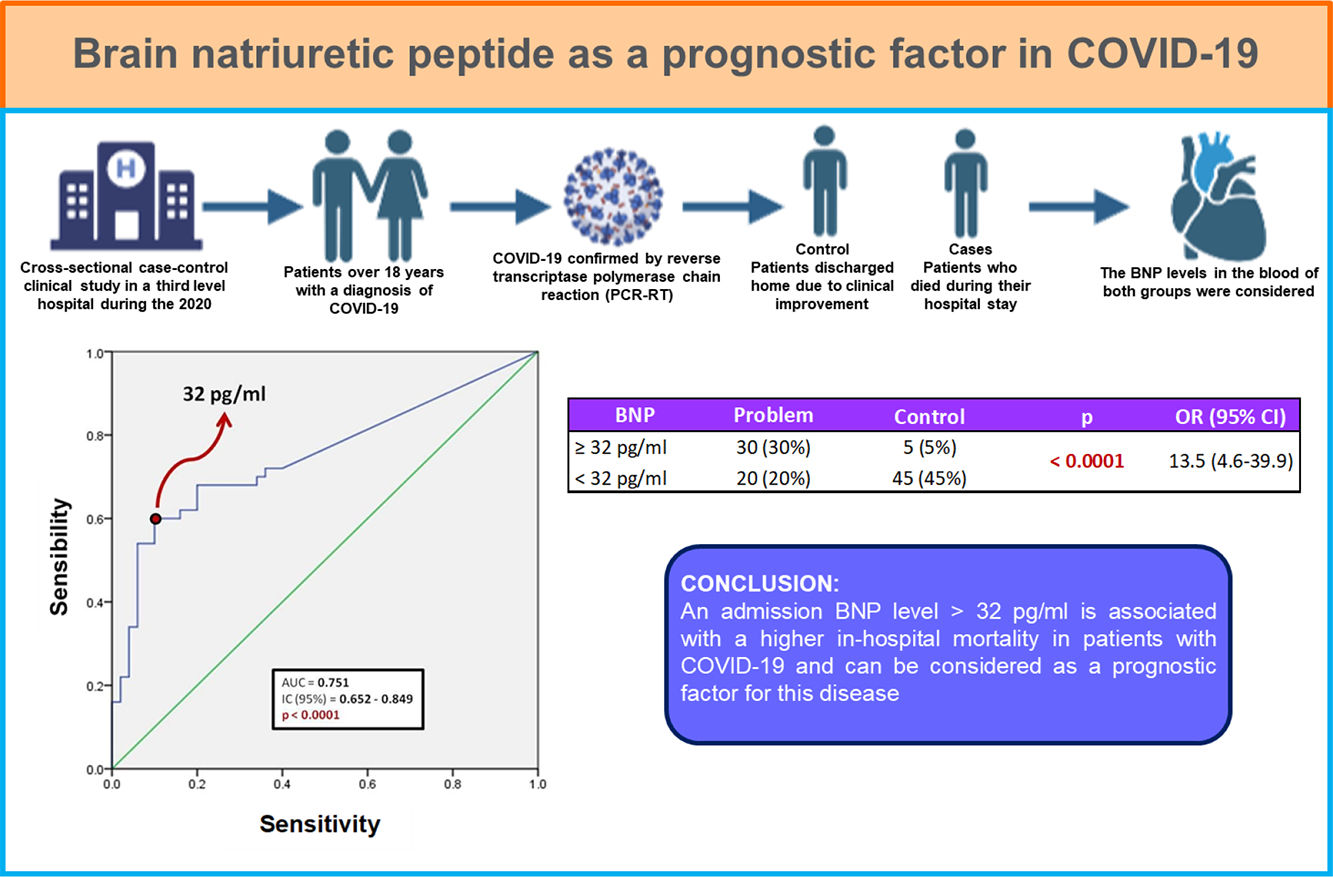
Actualmente la infección por SARS-CoV-2 ha provocado la muerte de 6.5 millones de personas a nivel mundial. La COVID-19 es una enfermedad que afecta primordialmente al sistema respiratorio y puede llegar a provocar falla multiorgánica. Debido a la mortalidad que ha generado se han generado diferentes índices pronósticos para determinar que pacientes son más propensos a complicarse y fallecer. En BNP es una hormona peptídica sintetizada en los ventrículos del corazón y se ha usado como indicativo de insuficiencia cardiaca y como factor pronostico en pacientes con choque séptico. Por lo tanto, se ha planteado su uso como factor pronóstico en pacientes que presentan COVID-19.
Materiales y métodosSe llevó a cabo estudio retrospectivo de casos y controles que incluyó a 100 pacientes confirmados con infección por SARS-CoV-2 por PCR-RT, los cuales fueron egresados a domicilio o fallecido hasta el 30 de mayo de 2020. Utilizando el expediente clínico electrónico de estos pacientes, se obtuvieron datos demográficos, clínicos y bioquímicos para realizar la comparación entre los sobrevivientes y los pacientes que fallecieron. Se realizó análisis estadístico con curva ROC para determinar el nivel de BNP al ingreso, que se asoció con mayor mortalidad intrahospitalaria.
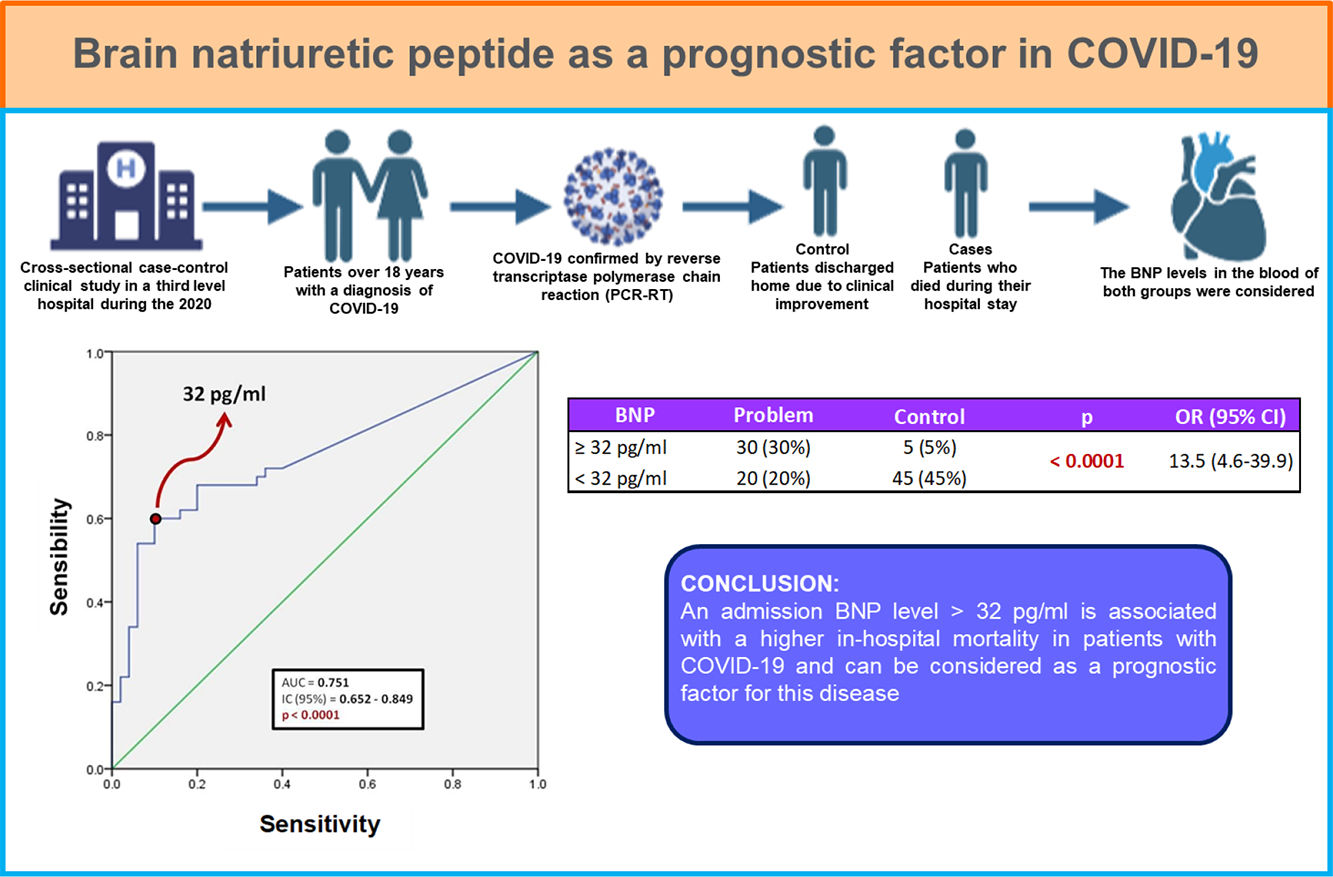
Resultados y conclusionesDe los 100 pacientes incluidos, 50 fueron egresados a domicilio y 50 fallecieron durante su estancia intrahospitalaria. El 87% de los pacientes presentaba al menos una comorbilidad, siendo la obesidad la más frecuente (38 pacientes, 38%), seguido de hipertensión (25 pacientes, 25%). Existió diferencia estadísticamente significativa entre ambos grupos en las siguientes características: edad, género masculino, saturación de oxígeno por pulsioximetría, conteo de leucocitos, recuento de neutrófilos, deshidrogenasa láctica y proteína C reactiva. Respecto al BNP, se encontró que un punto de corte mayor a 32 pg/ml puede utilizarse como factor predictor de mortalidad intrahospitalaria (AUC 0.751) con una sensibilidad de 60% y especificidad de 90%; así como odds ratio de 13.5 (IC 95%; 4.6–39.9).
Un nivel de BNP, al ingreso, mayor de 32 pg/ml se asocia con mayor mortalidad intrahospitalaria en pacientes con COVID-19.
Currently, coronavirus type 2 (SARS-CoV-2) has been implicated as the etiological agent of Coronavirus Disease 2019 (COVID-19). The infectious clinical spectrum of SARS-CoV-2 goes from an asymptomatic process to a severe respiratory failure that can lead to death. Effects of SARS-CoV-2 virus is due to its property to bind with the human angiotensin I converting enzyme 2 (ACE2) receptor. Infections with this virus can lead to multiple organ failure because ACE2 receptor is expressed in almost all organs of the human body.1
Brain natriuretic peptide (BNP) is a hormone mainly produced at the heart ventricles.2 Its synthesis and release is secondary to heart wall distension, ventricular dilation and/or increased pressure in the cardiac chambers. BNP elevated blood levels promote vasodilation, increased diuresis, and produces suppression of the renin-angiotensin-aldosterone system in order to decrease heart preload.3
BNP, which is a recognized heart failure biomarker, has also been used as a prognostic factor in patients with sepsis and septic shock, regardless of their previous cardiovascular condition. This study aims to determine if BNP can be also used as a prognostic factor in patients with SARS-CoV-2 infection.
MethodologyA retrospective, observational, analytical, and cross-sectional case-control clinical study was designed, in which all patients over 18 years with a diagnosis of COVID-19 confirmed by reverse transcriptase polymerase chain reaction (PCR-RT) were included. Patients were admitted to the internal medicine service of a third level hospital in a one month period during the 2020 pandemia. Those with a previous diagnosis of chronic heart failure, chronic kidney disease, hyperthyroidism, chronic liver disease, malignancy, and a history of subarachnoid hemorrhage in the last 3 months were excluded. Chronic kidney disease during hospital stay, serum creatinine levels >1.5 mg/dl or blood glucose >250 mg/dl were considered as elimination criteria for this study.
Patients were divided in two groups: the ones who were discharged home due to clinical improvement (controls), and those who died during their hospital stay (cases). Clinical background records were collected, specially those referred to comorbidities and medication intake, as well as vital signs and anthropometric measurements to determine body mass index (BMI). Laboratory studies such as hematic biometrics, blood chemistry, liver function tests, ultrasensitive C-reactive protein, procalcitonin, ferritin, and blood BNP levels were considered.
Clinical and laboratory data were collected from clinical records within the first 24 hours of hospital admission. This information was recorded into an Excel spreadsheet for further analysis using SPSS version 21 software. Quantitative variables are expressed as mean ± standard deviation and categorical variables are expressed as frequency and percentage in relation to the population at risk. BNP values were analyzed using the ROC curve and then Odds ratio (OR) values were calculated.
ResultsA sample of 100 patients was obtained out of total of 204 clinical récords studied. Fifty patients were randomly assigned to each study group (cases and controls) using an Urna Software.
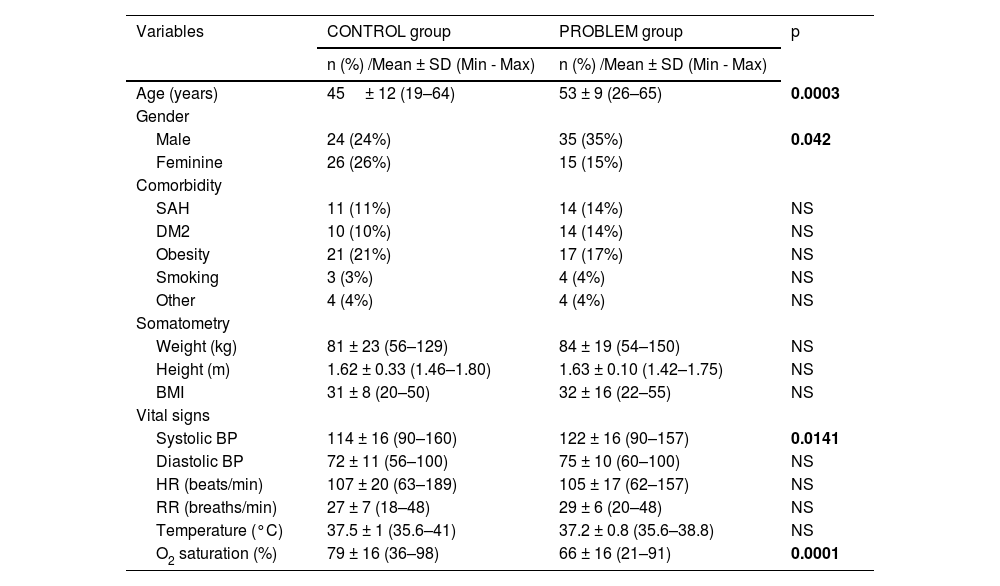
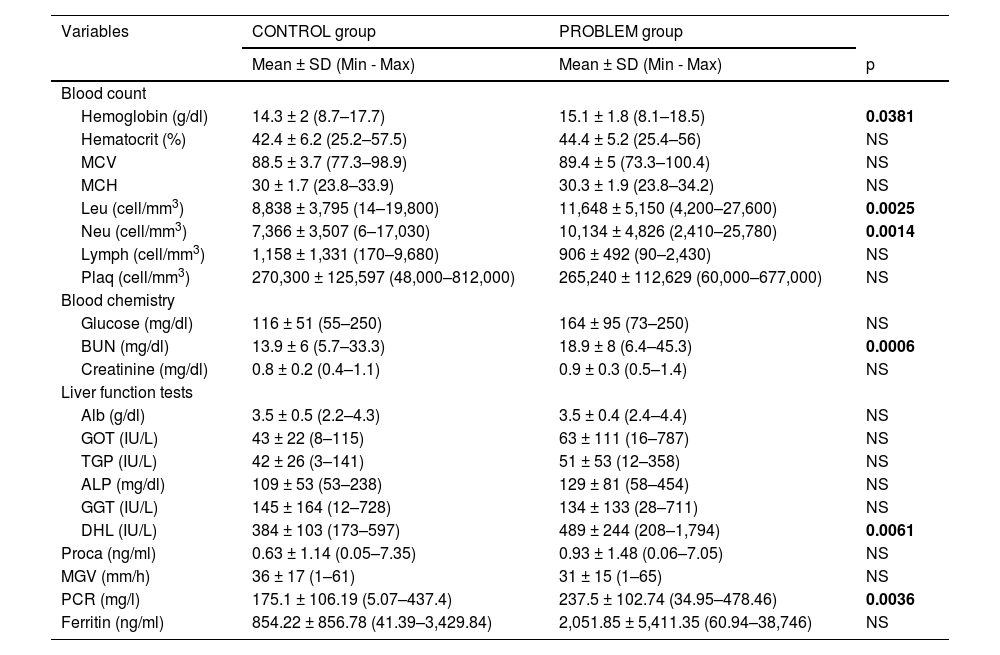
Table 1 shows sociodemographic variables, somatometry and vital signs. Male gender was predominantly observed at the problem group, which were eight years older in relation to the control group. Despite the fact that there were no significant differences in comorbidities and somatometry, the most frequent comorbidities in both groups were obesity, arterial hypertension and type 2 diabetes mellitus. There were no differences in vital signs, diastolic blood pressure, heart rate, respiratory rate and temperature at both studied groups. However, systolic blood pressure was higher in the problem group and oxygen saturation (SaO2) was higher in the control group. In Table 2 laboratory values are shown, and we can see higher levels in the problem group related to hemoglobin, leukocytes and absolute neutrophils, as well as blood urea nitrogen (BUN), levels of lactic dehydrogenase (LDH) and C-reactive protein.
Comparison of the sociodemographic variables, somatometry and vital signs of the studied groups.
| Variables | CONTROL group | PROBLEM group | p |
|---|---|---|---|
| n (%) /Mean ± SD (Min - Max) | n (%) /Mean ± SD (Min - Max) | ||
| Age (years) | 45± 12 (19–64) | 53 ± 9 (26–65) | 0.0003 |
| Gender | |||
| Male | 24 (24%) | 35 (35%) | 0.042 |
| Feminine | 26 (26%) | 15 (15%) | |
| Comorbidity | |||
| SAH | 11 (11%) | 14 (14%) | NS |
| DM2 | 10 (10%) | 14 (14%) | NS |
| Obesity | 21 (21%) | 17 (17%) | NS |
| Smoking | 3 (3%) | 4 (4%) | NS |
| Other | 4 (4%) | 4 (4%) | NS |
| Somatometry | |||
| Weight (kg) | 81 ± 23 (56–129) | 84 ± 19 (54–150) | NS |
| Height (m) | 1.62 ± 0.33 (1.46–1.80) | 1.63 ± 0.10 (1.42–1.75) | NS |
| BMI | 31 ± 8 (20–50) | 32 ± 16 (22–55) | NS |
| Vital signs | |||
| Systolic BP | 114 ± 16 (90–160) | 122 ± 16 (90–157) | 0.0141 |
| Diastolic BP | 72 ± 11 (56–100) | 75 ± 10 (60–100) | NS |
| HR (beats/min) | 107 ± 20 (63–189) | 105 ± 17 (62–157) | NS |
| RR (breaths/min) | 27 ± 7 (18–48) | 29 ± 6 (20–48) | NS |
| Temperature (°C) | 37.5 ± 1 (35.6–41) | 37.2 ± 0.8 (35.6–38.8) | NS |
| O2 saturation (%) | 79 ± 16 (36–98) | 66 ± 16 (21–91) | 0.0001 |
Abbreviations: SAH=systemic arterial hypertension, DM2=type 2 diabetes mellitus, Kg=kilograms, m=meters, BMI=body mass index, BP=blood pressure, HR=heart rate, min=minutes, FR= respiratory rate, °C=Celsius degrees and O2=oxygen
Laboratory variables of the study groups.
| Variables | CONTROL group | PROBLEM group | |
|---|---|---|---|
| Mean ± SD (Min - Max) | Mean ± SD (Min - Max) | p | |
| Blood count | |||
| Hemoglobin (g/dl) | 14.3 ± 2 (8.7–17.7) | 15.1 ± 1.8 (8.1–18.5) | 0.0381 |
| Hematocrit (%) | 42.4 ± 6.2 (25.2–57.5) | 44.4 ± 5.2 (25.4–56) | NS |
| MCV | 88.5 ± 3.7 (77.3–98.9) | 89.4 ± 5 (73.3–100.4) | NS |
| MCH | 30 ± 1.7 (23.8–33.9) | 30.3 ± 1.9 (23.8–34.2) | NS |
| Leu (cell/mm3) | 8,838 ± 3,795 (14–19,800) | 11,648 ± 5,150 (4,200–27,600) | 0.0025 |
| Neu (cell/mm3) | 7,366 ± 3,507 (6–17,030) | 10,134 ± 4,826 (2,410–25,780) | 0.0014 |
| Lymph (cell/mm3) | 1,158 ± 1,331 (170–9,680) | 906 ± 492 (90–2,430) | NS |
| Plaq (cell/mm3) | 270,300 ± 125,597 (48,000–812,000) | 265,240 ± 112,629 (60,000–677,000) | NS |
| Blood chemistry | |||
| Glucose (mg/dl) | 116 ± 51 (55–250) | 164 ± 95 (73–250) | NS |
| BUN (mg/dl) | 13.9 ± 6 (5.7–33.3) | 18.9 ± 8 (6.4–45.3) | 0.0006 |
| Creatinine (mg/dl) | 0.8 ± 0.2 (0.4–1.1) | 0.9 ± 0.3 (0.5–1.4) | NS |
| Liver function tests | |||
| Alb (g/dl) | 3.5 ± 0.5 (2.2–4.3) | 3.5 ± 0.4 (2.4–4.4) | NS |
| GOT (IU/L) | 43 ± 22 (8–115) | 63 ± 111 (16–787) | NS |
| TGP (IU/L) | 42 ± 26 (3–141) | 51 ± 53 (12–358) | NS |
| ALP (mg/dl) | 109 ± 53 (53–238) | 129 ± 81 (58–454) | NS |
| GGT (IU/L) | 145 ± 164 (12–728) | 134 ± 133 (28–711) | NS |
| DHL (IU/L) | 384 ± 103 (173–597) | 489 ± 244 (208–1,794) | 0.0061 |
| Proca (ng/ml) | 0.63 ± 1.14 (0.05–7.35) | 0.93 ± 1.48 (0.06–7.05) | NS |
| MGV (mm/h) | 36 ± 17 (1–61) | 31 ± 15 (1–65) | NS |
| PCR (mg/l) | 175.1 ± 106.19 (5.07–437.4) | 237.5 ± 102.74 (34.95–478.46) | 0.0036 |
| Ferritin (ng/ml) | 854.22 ± 856.78 (41.39–3,429.84) | 2,051.85 ± 5,411.35 (60.94–38,746) | NS |
Abbreviations: MCV=mean corpuscular volume, MCH=mean corpuscular hemoglobin; Leu=leukocytes, Neu=absolute neutrophils, Lymph=absolute lymphocytes, Plaq=platelets, BUN=blood urea nitrogen, Alb=albumin, GOT=glutamic-oxaloacetic transaminase, GGT=glutamic–pyruvic transaminase, ALP=alkaline phosphatase, GGT= gamma- glutamyl transpeptidase, LDH=lactic dehydrogenase, Proca=procalcitonin, ESR=erythrocyte sedimentation rate, CRP= c-reactive protein, g=grams, dl=deciliter, mm=millimeters, mg= milligrams, IU= international units, L=liters, ng=nanograms, h=hour
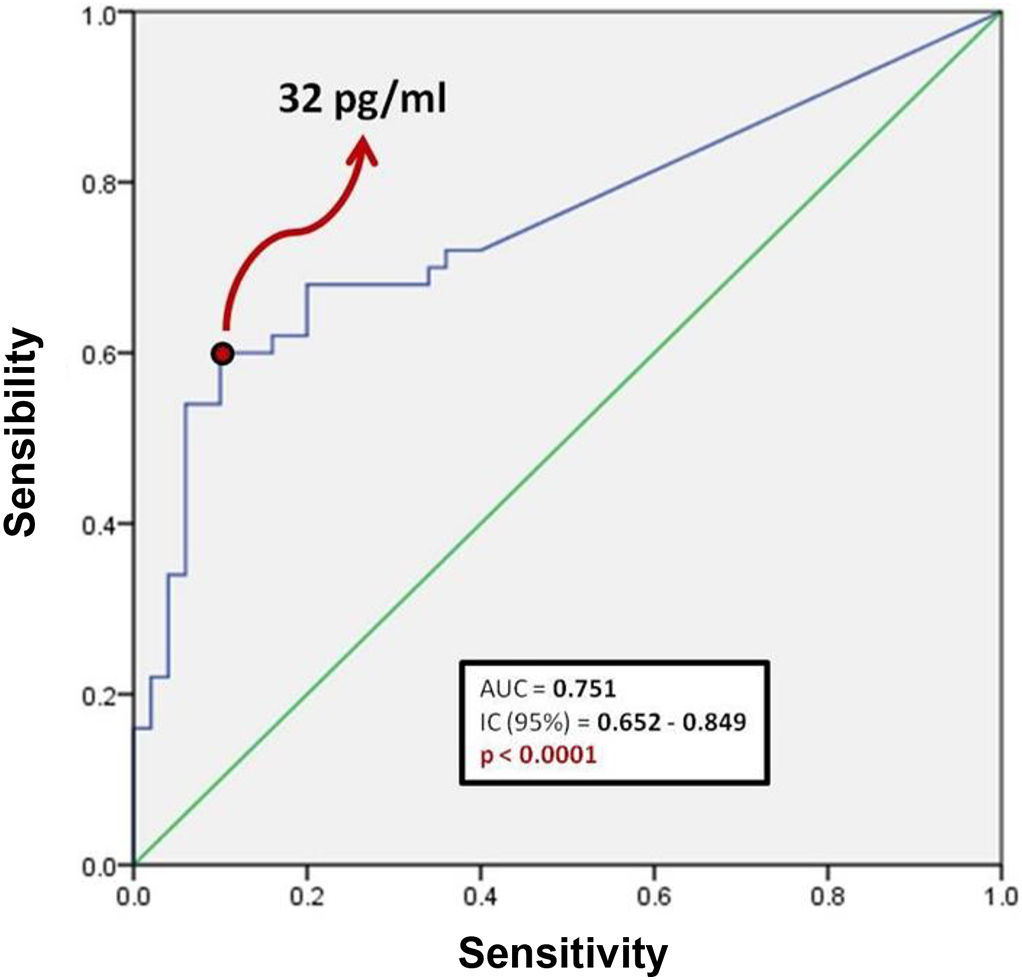
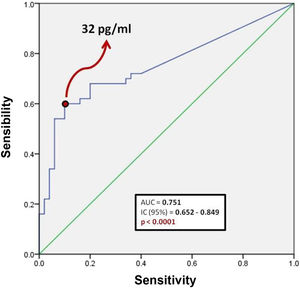
Fig. 1 shows a ROC curve for BNP values of the problem group. It was found that the cut-off point for serum BNP was 32 pg/ml, which represented the highest sensibility and specificity for the end point of death in patients with COVID-19. Therefore, a patient who died showed a 60% probability of having a serum BNP level > 32 pg/mL at admission. A patient who was discharged from hospital showed a 90% probability of having an initial BNP serum level < 32 pg/ml. It was determined that the positive predictive value of BNP for intrahospital mortality from COVID-19 is 0.85 and the negative predictive value is 0.69. The area under the curve (AUC) of the BNP as a prognostic tool for in-hospital mortality due to COVID-19 was 0.751. Since the confidence interval does not include the value of 0.50, we can affirm that the AUC of the BNP concentration is different from non-discrimination.
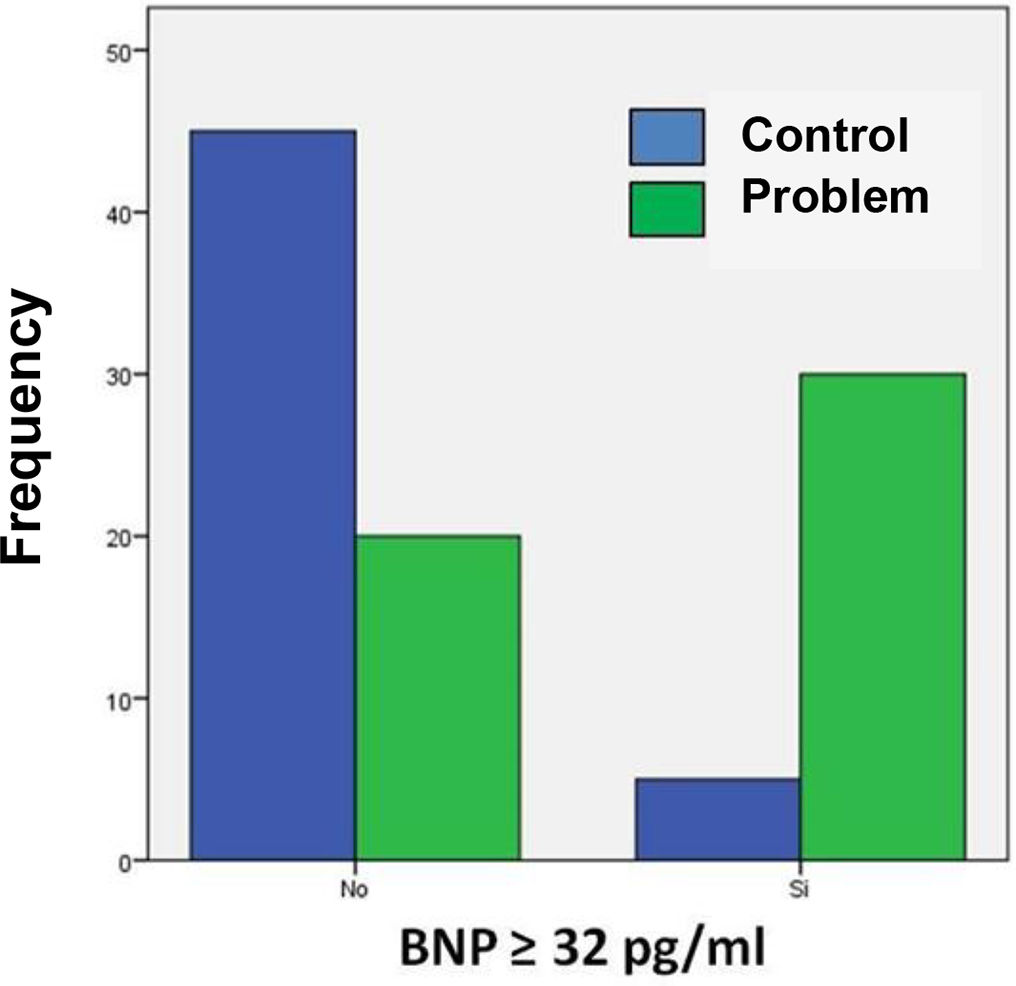
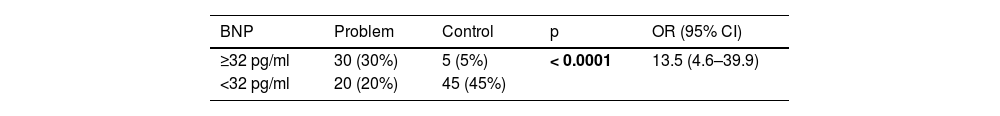
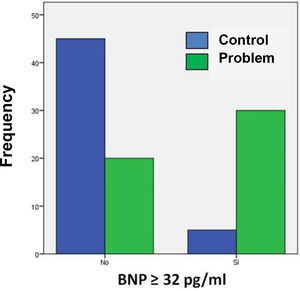
Table 3 shows the number of patients in both groups who presented both higher and lower BNP values on admission, taking 32 pg/ml as the cut-off point. The calculated OR was 13.5, so that patients with COVID-19 and BNP ≥ 32 pg/mL are 13 times more likely to die during their hospital stay compared to those patients with a BNP level below this cut-off point. In turn, the 95% confidence interval was between 4.6 and 39.9.
Contingency table of the frequency of BNP values at the cut-off point established by the ROC curve in both study groups.
| BNP | Problem | Control | p | OR (95% CI) |
|---|---|---|---|---|
| ≥32 pg/ml | 30 (30%) | 5 (5%) | < 0.0001 | 13.5 (4.6–39.9) |
| <32 pg/ml | 20 (20%) | 45 (45%) |
Abbreviations: pg=picograms, ml=milliliters, OR=odds ratio, CI=confidence interval
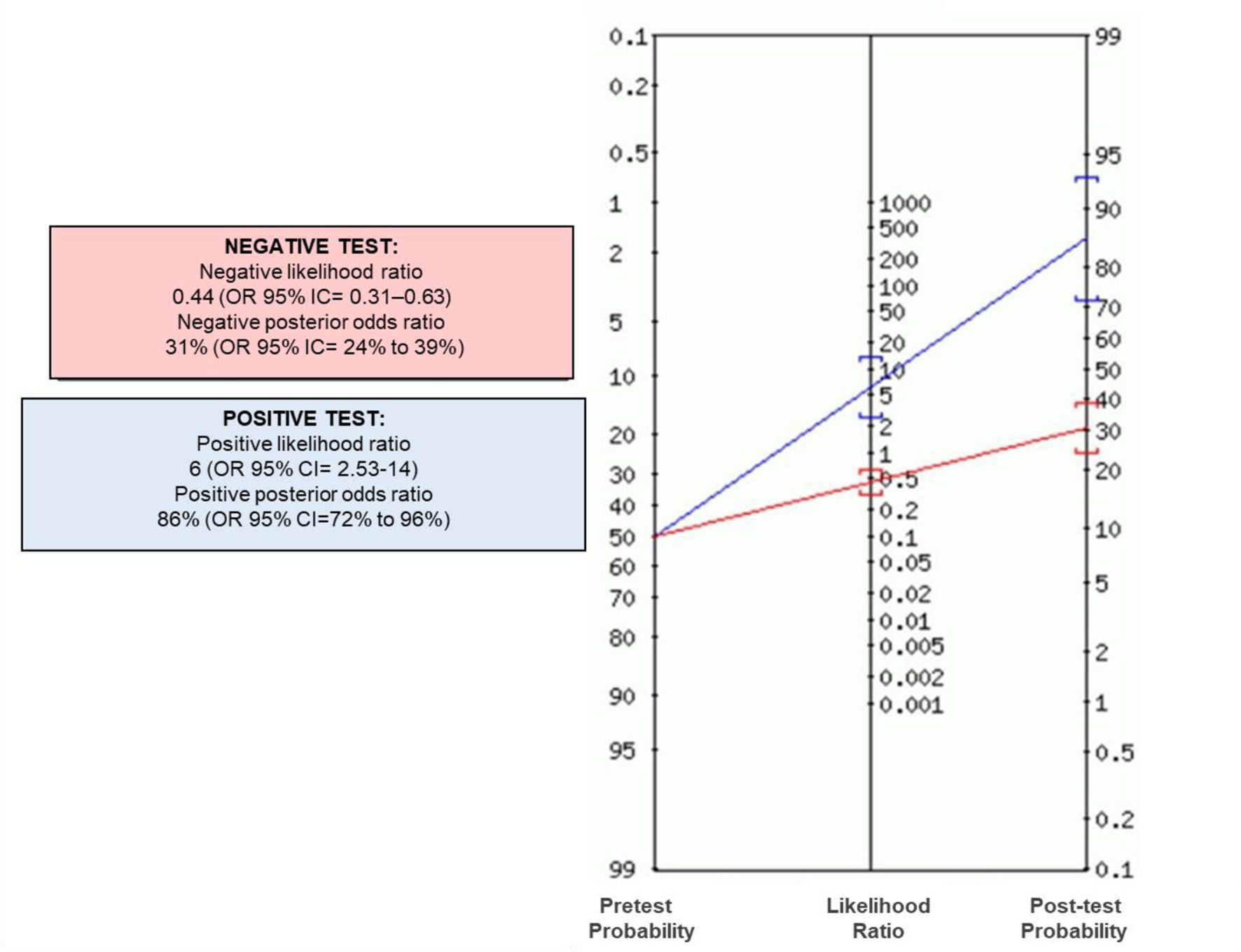
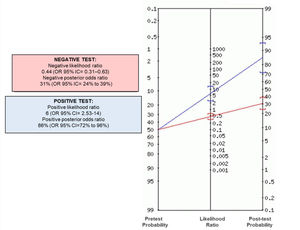
Fig. 2 shows that most of the patients in the problem group had BNP concentrations ≥ 32 pg/ml compared to the control group. Positive and negative odds ratios were calculated. The positive likelihood ratio (CPP) resulted in 6, while the negative likelihood ratio (CPN) was obtained in 0.44. Fig. 3 shows the Fagan nomogram. The first line shows the pre-test probability, which is represented by intra-hospital mortality in patients with COVID-19. Starting from the prevalence value, which in this study is 50, a line was drawn that intersects the values of the probability ratios, both positive and negative, and finally extends to the post-test probability axis. A positive post-test probability value of 86% was obtained. The negative post-test probability value was 31%. Therefore, in a clinical scenario with the prevalence of our study, a patient with COVID-19 and BNP on admission greater than 32 pg/ml has an 86% probability of dying and, conversely, if the BNP result is less than this cut-off value, the patient has a 31% probability of being discharged at home.
Morbidity and mortality due to COVID-19 disease increased significantly all worldwide. Therefore, it was necessary to develop scales and search for prognostic factors that would allow the identification of patients with the highest risk for complications and death. BNP is a biomarker that has been used as a prognostic factor in patients with sepsis and septic shock, because its short term elevation is a mortality predictor.4 Currently, there are few researches related to the prognostic value of BNP as a biomarker in COVID-19, since the N-terminal prohormone of brain natriuretic peptide (NT-proBNP) has mostly been used as a prognostic biomarker. Elevated NT-proBNP concentrations at admission in COVID-19 patients have been shown to be associated with higher rates of mechanical ventilation, intensive care unit (ICU) admissions, and a twice probability risk of in-hospital mortality, regardless of a clinical background of a previous heart failure.5,6 It has been stated that the NT-proBNP cut-off point to predict mortality in patients with COVID-19 is 88.64 pg/mL, with a 100% sensibility and a 66.67% sensitivity. Patients with NT-proBNP blood concentrations greater than that value have a lower cumulative survival rate compared to those with lower levels.7 In the present study, BNP was used as a mortality predictor biomarker that showed a relationship between elevated levels and in-hospital mortality in patients with COVID-19, with a 32 pg/ml as a cut-off point. The elevation of BNP/NT-pro-NBP has not yet been studied, but it is thought to be multifactorial.8 Currently, viral RNA has been associated with direct damage of the virus at the myocardial tissue. This tropism is associated with the expression of the angiotensin-converting enzyme receptor 2 (ACE2) in cardiac cells, which allows SARS-CoV-2 to infect and replicate within cardiomyocytes, leading to infiltration of monocytes, lymphocytes and plasma cells, and developing myocarditis due to COVID-19 with increased cardiac biomarkers.9,10
In addition to the direct cytopathic effect of SARS-CoV-2 on cardiomyocytes, it has also been proposed that myocardial damage is mediated by the uncontrolled release of cytokines. Cytokine storm is the main cause of severity and death in patients with COVID-19. It is the consequence of an excessive immune response that can be fatal. There is an imbalance in the cellular response of T helper 1 lymphocytes, excessive production of proinflammatory cytokines (Interleukin-6, tumor necrosis factor-α) and chemical mediators, that are capable of damaging the myocardium and causing cardiac dysfunction.10,11 In the problem group, the numbers of leukocytes, absolute neutrophils, and ultrasensitive C-reactive protein levels were significantly higher, which is associated with a great inflammatory response induced by SARS-CoV-2 infection. These data also show that myocardial injury and the consequent elevation of B-type natriuretic peptide are strongly associated with an inflammatory pathogenic substrate. Leukocytes are attracted by inflammatory signals to cardiac tissue, producing numerous reactive oxygen species at the site of injury and increased oxidative stress, which leads to cardiomyocyte deterioration associated with modification of contractile proteins and dysregulation of cellular redox states. These changes promote apoptosis of cardiomyocytes and determine a state of reparative fibrosis.12,13
In this study, no statistically significant difference was documented in lymphocyte count between both groups. Our data differ from most published studies in which lymphopenia is a typical profile in patients with COVID-19.14 Lymphopenia represents an independent risk factor for mortality and has even become part of some indices or scores that try to predict the progression of COVID-19 towards severe forms, such as the CALL score or the COVID-gram score.15
Multiple risk factors leading to severe COVID-19 disease have currently been identified. Advanced age (>65 years), male gender, chronic degenerative diseases (diabetes, arterial hypertension, obesity, and cardiovascular diseases) are significant risk factors for disease severity, complications, and poor prognosis. Since the beginning of the pandemic, it has been determined that older people have a more severe presentation of the disease from COVID-19 and a higher mortality. The processes of aging and cellular senescence of the immune system are factors that leave the elderly patient in a vulnerable state.17 In this study it was observed that in the problem group most of the patients were in the fifth decade of life and although they were not older than 60 years, it is corroborated that age is a prognostic factor for the outcome of COVID-19. Age has a directly proportional relationship with the increase in mortality. This is evident in patients ≥ 60 years, where the highest mortality occurs in patients ≥ 80 years in whom the risk of death is six times higher compared to young patients.18 Similarly, it has been shown that the male gender predicts a higher mortality rate compared to the female gender.19 In the problem group, the number of men was significantly higher compared to the control group, which supports that the male gender is a predictor of increased risk of death in adults with COVID-19. Sex hormones are involved in the immune response to SARS-CoV-2 infection. In women, estrogens are a protective factor and in infectious disease promote the proliferation of T cells and therefore a stronger immune response. In men, androgens, such as testosterone and dihydrotestosterone, increase the count and function of the main cells responsible for cytokine storm syndrome, the neutrophils. Therefore, there is a greater predisposition to develope an exaggerated immune response and greater complications. In general, male sex hormones facilitate the entry of the virus into tissues, since they increase the activity of the ACE2 receptor and favor the expression of the transmembrane protease serine 2 (TMPRSS2).20,21
Regarding vital signs, the problem group presented significantly lower SaO2. Hypoxemia occurs when the oxygen content in arterial blood is decreased. Hypoxemia in patients with COVID-19 is a consequence of alterations between ventilation/perfusion (V/Q) caused by the presence of pulmonary edema and loss of alveolar elasticity, which produces alveolar collapse and therefore alterations in hematosis.22 In patients with COVID-19, the severity of hypoxemia is an independent predictor of mortality, respiratory complications, and use of mechanical ventilation. In the presence of hypoxia, various compensatory mechanisms occur, such as the increase in the red blood cell count. Under hypoxic conditions, the juxtaglomerular cells of the kidney are stimulated by factor 1-alpha to favor the secretion of erythropoietin, which in the bone marrow favors the production of red blood cells, of which main component is hemoglobin (23). This explains why the patients in the problem group with considerably low oxygen saturation have significantly higher hemoglobin levels compared to the control group.
This study becomes important as no articles have been published that relate B-type natriuretic peptide with in-hospital mortality in COVID-19 population. It suggests, as well, a new low-cost and useful prognostic factor for SARS-CoV-2 infection.
Conflicts of interestThe authors have no conflicts of interest to declare.