COVID-19 is a health crisis that triggered the need to find a rapid and sensitive tool to screen populations with a high risk of complications. Lactate dehydrogenase (LDH) is an enzyme found in almost all body cells, particularly pneumocytes, and appears to be associated with worst outcome. Pneumomediastinum (PM), which results from ruptured alveoli, can occur in non-ventilated patients. Acute pneumocytes injury induces the release of serum LDH.
ObjectiveThis study evaluates the role of baseline serum LDH levels in predicting COVID-19 lung necrosis.
MethodsThis retrospective study was conducted among 524 COVID-19 patients admitted to Hôtel-Dieu de France university hospital, Lebanon, between March 2020 and March 2021. Baseline serum LDH was retrieved from patients’ medical records. Radiological severity outcomes were assessed at admission and during follow-up by non-contrast computed tomography (NCCT) of the chest.
ResultsThe mean age of participants was 63 ± 16 years, with 359 males (68.5%) and median (IQR) LDH levels upon admission of 328 (248–430). LDH was correlated with lobar involvement at both admission and NCCT follow-up (Spearman’s rho 0.527 and 0.264, respectively) and the development of a PM (p = 0.035) in 3% of the patients. Using ROC analysis, a baseline LDH value higher than 395 U/L was associated with the presence of a PM on admission and follow-up chest CT, with a sensitivity of 75% and a specificity of 60.1%.
ConclusionBaseline LDH levels could serve as a tool for early diagnosis of severe pulmonary injury with poor radiological outcomes in hospitalized COVID-19 patients.
El COVID-19 es una crisis sanitaria que desencadenó la necesidad de encontrar una herramienta rápida y sensible para el cribado de poblaciones con alto riesgo de complicaciones. La lactato deshidrogenasa (LDH) es una enzima que se encuentra en casi todas las células del cuerpo, particularmente en los neumocitos, y parece estar asociada con el peor resultado. El neumomediastino (PM), que resulta de la ruptura de los alvéolos, puede ocurrir en pacientes no ventilados. La lesión aguda de neumocitos induce la liberación de LDH sérica.
ObjetivoEste estudio evalúa el papel de los niveles séricos basales de LDH en la predicción de la necrosis pulmonar por COVID-19.
MétodosEste estudio retrospectivo se realizó entre 524 pacientes con COVID-19 ingresados en el hospital universitario Hôtel-Dieu de France, Líbano, entre marzo de 2020 y marzo de 2021. La LDH sérica basal se recuperó de los registros médicos de los pacientes. Los resultados de gravedad radiológica se evaluaron al ingreso y durante el seguimiento mediante tomografía computarizada (TCNC) sin contraste del tórax.
ResultadosLa edad media de los participantes fue de 63 ±16 años, con 359 varones (68,5%) y mediana (IQR) niveles de LDH al ingreso de 328 (248-430). La LDH se correlacionó con la afectación lobar tanto al ingreso como al seguimiento de la NCCT (rho de Spearman 0,527 y 0,264, respectivamente) y el desarrollo de un PM (p=0,035) en el 3% de los pacientes. Mediante el análisis ROC, se asoció un valor basal de LDH superior a 395 U/L con la presencia de un PM al ingreso y seguimiento de la TC de tórax, con una sensibilidad del 75% y una especificidad del 60,1%.
ConclusiónLos niveles basales de LDH podrían servir como una herramienta para el diagnóstico precoz de lesión pulmonar grave con malos resultados radiológicos en pacientes hospitalizados con COVID-19.
Since December 2019, the novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has triggered a global health crisis that has affected the populations and spread rapidly worldwide.1 Until October 2021, more than 244 million confirmed COVID-19 cases and 5 million deaths had been reported. This virus can cause symptoms ranging from mild (with low-grade fever and cough) to severe (dyspnea and high-grade fever), which can be fatal due to pneumonia and acute respiratory distress syndrome (ARDS).2
Several studies have been conducted to identify quick and sensitive ways to screen patients at high risk of complications. At present, biological predictors of severe disease and prognosis are not well understood.3 The severity of the disease was associated with lymphopenia and cytokine release syndrome (CRS),1,4 while monocyte count could be normal or increased.5 SARS-CoV-2 is known to have broad cell tropism, and its binding to the angiotensin-converting enzyme 2 (ACE2) receptor is crucial in the physiology of virus entry into the host cell. ACE2 is mainly expressed in type II pneumocytes, the principal defender of the alveolus.6
The virus penetration into the pneumocytes causes vasoconstriction, inflammation, apoptosis, and lung tissue fibrosis, thus inducing the release of LDH.7
COVID-19 patients with high LDH serum levels on admission present a higher risk of ARDS and complications.8 An LDH serum threshold of 450 IU suggests pneumocystosis (PCP) in HIV patients with pulmonary problems, whereas normal values make that diagnosis less likely.9
A national-level tertiary children's hospital in Shanghai used LDH as a biomarker of refractory pulmonary pathology, where a threshold of 379 IU/L predicted refractory M. pneumoniae at the early stage of hospitalization.10
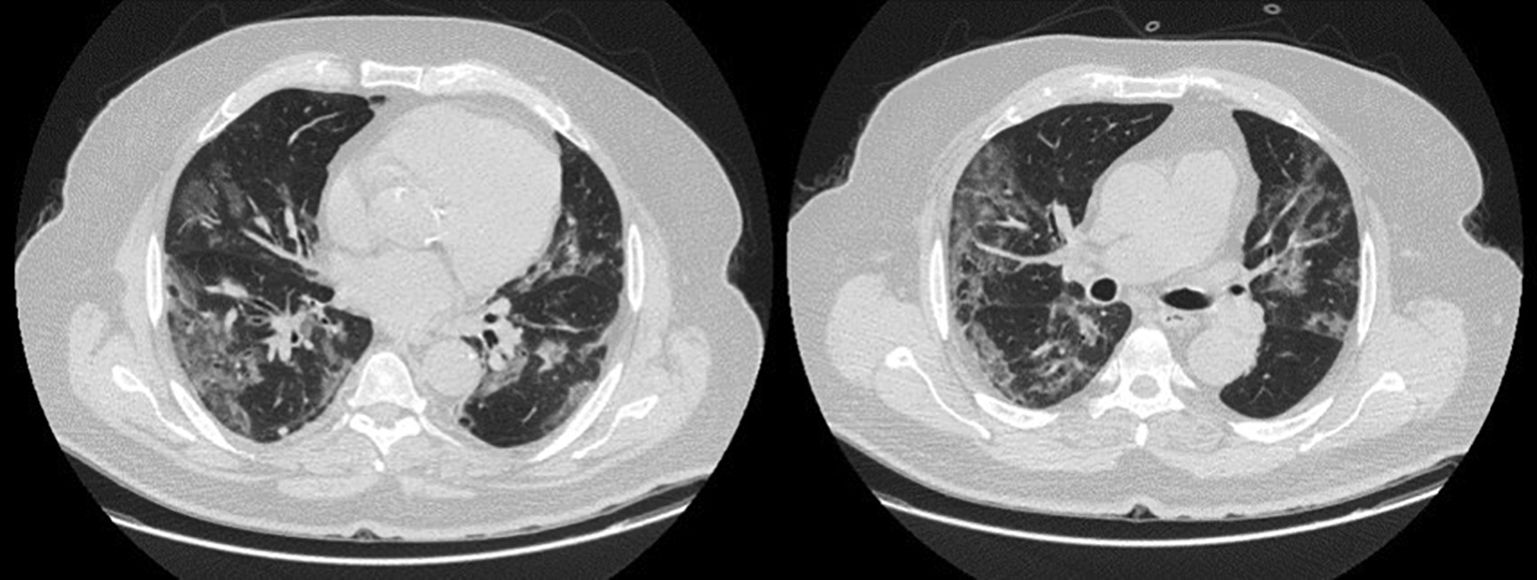
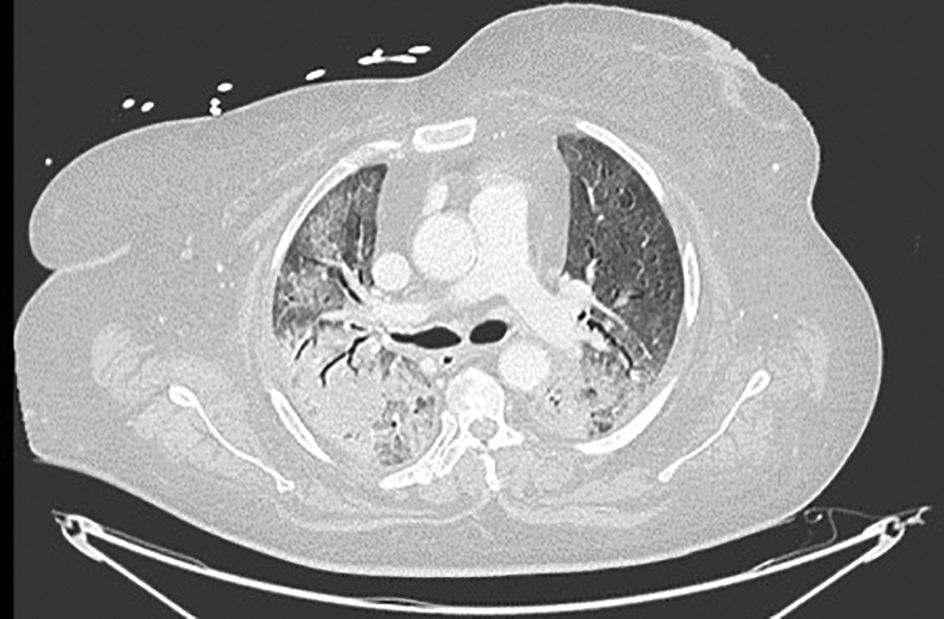
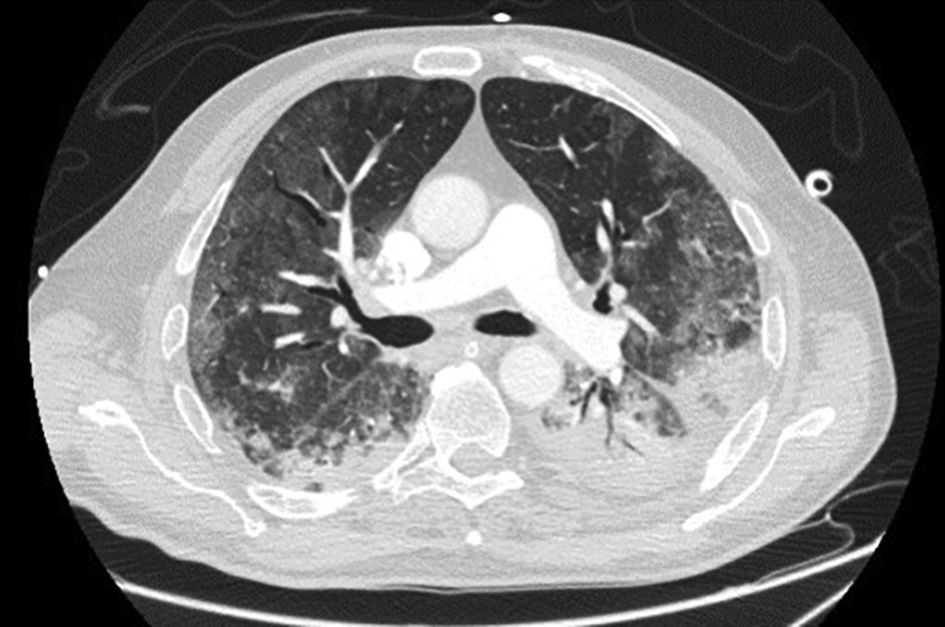
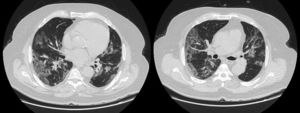
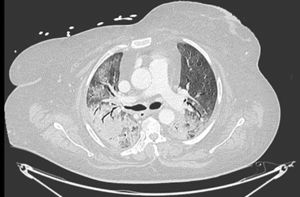
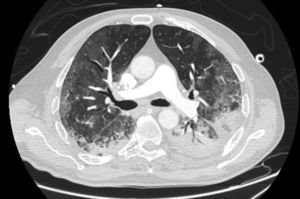
LDH has a significant positive correlation with the severity of pneumonia quantified on initial computed tomography (CT) in COVID-19 patients.11 Patients with severe disease presented higher levels at admission than those with a non-severe disease.12 Hence, LDH indicates the severity of tissue damage and the risk of worsening. The most characteristic chest CT abnormalities in COVID19 pneumonia were pure ground-glass opacities (GGO) (Supp. Figure 2) or mixed with consolidation (supp. Figures 3-4) or a reticular pattern, usually multifocal, asymmetric, bilateral, and peripheral, most commonly located in the inferior lobes.13 GGO is a radiological term revealing an area of increased, hazy lung opacity through which bronchial structures and vessels are still visible, consolidation was defined as the presence on a chest CT of opacities with obscuration of the pulmonary vessels’ airway walls.
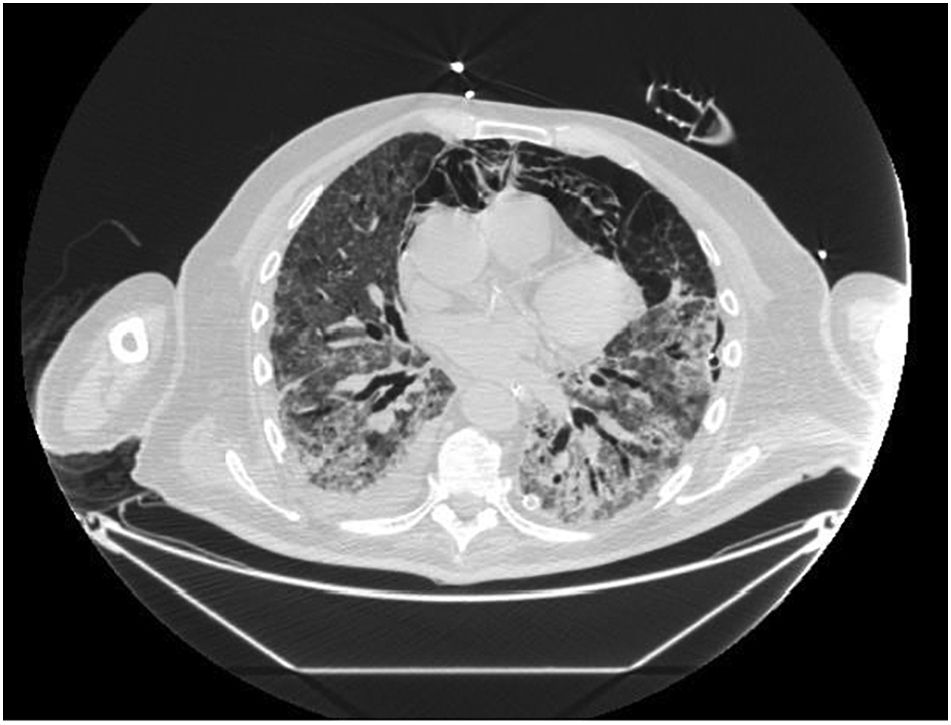
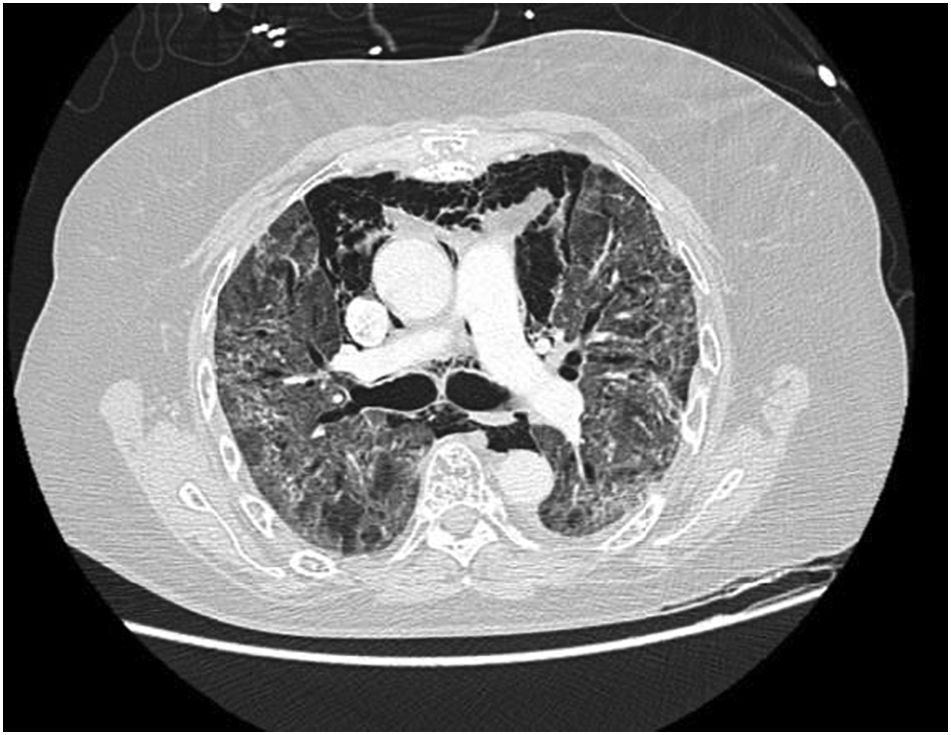
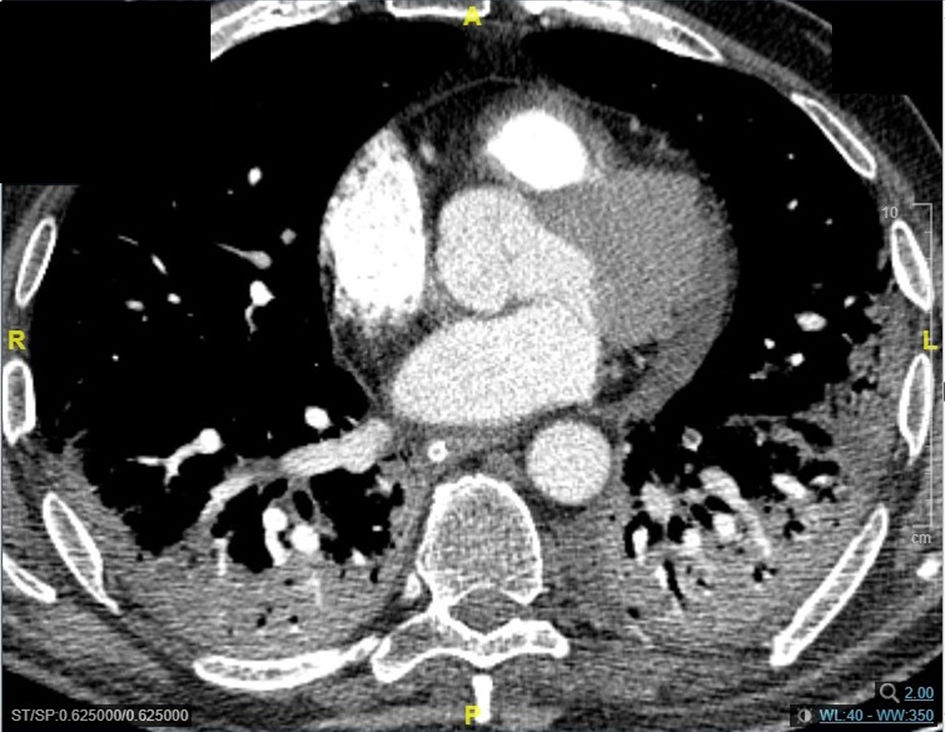
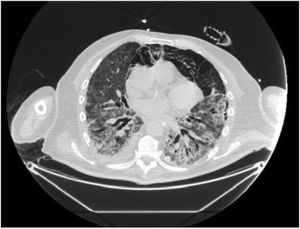
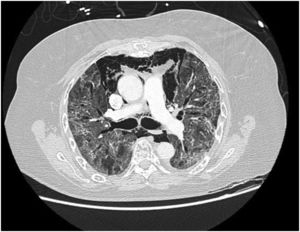
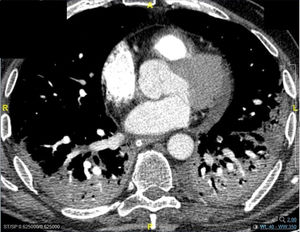
Pneumomediastinum (PM) is a condition where the air is present in the mediastinum. Free air leaks from ruptured alveoli, dissecting along the bronchovascular sheaths towards the mediastinum. PM, as seen on chest CT, elevates the mediastinal pleura and can extend into the neck or chest wall.14 One of the first signs that simulate a pneumomediastinum is the Mach band effect. This optical effect at the margin between areas of faintly different density15 can mimic various other pathologies (pneumopericardium, fracture), and makes it possible to suspect PM in COVID-19 patients. Non-traumatic PM is a rare complication of COVID-19 pneumonia.16 Its development in COVID-19 infection is also considered a possible indicator of disease worsening, which may be or not be associated with invasive ventilatory support. Spontaneous PM seemed to be a frequent complication of severe acute respiratory syndrome (SARS).17 In 2004, a high peak LDH level of 863 IU/L had been correlated with spontaneous PM in SARS patients, likely due to significant remodeling of the lung tissue causing leakage and vessel wall dissection.17 Most patients with PM had lung involvement higher than 50% at the moment of the diagnosis,18 corroborating the increased risk of rupture and PM development. This study aimed to evaluate the association between baseline serum LDH levels and COVID19 lung necrosis outcome based on the extent of lobar involvement and PM development.
Patients and methodsThis single-center retrospective analysis was conducted at Hôtel-Dieu de France (HDF) Hospital in Beirut, Lebanon, among patients hospitalized between March 2020 and March 2021. Patients admitted to the hospital were eligible for the study if they were adults (≥18 years of age) and had a confirmed diagnosis of COVID-19 with real-time polymerase chain reaction (rRT-PCR) assay.
The following data were collected from the database of COVID-19 patients: demographic characteristics, including age and sex; medical history (presence of arterial hypertension, diabetes mellitus, immunosuppression, cardiovascular diseases, and chronic renal failure); admission laboratory results; admission and follow-up chest imaging findings; admission and follow-up radiological severity outcomes.
Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m3. Immunosuppression was defined as any condition that could compromise immunity, including treatment with immunosuppressants or oral glucocorticoids (dexamethasone more than 3.0 mg per day) for more than 1 month before hospital admission, chemotherapy within 2 weeks of hospital admission, impaired immunodeficiency, and HIV. Cardiovascular disease included conditions affecting the heart or blood vessels, such as coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis, pulmonary embolism, and heart attacks or strokes. Admission laboratory results included baseline serum LDH (U/L), ferritin (ng/mL), CRP (mg/L), procalcitonin (μg/L), lymphocyte (/μL), and D-dimers (μg/mL). Chest imaging findings at admission and during follow-up comprised the percentage of ground glass, the presence of lobar consolidation, pulmonary embolism, and pneumomediastinum. Radiological severity outcomes were assessed upon admission and during follow-up using Non-Contrast Computed Tomography (NCCT), which included a semi-quantitative CT score based on the extent of lobar involvement. All CT images were reviewed by a senior radiologist expert in thoracic imaging and 2 fellowship-trained radiologists with approximately 3 years of experience in the Radiology Department at HDF. The baseline chest NCCT was systematically performed, while a contrast CT was ordered in case of clinical suspicion of pulmonary embolism (supp. Figure 1). A follow-up chest CT was performed whenever the patient clinical status worsened despite optimal treatment, pending transportability in critical situations. We evaluated the extension of the ground-glass opacities on chest CT, the number of patients with consolidation and with a mixed pattern. A mixed pattern was defined in our study as a GGO extension greater than or equal to 25%, along with a consolidation pattern.
A chest CT severity score was used in our study to evaluate the severity of pulmonary involvement, as proposed by Chung et al.19 The parameter used in this score was lung lobar extension. The GGO extension in each of the 5 lobes of the lung was scored as follows: no involvement (0), minimal involvement (1), mild (2), moderate (3), and severe involvement (4). A total severity score is thus obtained by adding the scores of each of the 5 lobes (range 0–20). The total was then multiplied by 5 to have a percentage.
In our study, CT was used to identify and confirm spontaneous PM and PM relative to mechanical ventilation. The scannographic signs of PM relied on the anatomical region occupied by the air as it exits the mediastinum.20 (Figs 1, 2).
Data were analyzed using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows) version 26. Quantitative variables not departing from normality assumptions were presented as mean ± standard deviation. Laboratory results on admission, including LDH levels, were right-skewed and expressed as median with their interquartile range (Q1–Q3). Qualitative variables were presented as percentages. The Spearman correlation coefficient and the Mann–Whitney tests were used to assess the association between LDH and NCCT radiological aspect, lobar consolidation, pulmonary embolism, and PM, as appropriate. A p-value less than 0.05 was considered significant. No correction for multiplicity was performed.
For graphical purposes, given its right-skewed, LDH was log-transformed to build the scatter plot with the extent of radiological damage. ROC analysis was performed to find the baseline LDH level cut-off with the best sensitivity and specificity to predict PM.
This study was approved and written informed consent was waived in the context of the COVID-19 pandemic by the ethics committee of HDF and the Saint Joseph University of Beirut, Faculty of Medicine (approval number: CEHDF-1630). This study was performed in accordance with the Declaration of Helsinki.
ResultsBetween March 2020 and March 2021, 524 COVID-19 hospitalized patients had a baseline serum LDH, with median values of 328 U/L (248–430 U/L). The mean age of participants was 63 ± 16 years, with 359 males (68.5%). Hypertension (55.9%), diabetes (28.1%), and cardiovascular disease (25.8%) were the most prevalent comorbidities. A chest CT on admission was performed in 451 (86%) patients, and a follow-up chest CT was ordered for 210 (40%) patients (Table 1) at a median of 9 days (IQR 5–22 days).
Patients characteristics.
| Total (n = 524) | |
| Age (years) | 63 (47–79) |
| Male | 359 (68.5%) |
| Female | 165 (31.5%) |
| Hypertension | 293 (55.9%) |
| CVD | 135 (25.8%) |
| Diabetes mellitus | 147 (28.1%) |
| Immunosuppression | 47 (9%) |
| Chronic renal failure | 83 (15.8%) |
| Baseline chest CT | Total (n = 451, 86%)a |
| Ground-glass estimation (%) | 26 (8%–44%) |
| Lobar consolidation | 38 (8.4%) |
| PE | 3 (0.7%) |
| PM | 2 (0.4%) |
| Mixed pattern | 21 (4.5%) |
| Follow-up chest CT | Total (n = 210, 40%)b |
| Ground glass estimation (%) | 40 (14%–66%) |
| Lobar consolidation | 30 (14.3%) |
| PE | 10 (4.8%) |
| PM | 12 (5.7%) |
| Baseline and follow up chest CT | Total (n = 524) |
| PM | 14 (2.7%)c |
| Laboratory on admission | Total (n = 524) |
| Lactate dehydrogenase (U/L) | 328 (248–430) |
| Ferritin (ng/ml) | 728 (353–1299) |
| CRP (mg/L) | 83 (34–148) |
| Procalcitonin (μg/L) | 0.17 (0.08–0.45) |
| Lymphocyte (/L) | 835 * 109 (560-109–1225*109) |
| D-dimer (μg/ml) | 0.77 (0.45–1.77) |
CT: Computed tomography, CVD: Cardiovascular disease, CRP: C-Reactive Protein, Mixed Pattern: More than 25% of Ground Glass opacity on Chest –CT with lung consolidation PE: Pulmonary Embolism, PM: Pneumomediastinum.
GGO occupied 26% (8%–44%) of the lung area on baseline chest CT and 40% (14%–66%) on follow-up. Lobar consolidation was found in 38 (8.4%) patients on baseline chest CT and 30 (14.3%) patients on follow-up. Follow-up chest CT showed a higher number of patients with PE and PM than on admission: PE was found in 3 (0.7%) on admission versus 10 (4.8%) patients on follow-up, noting that a contrast chest CT was only done in case of high suspicion of PE. Two (0.4%) patients presented a PM on admission, and 12 (5.7%) had PM on follow-up. A mixed pattern was detected in 21 (4.5%) patients on admission chest CT.
The median baseline values were as follows: ferritin 728 ng/mL (353–1299 ng/mL), CRP 83 mg/L (34–148 mg/L), procalcitonin 0.17 μg/L (0.08–0.45 μg/L), lymphocytes 835*109/L (560*109–1225*109/L), and D-dimer 0.77 μg/mL (0.45–1.77 μg/ml) (Table 1).
Baseline serum LDH was fairly correlated with the percentage of NCCT ground glass on admission (Spearman’s rho 0.527; 95% CI 0.451–0.591, p < 0.001) and follow-up (Spearman’s rho 0.264; 95% CI 0.127–0.390, p < 0.001).
Of the total sample, 2.7% had PM on CT (Table 1), of which 1.35% were ventilated/intubated, and 1.35% had a spontaneous PM.
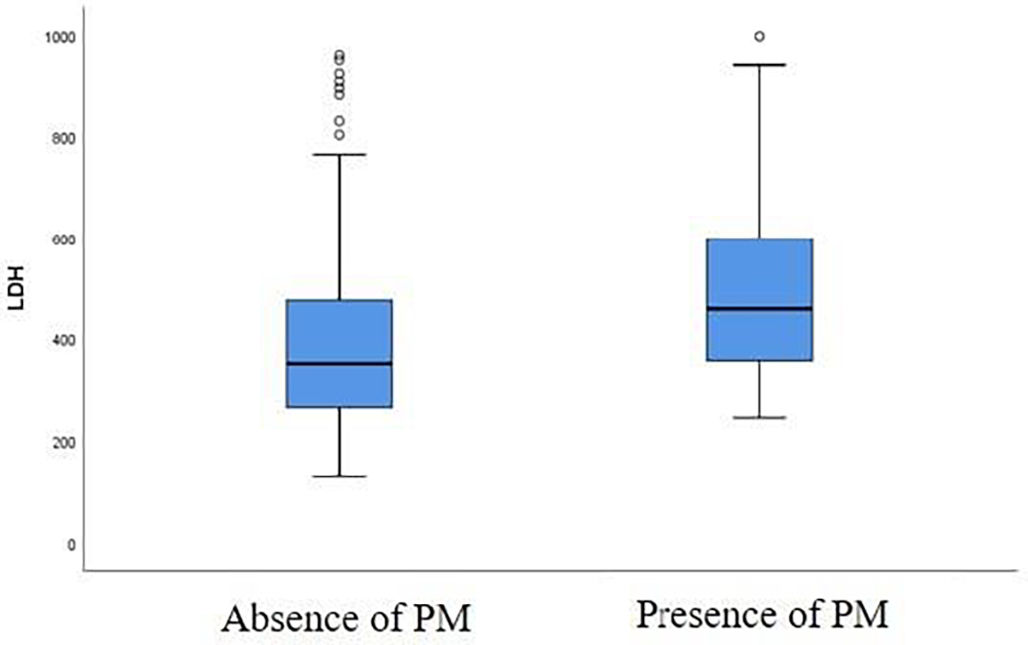
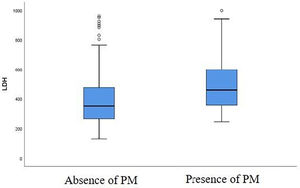
A correlation was found between the baseline LDH level and the risk of developing a PM at first follow-up chest CT. LDH value was higher in patients with PM at first follow-up chest CT compared to patients without a PM (Fig. 3). There is a significant difference between LDH level at admission (459 U/L [357–599 U/L]) in patients presenting a PM at first follow-up chest CT and in patients without this abnormality (351 U/L [265–476 U/L]) at follow-up chest CT, with a p-value of 0.035 (Table 2).
Serum LDH level on admission in patients with and without the abnormalities described on admission and follow-up chest CT.
| Chest computed tomography | Subject with the defect (prevalence) | Baseline serum LDH (U/L) with the chest CT abnormality | Baseline serum LDH (U/L) without the chest CT abnormality | p-valueb |
|---|---|---|---|---|
| Baseline chest CT (n = 451) | ||||
| Lobar consolidation | 38 (8.4%) | 335 (216–505) | 334 (251–431) | 0.753 |
| PM | 2 (0.4%) | 286 (280–292) | 335 (250–436) | 0.509 |
| PE | 3 (0.7%) | 335 (247–648) | 334 (251–435) | 0.737 |
| Follow-up chest CT (n = 210) | ||||
| Lobar consolidation | 30 (14.3%) | 404 (260–572) | 351 (270–473) | 0.238 |
| PM | 12 (5.7%) | 459 (357–599) | 351 (265–476) | 0.035a |
| PE | 10 (4.8%) | 397 (335–454) | 354 (266–488) | 0.430 |
Baseline serum LDH is given as median (interquartile range), CT: computed tomography, PM: Pneumomediastinum, PE: Pulmonary embolism.
In contrast, there was no correlation between LDH level and lobar consolidation or pulmonary embolism at baseline (p = 0.753 and p = 0.737, respectively) and at first follow-up (p = 0.238 and p = 0.43, respectively). There was no association between PM on admission and LDH baseline levels (p = 0.509) (Table 2).
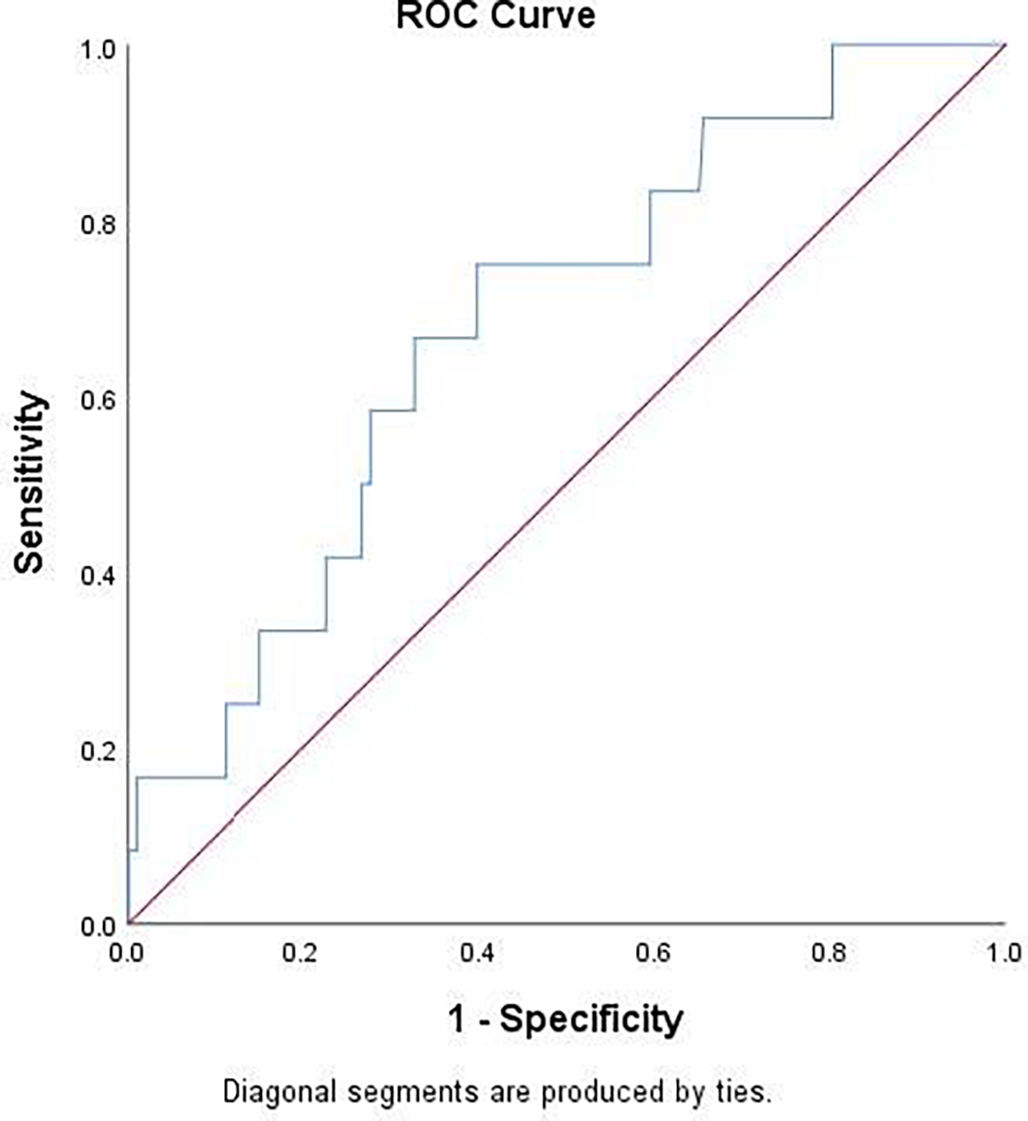
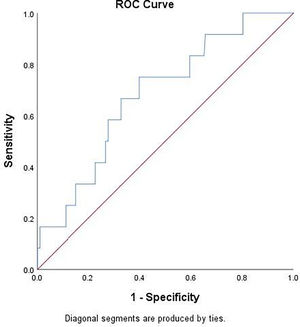
Table 2 showed that baseline LDH value was significantly higher in patients with PM at follow-up chest CT. Using ROC curve analysis (Fig. 4), LDH had an AUC of 0.681 [95% CI 0.537–0.825]; p = 0.0351) for detecting PM. Youden’s index identified the threshold of LDH at 395 U/L, yielding a sensitivity of 75%, specificity of 60.1%, a positive-predictive value (PPV) of 5% (prevalence of PM was 2.7%) and a negative-predictive value (NPV) of 98%.
In our series, 14 patients presented with PM, of whom, 7 had spontaneous PM. Among those, 2 had PM at baseline chest CT and 5 on follow-up chest CT. The follow-up chest CT done after a median of 16 days (Q1=11.5–Q3=21.5) showed that 8 patients intubated with a control plateau pressure presented a PM, of whom, 7 developed PM post-intubation, with a median of 7 days (Q1=2–Q3=18).
DiscussionThis study was conducted at one of the biggest multidisciplinary medical centers in Lebanon and one of the pioneers in treating COVID-19 patients, i.e., Hôtel-Dieu de France (HDF) hospital. It helped define a serum LDH threshold to predict the risk of developing a pneumomediastinum.
Our results reaffirm previous findings showing that the progression of CT pulmonary involvement was correlated with elevated LDH levels on admission.21 Furthermore, a positive correlation was found between serum LDH and CT staging.22 Previous findings suggest that LDH levels allow for clinical severity distinction between COVID-19 patients,23 likely due to the presence of LDH in pneumocytes, which, once destroyed, results in higher LDH levels in patients with significant tissue or cell damage.24
There is an association between LDH levels on admission and ground-glass opacity on the first and follow-up CT scan, with high LDH levels predicting severe CT damage.
Our study shows no correlation between LDH levels on admission and the risk of pulmonary consolidation, so LDH cannot be used as a marker of an infectious process.
In COVID-19, PM is a rare complication 25 that can be overlooked if no early chest CT is performed. The exact pathophysiology of PM associated with COVID-19 is still unclear.26 The different hypotheses explaining the occurrence of pneumomediastinum are reported in this article. First, barotrauma resulting from ventilation at excessive pressure or volume in patients who already have low pulmonary compliance could be involved in the genesis of PM.27 However, several studies have shown that this hypothesis alone is not sufficient to explain all PM cases.28
Another hypothesis is the Macklin effect, where dry cough of patients would cause increased alveolar pressures, rupture, then air diffusion into the peribronchovascular interstitium, gaining the interstitial tissue of the mediastinum by this path of lower resistance.26 A final hypothesis would be that the diffuse alveolar damage secondary to inflammatory cytokines caused by the “cytokine storm” softens the lung tissue and leads through the Macklin effect to the formation of PM.26,27 This phenomenon was described in a previous study showing the epithelial damage caused by the cytokine storm in the alveoli of deceased COVID-19 patients.29
Of the total sample, 2.7% presented a PM, of which 1.53% were ventilated/intubated, and 1.15%, were no barotrauma where noted. Therefore, barotrauma alone does not explain the occurrence of PM in COVID-19 patients, and the physiology of PM during COVID-19 seems to be related to interstitial pneumonia.
This large and unique study could determine a threshold of LDH to predict PM. When PM occurs, especially in patients without a clear iatrogenic notion of barotrauma, there is a risk of clinical deterioration, probably with severe pulmonary parenchymal involvement.
In pulmonary parenchymal involvement during viral/bacterial or other aggression, vessel remodeling and a cytokine storm are to blame when PM occurs in the absence of barotrauma. One of the most severe complications of PM, especially in COVID-19 patients without pulmonary reserve, is the compression of the great vessels due to mediastinal emphysema, mimicking a state of cardiac tamponade.30 Since the formation of PM can have disastrous consequences, the prediction of such an evolution becomes crucial.
Determining a threshold for serum LDH on admission to predict the risk for developing PM was rarely described in the literature. Even if current studies show that PM is a rare complication during COVID-19, a rapid worsening of the respiratory condition in these patients should lead to the search for a PM. Chest CT should be performed systematically in patients admitted for COVID-19, especially with a rapidly worsening respiratory status. When a chest scan is not available, a baseline serum LDH level of 394 UI/L could predict the risk of developing PM.
Our study has some limitations: it is retrospective; baseline chest CT was not performed systematically and a follow-up chest CT is missing for half of the patient. Baseline CT of the chest was available for 86% of the patients included in the study. For these patients with a baseline CT, all the baseline laboratory variables, and more specifically LDH, were available. The patients with no baseline CT of the chest (14%) were not accounted for in main outcome analysis, and were thus implicitly discarded from outcome evaluation.
Pneumomediastinum incidence is rare, so some statistical comparisons were underpowered. Chest CT without contrast is not accurate for pulmonary embolism diagnosis and for the ROC curve an AUC is fair but not high (<0.9). Finally, a prospective validation may be needed with a pre-planned protocols to identify a threshold of serum LDH.
ConclusionPM, a rare complication during COVID-19, can be overlooked and rapidly worsen respiratory status. Hence, a fast, affordable, and sensitive tool is required for the early diagnosis of severe pulmonary injury in hospitalized COVID-19 patients, namely serum LDH levels.
The following are the supplementary data related to this article.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mcpsp.2022.100347.
Disclosure statementThe data in this paper have been published as an abstract in the European Respiratory Journal.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical considerationsThis study was approved and written informed consent was waived in the context of the COVID-19 pandemic by the ethics committee of Hôtel-Dieu de France Hospital affiliated to Saint Joseph University (approval number: CEHDF-1631). This study was performed in accordance with the Declaration of Helsinki.
Availability of dataThe data that support the findings of this study are available from the corresponding author on reasonable request.
Declarations of interestNone.
This work would not have been possible without the unwavering support of our visionary and humanistic administration, namely the company of Jesus, and all the medical and nursing staff. This policy was at the service of humans and for humans in the image of our noblest principles.
Authors would like also to thank Science PRO sarl for conducting critical review and editing of the article.