In the last two decades, the human race has suffered fatal infections from coronaviruses: severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2012. Recently, a new coronavirus has been identified, called SARS-CoV-2, whose infection in humans was named by the World Health Organization (WHO) as COVID-19.1
Based on a recent meta-analysis carried out in China (38 studies with a total of 3062 patients with COVID-19), the most frequent symptoms identified were: fever, fatigue, cough, dyspnoea and expectoration, and less frequent were: rhinorrhoea, headache and odynophagia, with a relatively low percentage of patients being asymptomatic (11%).1 The most common laboratory findings included lymphocytopaenia, as well as elevated levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), d dimer and erythrocyte sedimentation rate (ESR). The most characteristic finding in chest X-rays or computed tomography (CT) imaging is the ground glass pattern on its own or in combination with pulmonary consolidations, compatible with bilateral pneumonia. SARS-CoV-2 is currently diagnosed using the reverse transcriptase polymerase chain reaction (RT-PCR) assay which identifies the homologous nucleic acid sequence of the SARS-CoV-2 virus in nasopharyngeal and/or oropharyngeal swab samples.1,2
The susceptibility to symptomatic infection increases with age and compared to younger age groups so does the rate of pneumonia and fatality. Patient comorbidities, such as cardiovascular diseases including essential arterial hypertension and coronary heart disease, or diabetes mellitus, show a worse clinical course in patients with COVID-19.1
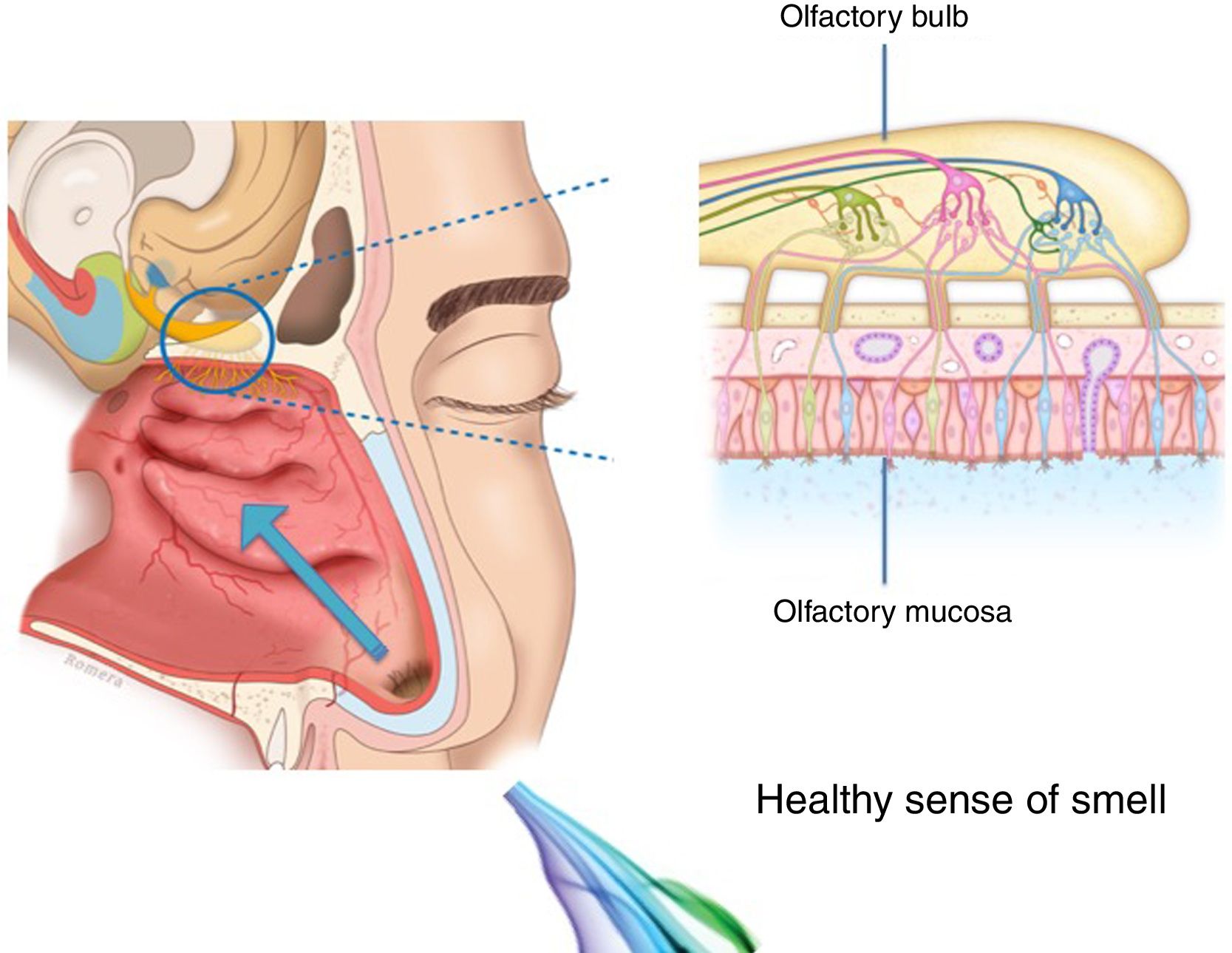
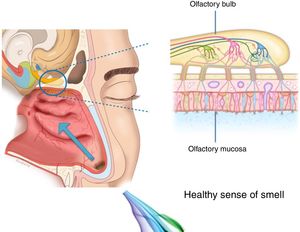
The importance and physiology of smellThe human olfactory neuroepithelium constitutes 1.25% of the nasal mucosa and covers an area of 8–10 cm2 located below the lamina cribrosa, the upper part of the nasal septum and the superior turbinates. Approximately 10 million dendrites of bipolar olfactory sensory neurons in the olfactory bulb project into the mucosa. The odorants that reach the olfactory neuroepithelium dissolve in the mucus layer and bind to activate specific olfactory receptors through a complex interaction that requires specific binding proteins. One odour is capable of activating multiple types of receptors to varying degrees.3
The olfactory sensory neurons send the chemical message by nerve impulses to the olfactory glomeruli in the olfactory bulb. This information is processed and integrated into the olfactory bulb, then it is projected to structures of the limbic system (emotions) and the hypothalamus (long-term memory), and finally to the primary olfactory cortex (inferior and medial areas of the temporal lobe). Smell is the only sense that does not synapse in the thalamus before connecting to the primary cerebral cortex. A human being has approximately 350 functional odorant genes that encode protein receptors. These receptors interact with their own subset of chemicals or substances leading to the complex mechanism of smell identification3 (Fig. 1).
Loss of smell aetiologySmell plays an important role in daily life. It influences food selection and nutrient intake, the identification and enjoyment of food, socialising, overall quality of life and the detection of potentially toxic and harmful substances, thereby proving its importance in the safety against food poisoning or intoxication.4
Olfactory dysfunction (OD) can be classified as quantitative which implies an alteration in intensity, or qualitative when there are changes in the quality of the perception of smells. While normal olfactory function is defined as normosmia, quantitative disorders are classified as partial (hyposmia) or total (anosmia) loss of smell.4 Physiologically, olfactory perception as in detection, memory, and identification, worsens with age. Although smell detection increases and peaks while a person is in their 30 s, the recognition/memory and identification of the different smells decreases once they are in their 60 s.5
The association between OD and smoking is controversial in current literature. Some studies describe a negative impact of smoking on the sense of smell, while others describe a positive impact.5 However, the olfactory function seems to improve when a person stops smoking. A study that included 3900 patients with olfactory loss, 521 active smokers and 316 ex-smokers, concluded that patients who were active smokers did not have significantly lower olfactory function.6
Many diseases can be linked to OD, as well as congenital causes, post-infectious disorders, rhinosinusal diseases, head trauma, and neurodegenerative diseases.3
- a)
Congenital anosmia (aplasia or hypoplasia of the olfactory bulbs and structures) is a very rare condition and it can occur as an isolated abnormality or as a syndrome. Therefore, it is recommended to rule out disorders such as Kallmann syndrome (hypogonadotropic hypogonadism) or Turner syndrome (altered X chromosome) as well as other congenital pathologies such as ciliopathies (Bardet–Biedl syndrome, Leber congenital amaurosis) or hereditary sensory autonomic neuropathy.
- b)
Post-infectious hyposmia/anosmia (common cold, flu) may impair the sense of smell due to a combined inflammatory/conductive and neurodegenerative disorder.
- c)
Inflammatory sinonasal diseases, such as allergic rhinitis or chronic rhinosinusitis/nasal polyps, can cause conductive and inflammatory disorders. They can prevent odorants from reaching the receptors of the olfactory epithelium or cause inflammatory neurodegeneration to the respiratory epithelium.
- d)
Neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, are neurosensory disorders that arise from poor reception or processing of stimuli by the olfactory receptors, olfactory neurons, or the pathways towards the olfactory centres of the central nervous system.
- e)
Olfactory dysfunction due to traumatic brain injury is a neurosensory disorder that is often overlooked after a serious accident, by both the patient and the healthcare personnel due to the focus being on patient stabilisation and the initial treatment.
There are numerous techniques and tools available to explore the olfactory chemosensory capacity both subjectively and objectively, all with advantages and disadvantages.
The olfactometric assessment depends on factors such as patient collaboration, culture (recognition of certain smells), the examiner, and the type of study. There is also a series of environmental factors that depend on the person, such as: age, sex (hormonal variations), toxic habits (alcohol, tobacco, cocaine, heroin), work, medical history, exposure time and social aspects (association of different smells to certain situations).
Subjective testsOlfactometry can include a smell threshold test, which determines the concentration at which the patient is able to identify an odorant (n-butanol, rose) and a smell supraliminal detection test (with odorant concentrations above the patient’s threshold of detection) that allow the detection, recognition/memory and identification of a certain odour to be tested. It is a test designed to assess the olfactory state in a normal or pathological situation, as well as to quantify and interpret the results. The olfactometry of the University of Pennsylvania Smell Identification Test (UPSIT) is the most widely used test in the world. This test comprises of smells (n = 40) that are released when a “scratch & sniff” label of microencapsulated odours on a page is scratched. This test enables the OD to be classified as either anosmia, or mild, moderate or severe hyposmia.7
All or just some of the substances can be studied, and the study can be quantitative or qualitative. A quantitative study determines the lowest concentration of an odour that can be reliably detected (olfactory threshold) and aims to study olfactory variations depending on the odour concentration and the quantity of odours detected, giving a result of anosmia (total loss), hyposmia (partial loss) or normosmia (normal smell). The qualitative study analyses the ability to describe odour qualities, evaluating the qualitative variations of the odorant substances used, analysing the error in the response of a known smell from a list of four or five alternatives, of which only one is correct (forced identification). These tests help to study the patient’s smell identification, olfactory discrimination and/or olfactory threshold. They are indicated for diagnosing OD and classifying its severity, as well as for monitoring progression or reversal of the loss of smell. The methodology must be validated and performed by a well-trained healthcare professional.
A simple, fast and easy tool to use is the visual analog scale (VAS). It is a horizontal line 10 cm long where 0 equals no loss of smell and 10 equals total loss of smell — and the patient marks on the line the point he/she feels represents his/her current state.
Objective testsObjective tests are especially used in research, and some have been developed in which the data does not depend on patient subjectivity.5–7 Tests to be highlighted include the electrophysiological tests of olfaction (electro-olfactogram, olfactory evoked potentials), olfactory structural and functional imaging tests, and biopsies of the olfactory region. The limitations are that these tests can only be performed in highly specialised centres; furthermore, they do not discriminate between the different causes of OD, and they do not provide information on the possible location of the damage.
- -
Electro-olfactogram (EOG). The EOG records the magnitude of the electrical activity of the nasal olfactory epithelium by applying olfactory stimuli while the activity is recorded using intranasal electrodes applied by the specialist. This test is generally poorly tolerated by patients.
- -
Olfactory evoked potentials (OEP). OEPs collect the electrical activity (of the olfactory bulb and/or the frontal cortex) using external electrodes while olfactory stimuli are applied. For a quality record it is essential to stabilise the room temperature, air humidity and the stimuli (they must be applied every 30–45 s), to overcome the difficulty of detecting a very slight signal.
- -
Computed tomography (CT). CT is very useful in imaging the sinonasal tract for evaluating inflammation. Its use is indicated in nasal obstruction (deviated nasal septum, inferior turbinate hypertrophy, nasal polyps or tumours).
- -
Magnetic resonance imaging (MRI). MRI evaluates the olfactory bulb and tract. It is the method of choice when the suspicion is CNS lesions, and it also allows direct visualisation of the olfactory bulb and extensions (Kallmann syndrome or agenesis), the skull base (tumour invasion), inflammatory lesions (encephalitis or multiple sclerosis), vascular lesions and other neoplasms. The Barcelona Olfactory Identification Score (BOIS) is a recently validated test which gives a score to the damage to the brain and olfactory structures associated with the loss of smell.
- -
Positron emission tomography (PET). PET indirectly measures the brain function using radioisotopes to visualise brain functions; it has the unique advantage of providing measurement in vivo of specific brain functions, such as the cortical responses to smells.
- -
Functional magnetic resonance imaging (fMRI). Unlike the previous technique fMRI does not require radioactive material and it detects the patient’s brain activity when performing psychocognitive, sensory or motor tasks. It identifies cortical areas that are activated in different parts of the brain when faced with olfactory stimuli: entorhinal cortex, amygdala, insular cortex, putamen and visual cortex.
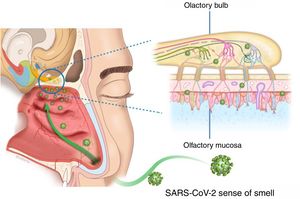
The presentation of OD in viral infections such as the common cold or flu is very common, and many viruses lead to OD due to an inflammatory reaction in the nasal mucosa, with increased production of mucus (rhinorrhea), and/or in the neuroepithelium olfactory. The commonly-known agents are rhinovirus, parainfluenza, Epstein–Barr virus and some coronaviruses. The follow-up of postviral olfactory loss showed that over 80% of patients had subjective recovery at one year. The exact pathophysiology of postviral OD remains under study. No specific upper respiratory symptoms allow COVID-19 to be reliably distinguished from other types of viral respiratory infections.3
There appear to be two likely causes: (a) during an upper respiratory infection the loss of smell occurs as a result of nasal swelling, mucosal oedema and obstruction of the airflow into the olfactory cleft, and/or (b) a postviral loss of smell is caused by infection and direct swelling of the olfactory mucosa, leading to the subsequent neurodegeneration of the olfactory neuroepithelium. Damage to and dysfunction of the peripheral olfactory system, revealed by hyposmia or anosmia, could be a relevant indicator of disease progression8 (Fig. 2).
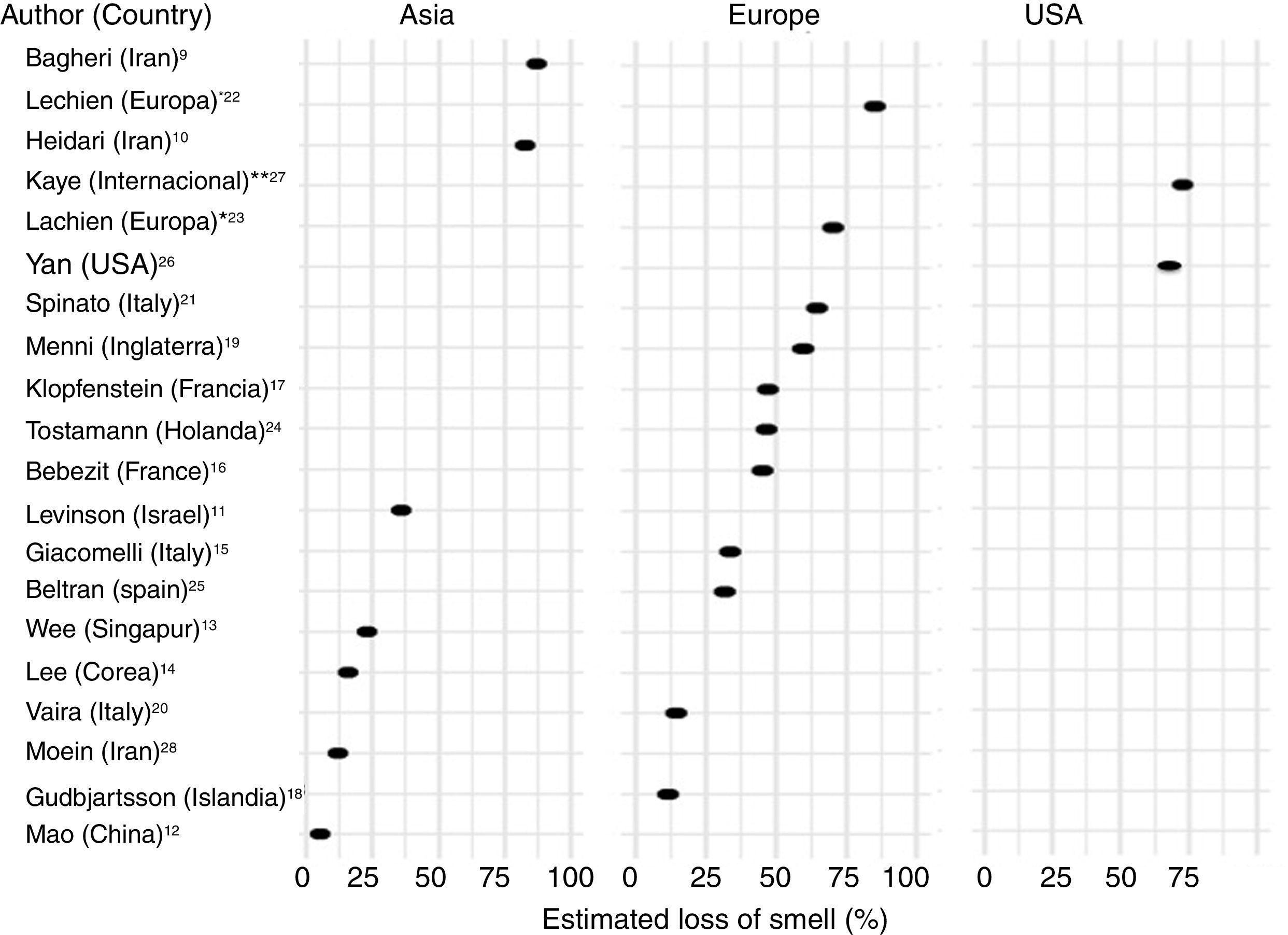
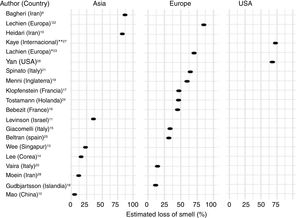
Frequency of olfactory dysfunction during the COVID-19 pandemicObservational studiesThere is a current increase in papers reporting on the frequency of OD in patients with COVID-19. These figures vary from 5% to 85% according to the country and the study (Fig. 3), and can be classified according to regions and continents: Asia (China, Singapore, Wuhan, Korea), Europe (Italy, France, United Kingdom, Spain) and America.
AsiaOne of the first published studies was from Iran, based on telephone surveys. It showed a significant increase in new-onset anosmia since the COVID-19 outbreak. It also reported a strong correlation between the number of self-reported hyposmia/anosmia cases and the COVID-19 cases, with the frequency of OD being 87%.9
Another case series (n = 23) also conducted in Iran, regarding smell in COVID-19 patients, showed that 83% of its patients presented OD,10 while another study (n = 42) conducted in Israel reported OD at 35.7%.11
A study in China of hospitalised COVID-19 patients with pneumonia showed a loss of smell and taste of only 5.1 and 5.6%, respectively.12 In this study, conducted by neurologists, data was collected retrospectively from medical records, which could have led to an underestimation of the true prevalence. These nasal symptoms may not have been reported spontaneously because the patients were not questioned in a targeted manner, and are consequently not reflected in the medical history.
Wee et al.13 in the study of an Asian cohort (n = 154) conducted in Singapore, concluded that self-reported OD had high specificity as a detection criterion for COVID-19. COVID-19 patients appeared to have higher odds of OD (22.7%) compared to those who were positive for other respiratory viruses.
In Korea, 3191 patients were included in a study by telephone interview and 15% of COVID-19 patients were found to have anosmia at the onset of the disease.14
EuropeThe first European study included 59 hospitalised patients in Italy. 33.9% reported a taste or smell disorder, and 18.6% both symptoms. 20.3% had symptoms before hospital admission, while 13.5% experienced the symptoms during the hospital stay.15 Benezit et al.16 studied 68 patients with COVID-19 and concluded that 45% had OD. Klopfenstein et al.17 analysed 114 patients confirmed with COVID-19 in France, who confirmed OD in 47% of cases, with taste alteration in 85%. In this study OD was the third symptom in 38% of cases. OD developed 4.4 days after the onset of infection. A selective and population-based evaluation conducted in Iceland in 528 COVID-19 patients demonstrated an OD of 11.5%.18
In another UK study, 579 positive and 1123 negative COVID-19 patients were included. The authors concluded that loss of smell and taste (59%) is a strong predictor of having been infected by the SARS-CoV-2 virus.19
In Italy, Vaira et al.20 reported an anosmia rate of 19.4% in 320 COVID-19 cases. However, the study does not provide information on the method used for measuring loss of smell. In another Italian cohort (n = 202), similar results were described, with an OD of 64.4%.21
A multicentre study of 417 patients with COVID-19 carried out in 12 European hospitals in France, Belgium, Italy and Spain described a frequency of OD of 85.6% (20.4% with hyposmia and 79.6% with anosmia) and with an impact on the quality of life, as well as 88.8% with taste disorders. OD appeared before (11.8%), after (65.4%) or at the same time (22.8%) as the local symptoms in the ear, nose and throat, with the particularity of not being associated with rhinorrhea.22
Lechien et al.,23 in a European multicentre study, included 1420 patients with mild-moderate COVID-19 symptoms who did not require hospitalisation and observed an OD of 70.2%.
In the Netherlands in a study of healthcare workers, anosmia was observed in 47% of SARS-CoV-2 positive cases.24
In Spain, Beltrán-Corbellini et al.25 included 79 patients with COVID-19 and observed anosmia in 45% of the cases.
AmericaIn the United States, Yan et al.26 included a total of 1480 patients with influenza-like symptoms who underwent PCR testing for SARS-CoV-2. Loss of smell and taste was reported in 68% and 71%, respectively, of COVID-19 positive patients (n = 59), compared to 16% and 17% of COVID-19 negative patients (n = 203) (p < 0.001).
An international cross-sectional study (United States, Mexico, United Kingdom and Italy) in 237 patients with COVID-19 reported an OD of 73%.27
Olfactometry studiesDue to the limitations related to the spread of the disease, emergency contingencies and the risk of contamination of healthcare personnel and patients, it is difficult to methodologically implant the performance of smell tests (olfactometry, VAS). Future studies using psychometric smell tests will be very important to corroborate these patient-reported subjective assessments of loss of smell. To date there are only two studies that have used a smell quantitative tool. In Iran, Moein et al.28 used the UPSIT identification test, which showed that 98% of COVID-19 patients (n = 60) presented some type of OD, with 33% severe loss and 58% anosmia. Another study in Italy used the Connecticut Chemosensory Clinical Research Center (CCCRC) olfaction test, finding an OD of 73.6% (53 patients) in patients with COVID-19 (n = 72).29
TreatmentThree out of four COVID-19 patients present an improvement in OD one month after diagnosis.26 This improvement over time suggests a competitive action of the virus on the smell and taste cell receptors or a local inflammatory phenomena, rather than permanent damage to the olfactory neuroepithelium. If OD persists, it may be reasonable to consider treatment. The efficacy of available treatments for postviral OD is unknown.
Olfactory training involves repetition and repeated exposure to a set of odours (lemon, rose, cloves and eucalyptus) for 20 s each (twice a day) for at least 3 months. Damm et al.30 conducted a randomised, controlled and multicentre study concluding that olfactory training is a safe procedure and useful in patients who begin the training within 12 months after the onset of OD. Olfactory training improves OD, and the use of odours in high concentrations is beneficial for olfactory improvement.
Oral and intranasal corticosteroids have been used to exclude an inflammatory component. However, they are not currently recommended for people with COVID-19 due to a lack of scientific evidence and risk of adverse effects. In contrast, patients who were using intranasal corticosteroids prior to developing COVID-19 should continue with this medication.4
Other medications that have shown promise in postviral anosmia include:
- -
Intranasal sodium citrate, which appears to modulate olfactory receptor transduction cascades.
- -
Intranasal vitamin A, which may act to promote olfactory neurogenesis.
- -
Omega-3, which may act through neuroregenerative or anti-inflammatory means.
However, to date, there is no evidence that these therapies are effective in patients with OD related to COVID-19. Long-term clinical studies are needed to investigate the spontaneous resolution and duration of OD and to define the therapeutic management of patients with permanent anosmia.
Conclusions and special recommendationsA sudden and severe OD in the context of the COVID-19 pandemic and in the absence of other respiratory diseases, such as allergic rhinitis, acute or chronic rhinosinusitis, should alert doctors to the possibility of a SARS-CoV-2 infection. Studying the sense of smell in people with a clinical suspicion of infection can be useful to identify patients who require isolation measures and/or initial treatment. In the face of a fast-onset and growing pandemic such as COVID-19, there is a need for similarly quick investigative techniques that use real-time data collection. Evaluating the loss of smell with olfactometry (predominantly for individualised use) or even with a VAS could help early detection of infected patients and could reduce the number of infections thereby avoiding the spread of the virus.
During the COVID-19 pandemic, patients with sudden and severe loss of the sense of smell are advised to initiate social distancing measures, preventive home isolation, and perform diagnostic tests for SARS-CoV-2 when possible. Finally, the possibility of olfactory training should be evaluated in patients with permanent loss of smell, after a month of lack of recovery of smell.
Conflict of interestsIsam Alobid is a consultant for Novartis, Roche, Menarini and Mylan. The rest of the authors declare that they have no conflict of interest.
Please cite this article as: Izquierdo-Domínguez A, Rojas-Lechuga MJ, Mullol J, Alobid I. Pérdida del sentido del olfato durante la pandemia COVID-19. Med Clin (Barc). 2020;155:403–408.