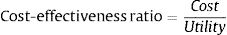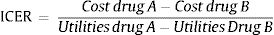Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosThe aim of this research is to evaluate the cost and utility of the use of risperidone or haloperidol combinations on schizophrenic patients in the cases at Prof. V.L. Ratumbuysang Psychiatric Hospital North Sulawesi Province of Indonesia.
MethodThis study was an observational study with a Cohort design. Sampling was done using a purposive sampling method for all 82 patients and finally obtained 22 patients for risperidone-combination group and 28 patients for haloperidol-combination group. Data was collected from patient's medical records by using retrospective approaches from April to July 2018 and prospectively by using a short form questionnaire. The utility based on the quality of life was assessed by the 36-item Short Form (SF-36) questionnaire. The quality-adjusted life years (QALY's) for haloperidol-combination were recorded as 0.433 which more high than risperidone-combination group, which recorded as 0.423 Average cost-effectiveness ratio (ACER) for the risperidone-combination group was IDR 5,813,716.13/QALY's, which more cost-effectively than the haloperidol-combination group of IDR 6,454,822.17/QALY's with the record of incremental cost-effective ratio (ICER) of IDR 33,573,600/QALY.
ResultsThe result of sensitivity test to 25% total cost increase for risperidone-combination group (IDR 7,267,145) and the 25% total cost decrease for haloperidol-combination group has change in ACER values with compared to the baseline of risperidone-combination group.
ConclusionRisperidone-combination is the dominant therapeutic choice related to cost and QALY's in the treatment for schizophrenic patients.
Schizophrenia is a chronic mental disorder that affects more than 21 million people worldwide, and furthermore changes the way people think, feel, and act.1,2 The incidence of schizophrenia is 2–5 cases per 1000 population per year, and its prevalence is 1% of the world's population. In Indonesia, especially in North Sulawesi Province, the prevalence of schizophrenia is about 0.8 per 1000 populations.3 Pharmacotherapy by using antipsychotics is the first-line choice in schizophrenia therapy.4,5 Meta-analysis studies showed that atypical-antipsychotics like risperidone is more effective than typical-antipsychotic like haloperidol.6 But there is a risk of side effects for either haloperidol or risperidone, thus in the practice, those antipsychotics were combined trihexyphenidyl.4 As a chronic disease, schizophrenia is associated with a decrease in productivity and a low quality of life.7 Various clinical factors related to the life quality of schizophrenic patients have been reported. A cross-sectional study stated that psychopathological symptoms have a stronger correlation with community function.8 Correlation between atypical antipsychotic with cognitive function also revealed there is a significant improvement.9 Even though atypical antipsychotic (risperidone) has been recognized as the first-line choice for schizophrenia therapy but in the real clinical practice, the typical antipsychotic is still given to schizophrenia patients (Fujimaki 2012),10 similar to the practice in Prof. Dr. V.L. Ratumbuysang Hospital where the typical antipsychotic is still the choice of clinician. This is associated with the high financial burden in the treatment of schizophrenia (Patel 2003),11 the cost of risperidone relatively higher than haloperidol. Hence, a pharmacoeconomic study related to the quality of life is important. Using the cost-utility analysis (CUA) method, this study aims to assess the cost-utility of using risperidone-combination and haloperidol-combination in the Prof. Dr. V.L. Ratumbuysang Psychiatric Hospital, North Sulawesi Province of Indonesia.
MethodStudy designThe design of this study was a non-experimental or observational with a cohort study design. Data were obtained retrospectively from the patient's medical record as a secondary data and prospectively by the assessment of patient's quality of life by using the 36-item Short Form (SF-36) as a primary data. Interviews for patients were conducted in two stages to obtain initial and final scores with two months interval. The design of this study is approved by the ethical consideration related toward all subjects were willing to fill the informed after received an explanation from the researcher.
Subjects of studyThe Subjects of this study were schizophrenic inpatients in Prof. Dr. V.L. Ratumbuysang Psychiatric Hospital in North Sulawesi Province of Indonesia, started from April to late July 2018. The number of subjects was obtained by using role of thum method accompanied by purposive sampling technique. Total subjects which obtained were 50 samples, 22 patients were using risperidone-combination and 28 patients using haloperidol-combination. Patients matched to the inclusion category were the patients diagnosed with schizophrenia and had been received risperidone-combination and haloperidol-combination for at least 2 months or 8 weeks. Haloperidol dose is 4–6mg/day, and risperidone dose is 5–15mg/day. Every patient also treated with anticholinergic trihexyphenidyl in dose 5–15mg/day as the combination for antipsychotics. Patients with incomplete medical record, refused to fill the informed consent and included in default criteria categorized in exclusion criteria in this study. The use of other medicines for agitation or insomnia (diazepam 2–10mg/day and lozepam 1–10mg/day); mood stabilizer (lithium carbonate 200–400mg/day); and vitamin (folic acid 0.4mg and vitamin B complex) also included in this study without interfering the effectivity of antipsychotic.
Assessment to the quality of lifeThe quality of life of patients was assessed by an international standard of Short Form 36 (SF-36) instrument.12 The SF-36 form has been widely used to assess function and health generally to the improvement of schizophrenic patient symptoms. The SF-36 form consists of 36 questions that covered 8 domains: physical function, physical role, emotional role, vitality, pain, mental health, general health and social function.12 Furthermore, these 8 domains grouped into 2 assessment components, such as mental component (consists of vitality, emotional role, social role, and mental health) and physical component (consists of physical function, general health, pain, and physical role).13 The SF-36 form assessment is done through two stages: the first stage is converted to the value into 0–100; the second stage is averaging convention values of every domain.13 Score above 50 is interpreted as good quality of life and score under 50 is interpreted as poor quality of life.14
CostDrugs (risperidone-trihexyphenidyl and haloperidol-trihexyphenidyl combinations) and other drug supplements, hospitalization, clinician visit, treatments, administration, and other supported, like laboratory costs were cost components which measured in this study. The drug costs were obtained from The 2018 Drug e-catalog which has been set by the government through department of LKPP. The costs of hospitalization, clinician visits, treatment and administration were based on the BPJS insurance perspective. The cost of laboratory included in support cost which has been set by the North Sulawesi Local Regulation No. 2 the Year 2016.
UtilityUtility unit used in this study was the quality-adjusted life years (QALYs) score. Utility score illustrates by the assessment of patient's quality of life set by The Ministery of Health of Indonesia.15 The assessment was carried out subjectively based on patients quality of life score associated with their health, which is, in this case, the quality of life score was obtained from the percentage of score increasing from the initial to the final treatments by using SF-36 questionnaire.
Cost utility analysisIn every group, the average cost-effectiveness ratio (ACER) is calculated by using a standard formula from ministry of health of Indonesia as follows15:
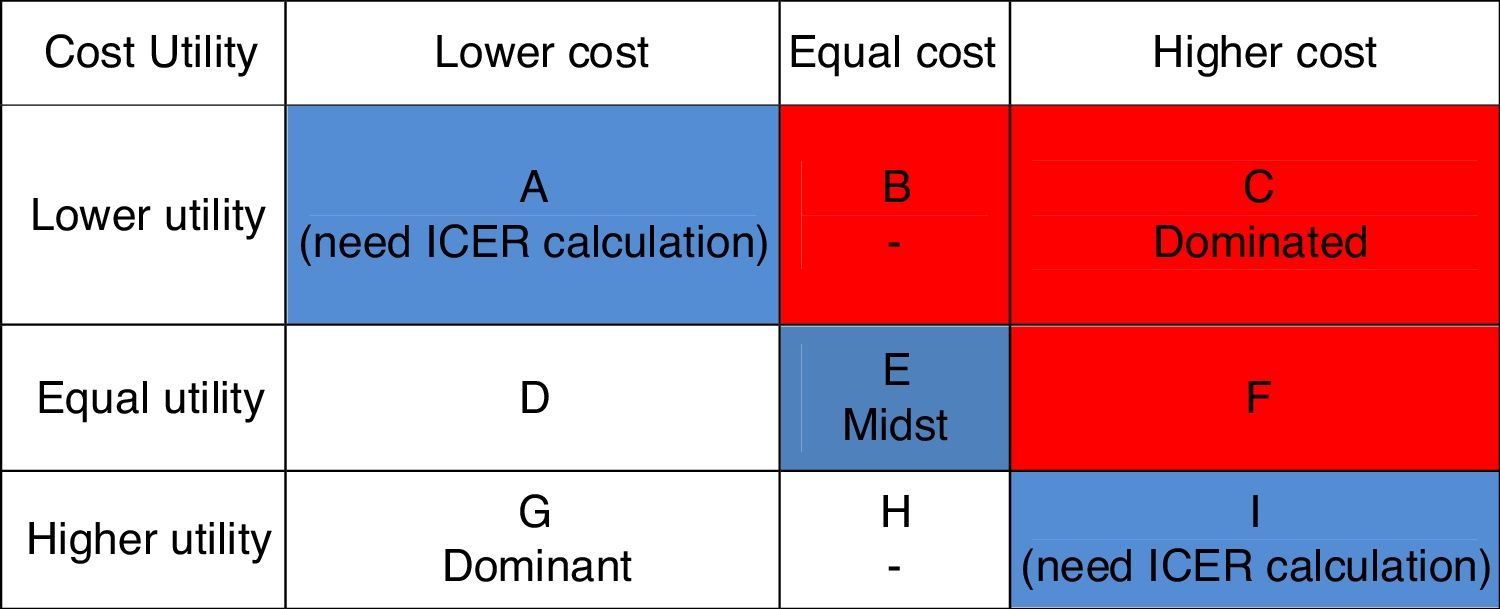
Furthermore, the alternative positioning of schizophrenia treatment based on cost utilitiy diagram was conducted (Fig. 1). If the treatment position was located in column A and column I then it was necessary to calculate incremental cost-effectiveness ratio (ICER) with the standard formula from ministry of health of Indonesia as follows15:
Sensitivity analysisSensitivity analysis is conducted to find out how the extent of change in cost or utility value was used to calculate cost-effective ratio and could affect the conclusion. Sensitivity analysis was carried out by varying 25% of increase and decrease of the cost-utility analysis of antipsychotic to the total cost.16
Statistical analysisThe patient's demographic data was shown in the form of a percentage (frequency), and the age of patients were analyzed by using Chi-Square Test, while the patient's gender, occupation, and education were analyzed by independent Sample T-Test as well. If the value of p>0.05 it was interpreted as not significantly different. The quality of life value based on SF-36 also processed by using Paired Sample T-Test when data distributed normally and wilcoxon test was used if the data were not distributed normally. If the value of p<0.05, it was interpreted as significantly different.
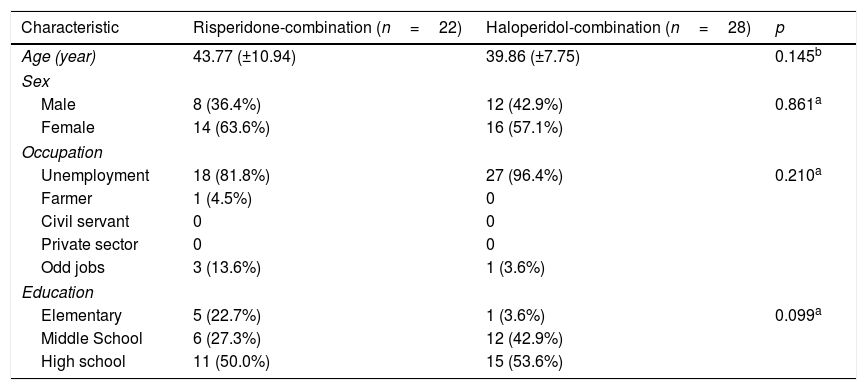
ResultsDemographic characteristic of sampleDemographic characteristics of the two groups of patients, namely 22 patients with risperidone combination and 28 patients haloperidol combination, could be seen in detail in Table 1. The average age of group risperidone-combination was 43.77 years (±10.94) and the group of haloperidol-combination was 39.86 years (±7.75). The group of risperidone-combination consisted of 63.6% female and 36.4% male, while in the group of haloperidol combination consisted of 57.1% female and 42.9% male. The occupation characteristics of the group risperidone combination were 81.8% unemployment and in the group of haloperidol combination was 96.4% unemployment. In the Education sector shows that 50.0% in the group of risperidone combinations were high school graduates, and 53.6% of the group haloperidol combination were high school graduates. Each variable from the two groups were compared to each other than statistically analyzed. There is no significant difference between two groups based on age, sex, occupation, and education characteristics.
Demographic characteristic of patients.
| Characteristic | Risperidone-combination (n=22) | Haloperidol-combination (n=28) | p |
|---|---|---|---|
| Age (year) | 43.77 (±10.94) | 39.86 (±7.75) | 0.145b |
| Sex | |||
| Male | 8 (36.4%) | 12 (42.9%) | 0.861a |
| Female | 14 (63.6%) | 16 (57.1%) | |
| Occupation | |||
| Unemployment | 18 (81.8%) | 27 (96.4%) | 0.210a |
| Farmer | 1 (4.5%) | 0 | |
| Civil servant | 0 | 0 | |
| Private sector | 0 | 0 | |
| Odd jobs | 3 (13.6%) | 1 (3.6%) | |
| Education | |||
| Elementary | 5 (22.7%) | 1 (3.6%) | 0.099a |
| Middle School | 6 (27.3%) | 12 (42.9%) | |
| High school | 11 (50.0%) | 15 (53.6%) | |
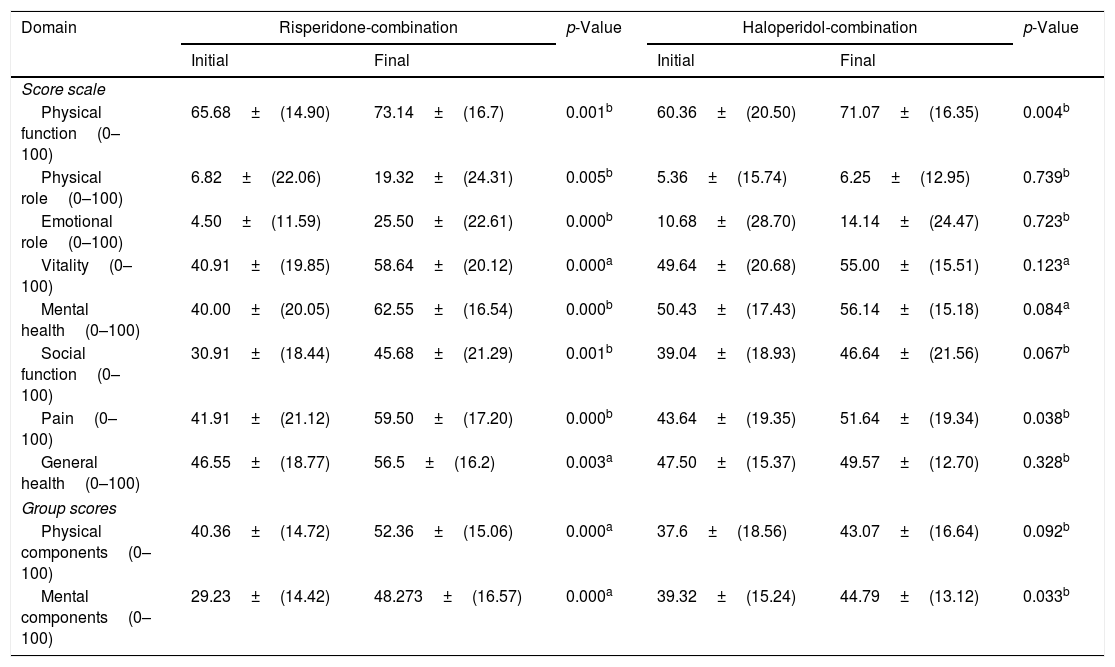
The result of initial and final scoring by using SF-36 questionnaire to each domain and component from both groups was presented in Table 2. In a risperidone-combination group, the final scores which were shown mean scores below to the normative score could only be found in physical role (19.32±24.31), emotional role (25.50±22.61) and social function domains (45.68±21.29), while other domains and components shows mean scores was above to normative score. These mean that there was a significant improvement of quality of life in each domain and component in risperidone-combination group with p-value<0.05. It was different result found on haloperidol-combination group which was the final scores shown mean scores to above normative score was physical role (6.25±12.95), emotional role (14.14±24.47), social function (46.64±21.56), general health (49.57±12.7), physical component (43.07±16.69) and mental component (44.78±13.15), while other domains were above to the normative score. These mean that there was a significant change in the physical function domain (p=0004), pain (p=0.038), and mental component (p=0.033).
SF-36 scores.
| Domain | Risperidone-combination | p-Value | Haloperidol-combination | p-Value | ||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | |||
| Score scale | ||||||
| Physical function(0–100) | 65.68±(14.90) | 73.14±(16.7) | 0.001b | 60.36±(20.50) | 71.07±(16.35) | 0.004b |
| Physical role(0–100) | 6.82±(22.06) | 19.32±(24.31) | 0.005b | 5.36±(15.74) | 6.25±(12.95) | 0.739b |
| Emotional role(0–100) | 4.50±(11.59) | 25.50±(22.61) | 0.000b | 10.68±(28.70) | 14.14±(24.47) | 0.723b |
| Vitality(0–100) | 40.91±(19.85) | 58.64±(20.12) | 0.000a | 49.64±(20.68) | 55.00±(15.51) | 0.123a |
| Mental health(0–100) | 40.00±(20.05) | 62.55±(16.54) | 0.000b | 50.43±(17.43) | 56.14±(15.18) | 0.084a |
| Social function(0–100) | 30.91±(18.44) | 45.68±(21.29) | 0.001b | 39.04±(18.93) | 46.64±(21.56) | 0.067b |
| Pain(0–100) | 41.91±(21.12) | 59.50±(17.20) | 0.000b | 43.64±(19.35) | 51.64±(19.34) | 0.038b |
| General health(0–100) | 46.55±(18.77) | 56.5±(16.2) | 0.003a | 47.50±(15.37) | 49.57±(12.70) | 0.328b |
| Group scores | ||||||
| Physical components(0–100) | 40.36±(14.72) | 52.36±(15.06) | 0.000a | 37.6±(18.56) | 43.07±(16.64) | 0.092b |
| Mental components(0–100) | 29.23±(14.42) | 48.273±(16.57) | 0.000a | 39.32±(15.24) | 44.79±(13.12) | 0.033b |
SF-36, 36-item Short From questionnaire.
Data are rounded mean±SD.
Determination of utility cost was based on the increase of the percentage of SF-36 which result could be seen in Table 3. There was no significant difference in the SF-36 average increase of value (p=0.681) between the two groups.
Mean/Average of initial, final, and increase value of SF-36.
| SF-36 | Risperidone-combination (n=22) | Haloperidol-combination (n=28) | p-Value |
|---|---|---|---|
| Initial | 0.413 | 0.430 | 0.739 |
| Final | 0.553 | 0.580 | 0.73 |
| Increase | 0.423 | 0.433 | 0.681 |
SF-36, 36-item Short Form questionnaire Statistical tested by using independent sample T-Test.
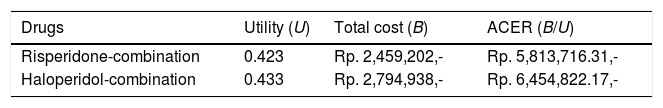
The Average Cost-Effectiveness Ratio (ACER) for both groups could be seen in Table 4. Risperidone-combination group yielded a lower ACER (IDR 5.813.822,13.-per-QALY) compared to haloperidol-combination group (IDR 6.454.822,17.-per-QALY). By placing the ACER alternative position of these two groups into diagram of alternative based on cost utility (Fig. 1), it was noted that risperidone-combination group was into column A which mean the column for low cost and low utility and haloperidol-combination group was into column I which mean column for high cost and high utility, thus an Incremental Cost-Effectiveness Ratio (ICER) calculation was necessary to proceed.
The result of Incremental Cost-Effectiveness Ratio (ICER) calculation could be seen in Table 5 as follows.
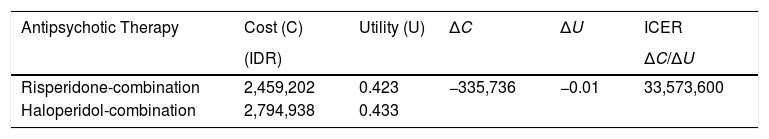
The result of ICER calculation for two months period of therapy to schizophrenic patients in Prof. DR. V. L. Ratumbuysang Psychiatric Hospital.
| Antipsychotic Therapy | Cost (C) | Utility (U) | ΔC | ΔU | ICER |
|---|---|---|---|---|---|
| (IDR) | ΔC/ΔU | ||||
| Risperidone-combination | 2,459,202 | 0.423 | −335,736 | −0.01 | 33,573,600 |
| Haloperidol-combination | 2,794,938 | 0.433 |
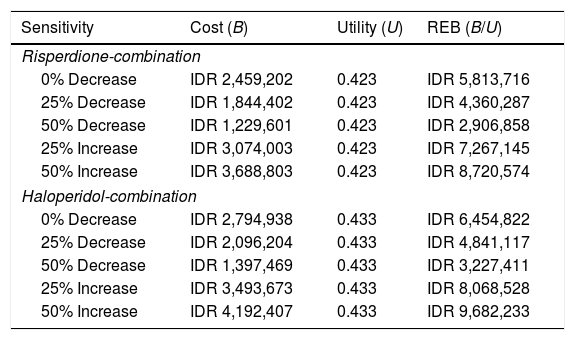
A sensitivity analysis has been done for both groups, as shown in Table 6. The results show that the risperidone-combination was sensitive to the 25% of cost-increase, and was also sensitive to the 25% of cost-decrease into haloperidol-combination group.
Sensitivity analysis of the risperidone-combination and haloperidol-combination to schizophrenia patients in Prof. DR. V.L. Ratumbuysang Psychiatric Hospital.
| Sensitivity | Cost (B) | Utility (U) | REB (B/U) |
|---|---|---|---|
| Risperdione-combination | |||
| 0% Decrease | IDR 2,459,202 | 0.423 | IDR 5,813,716 |
| 25% Decrease | IDR 1,844,402 | 0.423 | IDR 4,360,287 |
| 50% Decrease | IDR 1,229,601 | 0.423 | IDR 2,906,858 |
| 25% Increase | IDR 3,074,003 | 0.423 | IDR 7,267,145 |
| 50% Increase | IDR 3,688,803 | 0.423 | IDR 8,720,574 |
| Haloperidol-combination | |||
| 0% Decrease | IDR 2,794,938 | 0.433 | IDR 6,454,822 |
| 25% Decrease | IDR 2,096,204 | 0.433 | IDR 4,841,117 |
| 50% Decrease | IDR 1,397,469 | 0.433 | IDR 3,227,411 |
| 25% Increase | IDR 3,493,673 | 0.433 | IDR 8,068,528 |
| 50% Increase | IDR 4,192,407 | 0.433 | IDR 9,682,233 |
Several studies reported that schizophrenic patients obtain a better quality of life when using atypical antipsychotics than when they are using typical antipsychotics.17,18 In this study, the result of SF-36 score for both groups of patient, shows final score is below the normative score were domain of physical role, emotional role, social function and physical component associated with activity factor, and the side effect of therapy as well. The lack of activity and the utilization of free time to do activities in schizophrenic patients was lead to the poor quality of life, as well as the side effects of the therapy, like tardivedyskinesia, pseudoparkinson symptoms and akathisia that interfere the physical and social comforts of schizophrenic patients, and moreover affected the quality of life of schizophrenic patients.4,19 The interesting fact, that the lowest scores on initial and final assessment were domain in physical role and emotional role. Previous studies reported that physical role and emotional role are interrelated with each other, and the patient usually unable to distinguish where the source of his/her limitation is physically or mentally.20 Furthermore, this study showed that risperidone-combination group was significantly exhibited an increase in quality of life compared to the haloperidol-combination group. This is associated with the response of patients to the given therapy (Dipiro et al., 2008) which the effect of risperidone therapy was already seen in the fifth to eighth week of therapy,4 while the significant final result also indicated that the result could be maintained during therapy and there was an improvement in the symptoms of mental health.17,19
Previous studies also indicated that the atypical antipsychotic (risperidone) was more cost-effective than the typical antipsychotics (haloperidol), and it was an expectation that by using second generation of antipsychotic there will be decreased in the cost of every component related to schizophrenia therapy.21,22 This study showed that the average cost effectiveness ratio (ACER) value of risperidone-combination was lower than the haloperidol-combination. This indicated that risperidone-combination was more cost-effective compared to haloperidol-combination, which means that it is needed a cost of IDR 5.813.716,13 per quality-adjusted life-year (QALY). Based on the position resulted in Diagram of Alternative Groups Based on Cost Utilities, an incremental cost-effectiveness ratio (ICER) calculation is also necessary to be done. The ICER value obtained is the additional cost required per QALY in a therapy, and in this case, it was required an additional cost of Rp. 33.573.600, per QALY for the risperidone-combination group, but the patients in this group gained 0.423 additional times (survival years) or equally 5.08 months. Based on the result of sensitivity analysis, it was noted that in the change of 25% total cost-increase for risperidone-combination and 25% total cost-decrease for haloperidol-combination and causes a significant change in ACER values. A meta-analysis study in France showed that risperidone is the dominant choice compared to the use of haloperidol, and the use of risperidone saved $6.510 (CAN$) and yielded more than 0.04 QALYs than haloperidol.21 Risperidone also became a dominant choice for antipsychotic therapy, compared to haloperidol, in Spain.22
ConclusionRisperidone-combination is the dominant antipsychotic therapy choice compared to the haloperidol-combination. But the result of the sensitivity test of the 25% total cost decrease for risperidone-combination group and the 25% total cost decrease for haloperidol-combination group will affect the result of cost-utility.
Conflict of interestThe authors declare no conflict of interest.
Thank to our supervisor for their guidance and helpful comments on the draft. The authors thank to all staff of the Psychiatric Hospital of Prof. Dr. V.L. Ratumbuysang for their assistance during the study.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.