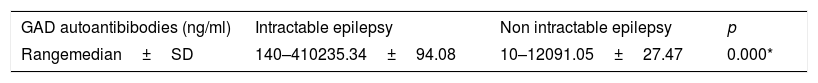Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosThe purpose of this study was to compare autoantibodies to glutamic acid decarboxylase serum levels in children suffering from intractable and non-intractable epilepsy at Dr. Soetomo General Hospital Surabaya, Indonesia.
MethodsA case–control study was conducted with children suffering from intractable and non-intractable epilepsy from November to December 2018 in the pediatric neurology outpatient clinic. Autoantibodies to glutamic acid decarboxylase serum levels were examined from both groups using ELISA; statistical analysis was performed using the Mann–Whitney U test non-parametric tests.
ResultA sample of 80 children was divided into two groups of 40 children each. Their average age was 5.4 years (range of 4 months–17 years). The average anti-epileptic drug treatment duration was 2.3 years (3 months–13 years). Glutamic acid decarboxylase autoantibody levels in the intractable group were higher compared to levels in the non-intractable group, ranging from 140 to 410ng/ml (median 243.3) and 10–120ng/ml (median 98.10) respectively with p values<0.05.
ConclusionChildren suffering from intractable epilepsy have higher levels of GAD-Ab compared to children with non-intractable epilepsy.
Intractable epilepsy is defined as inadequate seizure control despite compliance with effective AEDs and excluding non-epileptic events and poor compliance or failure of two adequate AEDs, either monotherapy or combination therapy, to achieve sustained seizure-free status, the occurrence of one seizure per month, and no more than a 3-month seizure-free period.1,2 Glutamic acid decarboxylase (GAD) is the main enzyme catalyst for the conversion of glutamic acid to gamma aminobutyric acid (GABA), the main neurotransmitter inhibitor in the GABAergic terminal in the brain. The presence of GAD have influence in balance equilibrium between inhibitors and excitation of neurotransmitters.3 Autoantibodies to glutamic acid decarboxylase (GAD-Ab) as one of the causes of epilepsy are found in patients with acute and chronic epilepsy. Most of the published reports concern adult patients, and the frequency of these antibodies in the pediatric epileptic population is unknown.4 In regard to intractable epilepsy, we found a higher concentration compared to the control as an effect of uncontrolled seizure activity. A previous study found 6.7% with autoantibodies>1000iu/ml in intractable epilepsy.5
The prevalence of GAD-Ab has been found to increase in adult epilepsy and temporal lobe epilepsy.6 Autoimmunity as an epilepsy etiology has a significant clinical impact, with immune therapy as part of a treatment plan that can offer the patient clinical improvement.7 Several studies have demonstrated the remission of epileptic seizures following the administration of immunosuppressant therapy, a finding that confirmed that epilepsy patients with GAD-Ab have a positive effect from immunosupressant therapy.8–11
Failure to control seizures in children has medical, social, and economic effects, as well as potential behavioral problems that may result in a negative effect on their education. Uncontrolled seizure activity has also been associated with cognitive impairment.12 Most clinicians do not consider autoimmunity as one of the causes of intractable epilepsy. Clinicians must consider that an immune etiology may be present, especially in patients with subacute seizures who do not appear to have structural abnormalities on magnetic resonance imaging.13
Materials and methodsPatientsThis study was conducted from November to December 2018. The author received written informed consent from the patient parent. Data collection included age, gender, regional AEDs prescribed, dosage and medication frequency, type of therapy and its duration, and seizure frequency. Inclusion criteria were children from 1 month to 17 years old, previously diagnosed with intractable epilepsy or non-intractable epilepsy, currently receiving anti-epileptic drug treatment, and a parent who agreed to have his/her child participate and who signed the informed consent. Exclusion criteria were a history of an autoimmune disease (e.g., type 1 DM, Crohn's disease, Hashimoto thyroiditis), neurological disease (e.g., encephalitis), chronic inflammatory disease, a history of refractory related to non-epileptic seizures, inadequate AEDs, non-compliance, and high-dose steroid therapy. Autoantibodies to glutamic acid decarboxylase serum were analyzed from peripheral blood collection. A total of 90 patients met the inclusion criteria for this study, but 10 refused blood collection and dropped out. The Institutional Review Board (IRB) at Dr. Soetomo Hospital, Surabaya, approved all protocols in this study (number 0585/KEPK/IX/2018).
MethodsThis was a case control study conducted at Dr. Soetomo Hospital, Surabaya. Data were collected in the pediatric neurology outpatient clinic)
MeasurementAll patients in this study were evaluated for GAD-Ab in serum. As a cut-off for increased GAD-Ab, we referred to a previous study that used>100mg/dl in serum.2 The evaluation of GAD-Ab was carried out using AED245Hu 96 Tests Enzyme-linked Immunosorbent Assay Kit For Anti-Glutamic Acid Decarboxylase Antibodies (Anti-GAD) (Cloud-Clone Corp., Houston, TX, USA) with a standard curve range of 2–600ng/ml. The examination results were in the form of quantitative data.
Statistical analysisIBM SPSS Statistics version 21.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. The Mann–Whitney U test was used to compare GAD-Ab levels in serum; a p value of <0.05 was considered to have statistical significance.
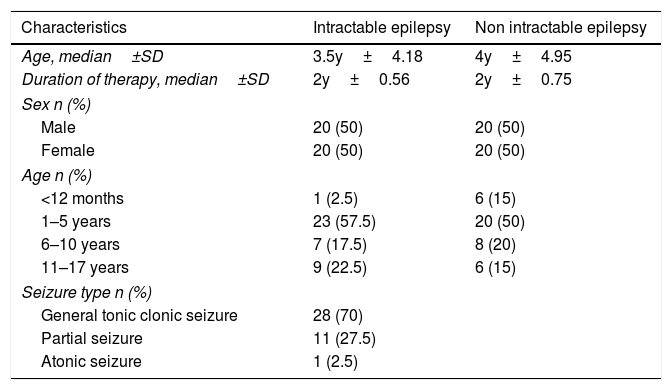
ResultA sample of 80 patients diagnosed with epilepsy were interviewed. Their parents also completed data collection sheets. Patients were divided into two groups, namely intractable epilepsy and non-intractable epilepsy. This study showed no differences between males and females. Patients whose ages were between 1 and 5 years old outnumbered other age categories. In the intractable group, the type of seizure experienced most often is the general tonic–clonic seizure, and all patients used 2 or 3 anti-epileptic drugs (Table 1). Based on the non-parametric Mann–Whitney U test, intractable epilepsy serum GAD-Ab levels were higher compared to those in the non-intractable epilepsy group, with p values=0.000 (p significant<0.05) (Table 2).
Patient characteristic.
| Characteristics | Intractable epilepsy | Non intractable epilepsy |
|---|---|---|
| Age, median±SD | 3.5y±4.18 | 4y±4.95 |
| Duration of therapy, median±SD | 2y±0.56 | 2y±0.75 |
| Sex n (%) | ||
| Male | 20 (50) | 20 (50) |
| Female | 20 (50) | 20 (50) |
| Age n (%) | ||
| <12 months | 1 (2.5) | 6 (15) |
| 1–5 years | 23 (57.5) | 20 (50) |
| 6–10 years | 7 (17.5) | 8 (20) |
| 11–17 years | 9 (22.5) | 6 (15) |
| Seizure type n (%) | ||
| General tonic clonic seizure | 28 (70) | |
| Partial seizure | 11 (27.5) | |
| Atonic seizure | 1 (2.5) | |
Autoantibodies to glutamic acid decarboxylase have been detected in CSF and serum in patients with epilepsy. Increased GAD-Ab is strongly associated with epilepsy and indicative of ongoing immune dysregulation.2 This study showed that GAD-Ab serum is higher in children with intractable epilepsy compared to children with non-intractable epilepsy. Although there were several neuronal autoantibodies suspected as etiologies of epilepsy, we compared GAD-Ab specifically between children with intractable and controlled forms of epilepsy. The increased prevalence of autoantibodies is more strongly associated with epilepsy, perhaps indicating that immune dysregulation may be commonly associated with this disorder.14
This study found a significant difference in GAD-Ab levels between the two groups, with a higher level of GAD autoantibodies in the group with intractable epilepsy. This result supports previous studies that found similar results; both found significantly higher levels of GAD-Ab in the intractable epilepsy group compared to the control group.2,15 One study confirmed that GAD-Ab was associated with several forms of epilepsy such as idiopathic and cryptogenic temporal epilepsy, and another found GAD-Ab up to 100 iu/ml, especially in drug-resistant patients with epilepsy.9,16
The highest GAD-Ab level was found in patients with intractable epilepsy, providing evidence for an autoimmune etiology and active immunological processes in the brain.16 Antibodies to glutamic acid decarboxylase induce suppression of GABA release, modulate inhibitory potential in interneurons, down-regulate GABA synthesis in neuronal cell terminals, decrease GABA release in post-synaptic purkinje cells, disrupt neuronal activity, and intervene in the regulation of brain neurotransmitters.7,17 This leads directly to intercellular protein mediated by T cell cytotoxicity, which causes inflamation and burnout in the hippocampal region.5,18 Antibodies to GAD play a role in the cerebellar pathway where irreversible lesions cause permanent neuronal damage and cerebellar atrophy.19
Our study has limitations that must be considered. The study examined GAD-Ab in blood. A high level of GAD-Ab is reported to have been found in cerebral spinal fluid; however, we did not use CSF for this study because the procedure for collecting it is invasive and uncomfortable, and the number of parents who would have rejected this procedure would, understandably, be quite high. The blood–brain barrier leak due to inflammation results in antineuronal antibodies being found in the blood; thus, we considered blood for the research material.20 In this study, the variation of epilepsy samples is wide, where the range of age is 1 month to 17 years; therefore, it required a larger sample number.
In conclusion, the GAD-Ab serum level is higher in children with intractable epilepsy compared to children with non-intractable epilepsy. Further studies of other neurological autoimmune antibodies to reveal autoimmunity in the etiology of epilepsy should be conducted.
ConclusionChildren suffering from intractable epilepsy have higher levels of GAD-Ab compared to children with non-intractable epilepsy.
Data availabilityThe data used to support the findings of this study are available from the corresponding author upon request.
Ethical approvalAll procedures in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The ethical approval of this study was granted from Ethical Committee, Faculty of Medicine, Universitas Airlangga.
ConsentInformed consent was obtained from all individual subjects included in the study.
Conflicts of interestNo potential conflict of interest relevant to this article was reported.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.