenia, and elevated alanine aminotransferase have been reported as adverse reactions. The most serious ADRs were serious infections, complications of diverticulitis, and pneumonia11.16.
Hypersensitivity reactions are rare and usually range from generalized delayed hives33 to anaphylaxis.34 There are also published cases of hypersensitivity caused by drug excipients.6
SarilumabIt is an immunosuppressant agent that, like tocilizumab, acts by inhibiting IL-6 receptors. In addition to its known use for rheumatoid arthritis, clinical trials (phase II/III) are underway to evaluate its efficacy in patients with severe COVID-19 infection,35 as IL-6 has been shown to play a significant role in SARS and MERS patients.36
The most common ADRs observed in clinical studies were neutropenia, thrombocytopenia, elevated transaminases, injection site erythema, upper respiratory tract infections, and urinary tract infections. The most common serious infections include pneumonia and cellulitis. Cases of opportunistic infections have also been reported.16
BaricitinibIt is a reversible and selective inhibitor of Janus kinases types 1 and 2 (JAK1 and 2). These enzymes transduce intracellular signals involved in haematopoiesis, inflammation, and immune function. As mentioned above, SARS-CoV-2 uses angiotensin converting enzyme to infect lung cells.12 For this reason, baricitinib has been proposed as a possible therapeutic mainstay in patients affected by SARS-CoV-2,37 since it inhibits protein kinase 1 (AAK1) and cyclin kinase-G (GAK), known to regulate endocytosis.38,39
Infections are among the adverse reactions,40 some of which can be serious, including tuberculosis and herpes zoster. Other clinically relevant adverse reactions include thromboembolic events, or neoplastic processes. Laboratory abnormalities such as elevated transaminases, creatine kinase (CK), lipids, neutropenia, lymphopenia, or anaemia have also been reported. Nausea is common with the use of this drug.
AnakinraIt is a human IL-1 receptor antagonist produced in cells of Escherichia coli by recombinant DNA technology.41 The IL-1 family is a group of pro-inflammatory cytokines, with IL-1〈 and IL-1® being those with the greatest inflammatory effect. They regulate and initiate the inflammatory response through the expression of integrins in leukocytes and endothelial cells.42 Anakinra neutralizes the biological activity of IL-1〈 and IL-1® by competitively inhibiting its binding to the type I receptor.
It is indicated to treat adults with rheumatoid arthritis in combination with methotrexate, in whom the latter, alone, has proven ineffective. It is also indicated (from infants from eight months) to treat both periodic syndromes associated with cryopyrin and Still's disease, including systemic juvenile idiopathic arthritis. It has been used as an alternative to colchicine in patients affected by familial Mediterranean fever, an autosomal recessive inflammatory disease.43
Relevant adverse reactions include serious infections, liver damage, thrombocytopenia, and neutropenia (it is recommended not to start treatment with counts below <1.5⋅109/L).44 Due to its immunosuppressant activity, its use in patients with malignant disease is not recommended.
Reactions in the puncture site, consisting of inflammation, erythema, pruritus and pain, are usually very common in biological treatments and, in the case of anakinra, it is due to the large amount of protein solution, which produces a local mast cell degranulation.41
Systemic reactions are usually rare with anti-IL-1 agents, but there are reported cases of immediate hypersensitivity.45–47
TacrolimusIt is a macrolide derived from a Streptomyces tsukubaensis bacterial sample (Tsukuba macrolide immunosuppressant). Inhibits signal transduction pathways in T lymphocytes through the inhibition of calcineurin, thus preventing the transcription of multiple genes related to pro-inflammatory cytokines, including IL-2, as well as type 1 interferons, decreasing the activity of T lymphocytes.48
Clinical trials are currently underway in severe SARS-CoV-2 pneumonia. Its possible efficacy is based on the ability to counteract excessive inflammation caused by a pro-inflammatory cytokine storm.49,50
It is a drug that requires monitoring its blood concentrations. The most common adverse reactions are neuro- and nephro-toxicity.48 Nephrotoxicity can occur acutely by a prerenal mechanism, with impaired glomerular filtration, or in its chronic form, with arteriolar lesions and interstitial fibrosis. Neurological effects include tremor, paresthesias, headaches, depression, confusion, or insomnia. Hypertension, dermatological complications, gingival hyperplasia, gastrointestinal symptoms, or glucose intolerance have also been reported.51 Due to its immunosuppressant effect, it can be associated with opportunistic infections or the reactivation of latent infections. Metabolic effects such as diabetes and haematological effects such as anaemia and leukopenia have also been attributed to it.
Cases of hypersensitivity to the drug's excipients6 and to the drug itself are well known. Although a possible cross-reactivity between macrolides and tacrolimus has been suggested, the cases published are quite rare.52
CyclosporineIt is a peptide produced by the Tolypocladium inflatum fungus with immunosuppressant activity. It prevents the transcription of genes related to pro-inflammatory cytokines by inhibiting the activity of calcineurin. It also acts on the mitochondria, inhibiting their apoptosis. For the same reasons as tacrolimus, this drug may be helpful for SARS-CoV-2 infection. At the same time, some articles suggest that its combination with interferon-〈-1 could be an effective synergistic antiviral activity.53
It is a drug that requires monitoring its blood concentrations. Among its adverse reactions, nephrotoxicity is common and dose dependent. Hypertension (associated with increased peripheral vascular resistance) and lipid abnormalities have also been reported.54 Due to its immunosuppressant effect, it can be associated with systemic or localized infections, especially by opportunistic microorganisms or the reactivation of latent infections.51 Because cyclosporine is partly eliminated through the sebaceous glands, pilosebaceous lesions in the form of hypertrichosis have been reported. Other adverse reactions include gingival hypertrophy, hypomagnesaemia, hyperkalaemia, or hyperuricaemia. It is also recommended to be careful with products that contain Hypericum perforatum (St. John's wort) as they can decrease the blood concentrations of cyclosporine. Cases of hypersensitivity to the drug's excipients have been published.6
GlucocorticoidsThey are chemicals produced in the adrenal cortex which are derived from cholesterol. The indication in COVID-19 would be determined by its ability to reduce the inflammatory response in the lung. Although, according to WHO, they would not be routinely indicated unless there was a concomitant exacerbation of COPD or refractory shock,55 different studies suggest that they can be a good strategy administered as pulses and for the shortest possible time. Recently, investigators from the Randomized Evaluation of COVid-19 thERapY (RECOVERY) clinical trial, which includes more than 11,500 COVID-19 infected patients in the UK, have reported that dexamethasone has decreased deaths in a third of patients on assisted mechanical ventilation and by a fifth in patients only on oxygen use.56 According to the researchers involved, these results will be published shortly.
The widely known side effects of glucocorticoids are basically untoward effects of their own glucocorticoid action or inhibition of the hypothalamic-pituitary-adrenal axis.
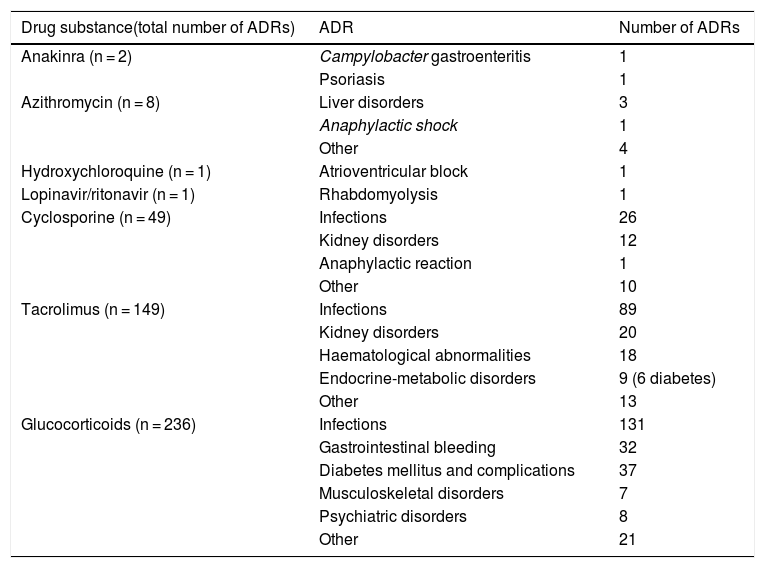
Main adverse drug reactions attributable to these drugsTable 1 details the suspected ADRs registered in the Pharmacovigilance Program (PFV) of the Hospital Universitari de Bellvitge.57 Suspected ADRs causing admission among patients seen in the emergency room and spontaneous notifications made by hospital personnel from 2007 to 2020 have been reviewed. Glucocorticoids are the drugs with the highest ADRs reported, followed by tacrolimus, cyclosporine, and azithromycin, while reports for anakinra, hydroxychloroquine, and lopinavir/ritonavir are rare. For other drugs being used in COVID-19, such as ivermectin, sarilumab, remdesivir, baricitinib or tocilizumab, no suspected ADRs have been reported yet.
Main adverse reactions according to the Pharmacovigilance Program of the Hospital Universitari de Bellvitge.57
| Drug substance(total number of ADRs) | ADR | Number of ADRs |
|---|---|---|
| Anakinra (n = 2) | Campylobacter gastroenteritis | 1 |
| Psoriasis | 1 | |
| Azithromycin (n = 8) | Liver disorders | 3 |
| Anaphylactic shock | 1 | |
| Other | 4 | |
| Hydroxychloroquine (n = 1) | Atrioventricular block | 1 |
| Lopinavir/ritonavir (n = 1) | Rhabdomyolysis | 1 |
| Cyclosporine (n = 49) | Infections | 26 |
| Kidney disorders | 12 | |
| Anaphylactic reaction | 1 | |
| Other | 10 | |
| Tacrolimus (n = 149) | Infections | 89 |
| Kidney disorders | 20 | |
| Haematological abnormalities | 18 | |
| Endocrine-metabolic disorders | 9 (6 diabetes) | |
| Other | 13 | |
| Glucocorticoids (n = 236) | Infections | 131 |
| Gastrointestinal bleeding | 32 | |
| Diabetes mellitus and complications | 37 | |
| Musculoskeletal disorders | 7 | |
| Psychiatric disorders | 8 | |
| Other | 21 |
This review has some limitations. The number of articles published in recent weeks and the speed with which they are published implies that the recommendations, and even the drugs tested, are constantly being modified, so it is likely that some will not appear in this review. The adverse reactions reported are found in the databases reviewed, but it is possible that certain adverse reactions have not been reported or published.
ConclusionsTo date, no treatment has been conclusively shown to improve the prognosis of COVID-19 patients. At the moment, most of the published articles are small observational studies or case series, without randomization or control group. Some drugs have shown activity in vitro, but their potential clinical benefits are unclear. At present, multiple randomized controlled clinical trials are being carried out, which are expected to provide more therapeutic evidence in the near future. It is to be expected that a better knowledge of the mechanisms of action of the virus will suggest new lines of treatment.
In any case, these treatment strategies should be based on the consideration that the benefit outweighs the associated risks of possible adverse reactions, which is why a therapeutic individualization that takes into account the risk of toxicity and the benefit-risk ratio is necessary.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Herrera-Lasso Regás V, Dordal Culla M, Lleonart Bellfill R. Reacciones adversas a fármacos utilizados en el tratamiento específico de la infección por SARS-CoV-2. Med Clin (Barc). 2020;155:448–453.
On behalf of the Grup de Treball Al.lergologia-Farmacologia Clínica Hospital Universitari de Bellvitge. The Group is composed of: Valeria Herrera-Lasso Regás, María Teresa Dordal Culla, Ramón Lleonart Bellfill, Jaume Martí-Garrido, Dolors Rodríguez Cumplido, Paula Vázquez Revuelta, Roser Llop Rius.