We aim to determine the effect of a fixed-dose combination (FDC) of tiotropium/olodaterol on Physical activity (PA) in patients with chronic obstructive pulmonary disease (COPD) in a real world setting.
MethodsCOPD patients were prospectively enrolled to evaluate the effect of a FDC of tiotropium/olodaterol inhaler therapy via the Respimat® Soft Mist™ inhaler (SMI) on the physical functioning scale (PF-10), and the general condition of the patient as assessed by the physician (Physician's Global Evaluation, PGE), and the patient's satisfaction after 6 weeks of treatment. The primary end-point was the percentage of patients with therapeutic success at 6th week follow-up, defined as a ≥10-points increase in the standardised PF-10 score from baseline.
ResultsA total of 257 patients from 57 sites were enrolled, and 234 completed the follow up. After 6 weeks of treatment, 155 out of 234 patients (66.2%) showed therapeutic success in the physical functioning score, coupled with significant improvement in PGE score: 78 (33.3%) patients with good/excellent PGE score at baseline, increasing to 172 (73.5%) at 6th week (p<0.0001). The patient's satisfaction was excellent: 77.2% reporting to be satisfied/very satisfied with the treatment, 79.9% with inhaling and 79.0% with the handling of SMI device. 1.6% of patients reported an investigator-defined drug-related adverse event.
ConclusionTreatment of COPD patients with a FDC of tiotropium/olodaterol SMI for 6 weeks resulted in significant improvements in the patients’ condition as assessed by patients and physicians, with no new safety findings.
Determinar el efecto sobre la actividad física (AF) de una combinación a dosis fija (CDF) de tiotropio/olodaterol en pacientes con enfermedad pulmonar obstructiva crónica (EPOC) en la práctica real.
MétodosSe reclutaron prospectivamente a pacientes con EPOC para evaluar el efecto de una CDF de tiotropio/olodaterol a través del inhalador Respimat® Soft Mist™ (SMI) en la escala de función física (PF-10), el estado general según la evaluación global del médico (EGM) y la satisfacción del paciente después de seis semanas de tratamiento. La variable primaria fue el porcentaje de pacientes con éxito terapéutico, definido como un aumento ≥10 puntos en la escala estandarizada de PF-10.
ResultadosSe incluyeron 257 pacientes de 57 centros, 234 completaron el seguimiento. Tras seis semanas, 155 de 234 pacientes (66,2%) mostraron un éxito terapéutico según la puntuación de función física, junto con una mejora significativa en la valoración de la EGM de 78 pacientes (33,3%) con una evaluación buena/excelente al inicio del estudio que aumentó a 172 (73,5%) a las seis semanas (p < 0,0001). La satisfacción del paciente fue excelente: el 77,2% refirió estar satisfecho/muy satisfecho con el tratamiento, el 79,9% con la inhalación y el 79,0% con el manejo del dispositivo. El 1,6% de los pacientes reportó un evento adverso definido por el investigador como relacionado con el fármaco.
ConclusiónEl tratamiento de pacientes con EPOC con una CDF de tiotropio/olodaterol a través un ISM durante seis semanas resultó en mejoras significativas en su condición evaluada por pacientes y médicos, sin nuevos hallazgos de seguridad.
Physical activity (PA) is believed to play a central role in the prognosis of patients with chronic obstructive pulmonary disease (COPD). Early in the disease progression, as early as GOLD Stage 2, PA can appear reduced1 and be instrumental in the development of extra-pulmonary effects, such as deconditioning, skeletal muscle atrophy, weakness, osteoporosis and cardiovascular disease.2 This in turn can directly or indirectly result in a steeper decline in lung function, as suggested by some epidemiological data,3 thus completing a deleterious vicious circle. Breathlessness and COPD-associated symptoms cause patients to reduce PA, leading to a physical deterioration and therefore a worse prognosis. COPD patients with low levels of PA have higher rates of hospital admission and mortality than those with moderate or high levels of PA.4
Long-acting bronchodilators, such as long-acting muscarinic antagonists (LAMAs) and β2-agonists (LABA), are the cornerstone of maintenance therapy for symptomatic patients with moderate to very severe COPD.5,6 GOLD guidelines recommend the combination of LAMA and LABA in patients suboptimally controlled on long-acting bronchodilator monotherapy.7 Fixed-dose combinations (FDC) of LAMA and LABA may be a safe, efficient and convenient option to treat these patients, similarly to FDC of short-acting bronchodilators.8 The advantages of a FDC of the LAMA tiotropium and the LABA olodaterol, a highly selective and effective β2 agonist,9,10 over its separate components have been tested in different phase III studies, evaluating lung function,11 quality of life (St. George's Respiratory Questionnaire -SGRQ),12 dyspnea (Transition Dyspnea Index, TDI)12 and exercise endurance time.13–16 There are still limited data about the effect of a FDC of tiotropium/olodaterol Respimat® Soft Mist™ inhaler (SMI) on physical activity in a primary care real world setting.17,17
MethodsStudy designThis is a prospective, open-label, observational, self-controlled, pre-post, multicentre study to appraise the effect of a FDC of tiotropium and olodaterol SMI on the physical functioning scale, as a surrogate for physical activity and exercise capacity, in COPD patients after 6 weeks of treatment. The patient's general condition (PGE) evaluated by the physician and patient's satisfaction, were secondary objectives.
Study populationPatients from 57 different primary care centres in Spain, meeting the following inclusion criteria were included into the study: (1) Diagnosis of COPD; (2) age ≥40 years old; (3) an indication for long-acting dual bronchodilation (LAMA+LABA) treatment with tiotropium/olodaterol (5/5μg) SMI according to GOLD guidelines. Main exclusion criteria were: (1) Clinical contraindication for LAMA or LABA therapy; (2) Treatment with a LAMA+LABA combination of any kind in the preceding 6 months; (3) Indication to continue treatment with a combination of LABA+inhaled corticosteroids (ICS); (4) Inability to obtain 6 weeks follow up at the enrolling site; (5) Patients in waiting list for lung transplantation. The protocol was written according to the GOLD recommendation version 2017.18
Patients meeting all the inclusion criteria and no exclusion criteria were considered eligible for the study. If the patient was on LABA+ICS and the combination could be replaced by ICS alone, the patient was considered immediately eligible for the study, with no mandatory blank out period. Consecutive enrolment of eligible patients was conducted to minimise selection bias.
The study was conducted according to the Declaration of Helsinki, the principles and standards of good clinical practice and Spanish Health Authorities regulation. The local Ethics Committee in each centre and regional Health Authorities approved the protocol and all patients signed specific informed consent for their participation. The study was approved by the Ethical Committees of the following Regional Health Authorities in Spain: Castilla y León, Cataluña, La Rioja, Murcia and País Vasco. The first Ethic Committee to approve the protocol was the CEI Hospital de Badajoz (21/09/2016), and the first Spanish Region to grant approval was Cataluña (21/10/2016). This study was registered in ClinicalTrials.gov (NTC02927795) and in the EU PAS (09/06/2016), with the number EUPAS13754.
Study protocol and data collectionAt baseline visit, besides standard parameters, the patients’ physical functioning was evaluated according to the PF-10 scores (primary objective), a sub-domain of the SF-36 Health Survey,19 consisting of 10 items that evaluate the patient's restrictions to carry out routine daily activities. After standardisation, the PF-10 score may range from 0 (very limited) to 100 (not limited at all). The patient's general condition as assessed by the physician (secondary objective) was evaluated by means of the Physician's Global Evaluation (PGE) score, ranging from 1 (very poor) to 8 (excellent). Breathlessness severity was evaluated with the modified Medical Research Council (mMRC) questionnaire.
Therapy with FDC tiotropium/olodaterol was administered at a dose of 5μg/5μg once a day, as two puffs of the Spiolto Respimat® Soft Mist™ inhaler (SMI) for a period of 6 weeks11 according to common clinical practice. Non-pharmacological actions, like exercise programs or cessation of smoking habits, were left at the treating physician's criterion, according to standard clinical practice. At the 6th week, patients were followed up (FU) at a face-to-face visit, in which physical functioning, general condition and breathlessness severity were re-evaluated, as previously described. In addition, during the FU visit safety information concerning adverse events (AEs) was collected, together with the patient's general satisfaction with the FDC treatment, evaluated by means of a custom-made questionnaire with scale ranging from 1 (very unsatisfied) to 7 (very satisfied); this questionnaire has not been properly validated, but due to its simplicity it has been previously used in previous studies.17,20 The questionnaire is available as supplementary material. Serious AEs were recorded, plus any adverse drug reactions with a reasonable probability of being caused by the study medication, irrespective of severity. Severe AE were defined as any untoward medical occurrence resulting in death, life-threat, significant disability, congenital anomaly or birth defect, or requiring or prolonging hospitalisation. AEs were adjudicated by local investigators onsite.
ObjectivesThe pre-specified primary objective of the study was the percentage of patients with therapeutic success at 6th week FU, defined as a ≥10-points increase in the standardised PF-10 score from baseline.
Secondary objectives were the change in PF-10 score from baseline to FU, the change in patient's general condition as assessed by the PGE score from baseline to FU and the patient's general satisfaction with the FDC treatment.
Statistical analysisDescriptive statistics for continuous variables following a Gaussian distribution were reported as mean±standard deviation (SD), whilst they were reported as median (Q1-Q3) whenever a normal distribution could not be assumed. Nominal variables were reported as count (percent). For the pre-specified primary and secondary objectives, a 95% confidence interval was estimated. Comparison between subgroups in continuous variables were performed using unpaired t-tests (for 2-groups comparison) or ANOVA (for>2 groups comparison) if a Gaussian distribution was assumed, or with Mann-Whitney U test (for 2-groups comparison) or Kruskal-Wallis test (for>2 groups comparison) if a Gaussian distribution could not be assumed. Comparison between subgroups in categorical variables was performed with Fisher's exact test. Changes pre-post between baseline and FU in the standardised PF-10 score were analysed with the non-parametric Wilcoxon Rank-sum test, whilst changes in the categorical PGE score were analysed with McNemar test. Statistical significance was set at the level of p≤0.05 No interim analysis was performed for this study. All statistical analysis were performed using the SAS (SAS Institute, Cary, NC, USA) software package, version 9.4.
Considering data from a previous study (NCT 006*99699), the therapeutic success for the current study was estimated as 50%.17 Under this premises, a sample size of 882 patients was calculated to estimate the therapeutic success with a precision of ±3.3% for the 95% CI, assuming a normal approximation of the binomial distribution. The sample size was rounded up to 900 patients to account for a 10% loss at FU. Sample size was calculated with PASS (NCSS-LCC, Kaysville, Utah, USA) software package, version 2011.
Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request.
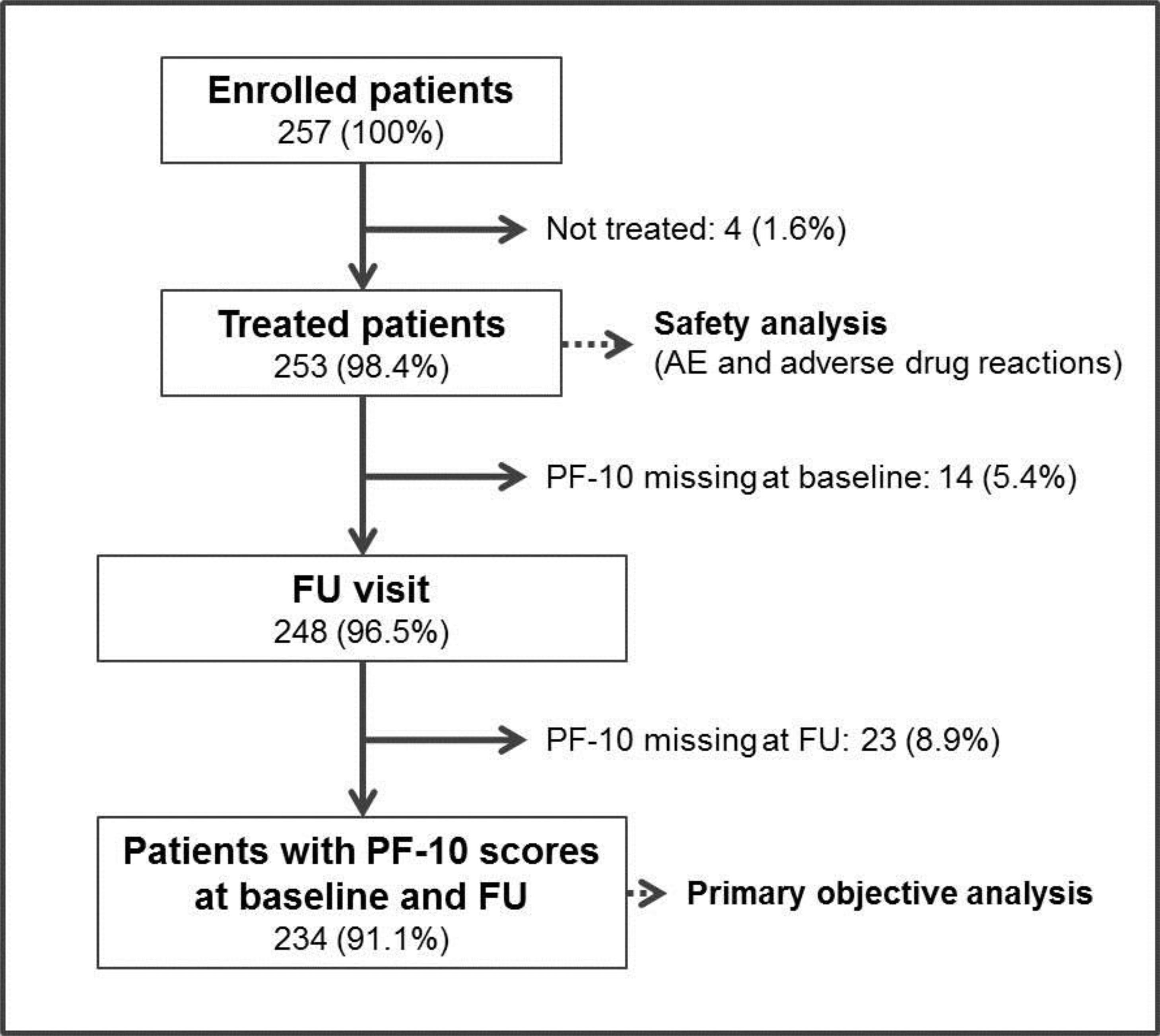
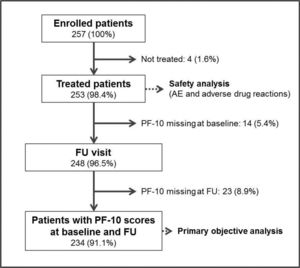
ResultsBetween the April 2017 and September 2018, 257 patients were enrolled into the study in 57 participating sites. The recruitment was prematurely stopped before completion of the predefined sample size due to logistic difficulties to include more subjects. Four enrolled patients (1.6%) were not finally treated and 9 patients (3.5%) were lost to follow-up. The final number of patients with PF-10 score recordings at baseline and FU for analysis of the primary objective was 234 (91.1%, Fig. 1).
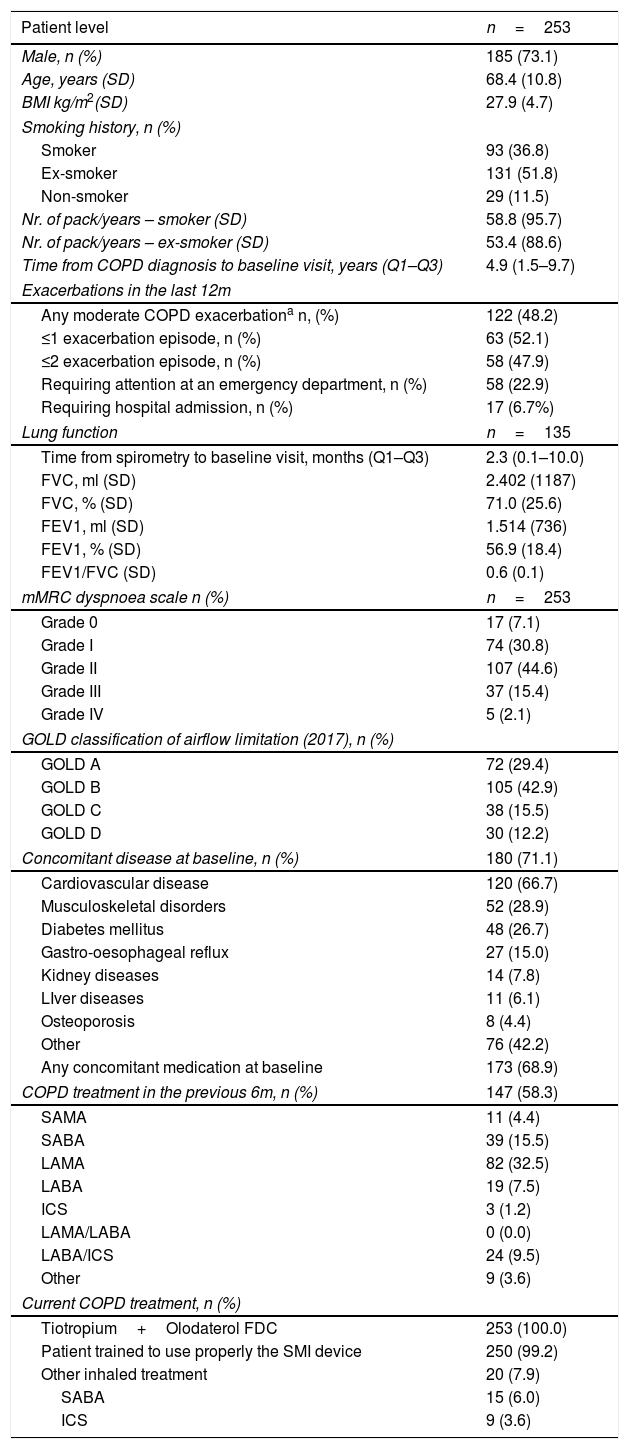
Table 1 summarises the sociodemographic, clinical and lung function characteristics of the patients. One hundred and eighty-five (73.1%) patients were male and the average age was 68.4 years-old. Only 11.5% of patients were non-smokers. The median time from COPD diagnosis to baseline visit was 4.9 months. One hundred and twenty-two patients (48.2%) had experienced one or more moderate COPD exacerbations in the preceding 6 months, defined as an episode requiring corticosteroids or systemic antibiotics. Among them, 47.9% experienced ≥2 exacerbation episodes, 22.9% required attention at an emergency department and 6.7% required admission to the hospital. According to the GOLD classification of airflow limitation, version 2017, the categories GOLD A and B were most prevalent: 72 patients (29.4%) and 105 patients (42.9%), respectively. The COPD treatment in the preceding 6 months is shown in Table 1, and Fig. 1S (supplementary information). In accordance with the study protocol, no patient was receiving a LAMA+LABA combination in the preceding 6 months and no patient continued the treatment with LABA+ICS during the study period.
Descriptive statistics of treated patients (n=253) at baseline.
| Patient level | n=253 |
|---|---|
| Male, n (%) | 185 (73.1) |
| Age, years (SD) | 68.4 (10.8) |
| BMI kg/m2(SD) | 27.9 (4.7) |
| Smoking history, n (%) | |
| Smoker | 93 (36.8) |
| Ex-smoker | 131 (51.8) |
| Non-smoker | 29 (11.5) |
| Nr. of pack/years – smoker (SD) | 58.8 (95.7) |
| Nr. of pack/years – ex-smoker (SD) | 53.4 (88.6) |
| Time from COPD diagnosis to baseline visit, years (Q1–Q3) | 4.9 (1.5–9.7) |
| Exacerbations in the last 12m | |
| Any moderate COPD exacerbationa n, (%) | 122 (48.2) |
| ≤1 exacerbation episode, n (%) | 63 (52.1) |
| ≤2 exacerbation episode, n (%) | 58 (47.9) |
| Requiring attention at an emergency department, n (%) | 58 (22.9) |
| Requiring hospital admission, n (%) | 17 (6.7%) |
| Lung function | n=135 |
| Time from spirometry to baseline visit, months (Q1–Q3) | 2.3 (0.1–10.0) |
| FVC, ml (SD) | 2.402 (1187) |
| FVC, % (SD) | 71.0 (25.6) |
| FEV1, ml (SD) | 1.514 (736) |
| FEV1, % (SD) | 56.9 (18.4) |
| FEV1/FVC (SD) | 0.6 (0.1) |
| mMRC dyspnoea scale n (%) | n=253 |
| Grade 0 | 17 (7.1) |
| Grade I | 74 (30.8) |
| Grade II | 107 (44.6) |
| Grade III | 37 (15.4) |
| Grade IV | 5 (2.1) |
| GOLD classification of airflow limitation (2017), n (%) | |
| GOLD A | 72 (29.4) |
| GOLD B | 105 (42.9) |
| GOLD C | 38 (15.5) |
| GOLD D | 30 (12.2) |
| Concomitant disease at baseline, n (%) | 180 (71.1) |
| Cardiovascular disease | 120 (66.7) |
| Musculoskeletal disorders | 52 (28.9) |
| Diabetes mellitus | 48 (26.7) |
| Gastro-oesophageal reflux | 27 (15.0) |
| Kidney diseases | 14 (7.8) |
| LIver diseases | 11 (6.1) |
| Osteoporosis | 8 (4.4) |
| Other | 76 (42.2) |
| Any concomitant medication at baseline | 173 (68.9) |
| COPD treatment in the previous 6m, n (%) | 147 (58.3) |
| SAMA | 11 (4.4) |
| SABA | 39 (15.5) |
| LAMA | 82 (32.5) |
| LABA | 19 (7.5) |
| ICS | 3 (1.2) |
| LAMA/LABA | 0 (0.0) |
| LABA/ICS | 24 (9.5) |
| Other | 9 (3.6) |
| Current COPD treatment, n (%) | |
| Tiotropium+Olodaterol FDC | 253 (100.0) |
| Patient trained to use properly the SMI device | 250 (99.2) |
| Other inhaled treatment | 20 (7.9) |
| SABA | 15 (6.0) |
| ICS | 9 (3.6) |
BMI: Body mass index; COPD: chronic obstructive pulmonary disease; ICS: Inhaled corticosteroids; FEV1: Forced expiratory volume in the 1st second; FDC: Fixed-dose combination; FVC: Forced vital capacity; LABA: long-acting beta-2 agonist; LAMA: long-acting muscarinic antagonist; mMRC: modified Medical Research Council; SABA: short-acting beta-2 agonist; SAMA: short-acting muscarinic antagonist; SD: standard deviation.
The primary outcome, therapeutic success, defined as an increase ≥10 points in the self-reported standardised PF-10 score between baseline and 6th week FU, was achieved in 155 patients (66.2%, 95% CI: 60.2–72.3, precision 6.05%).
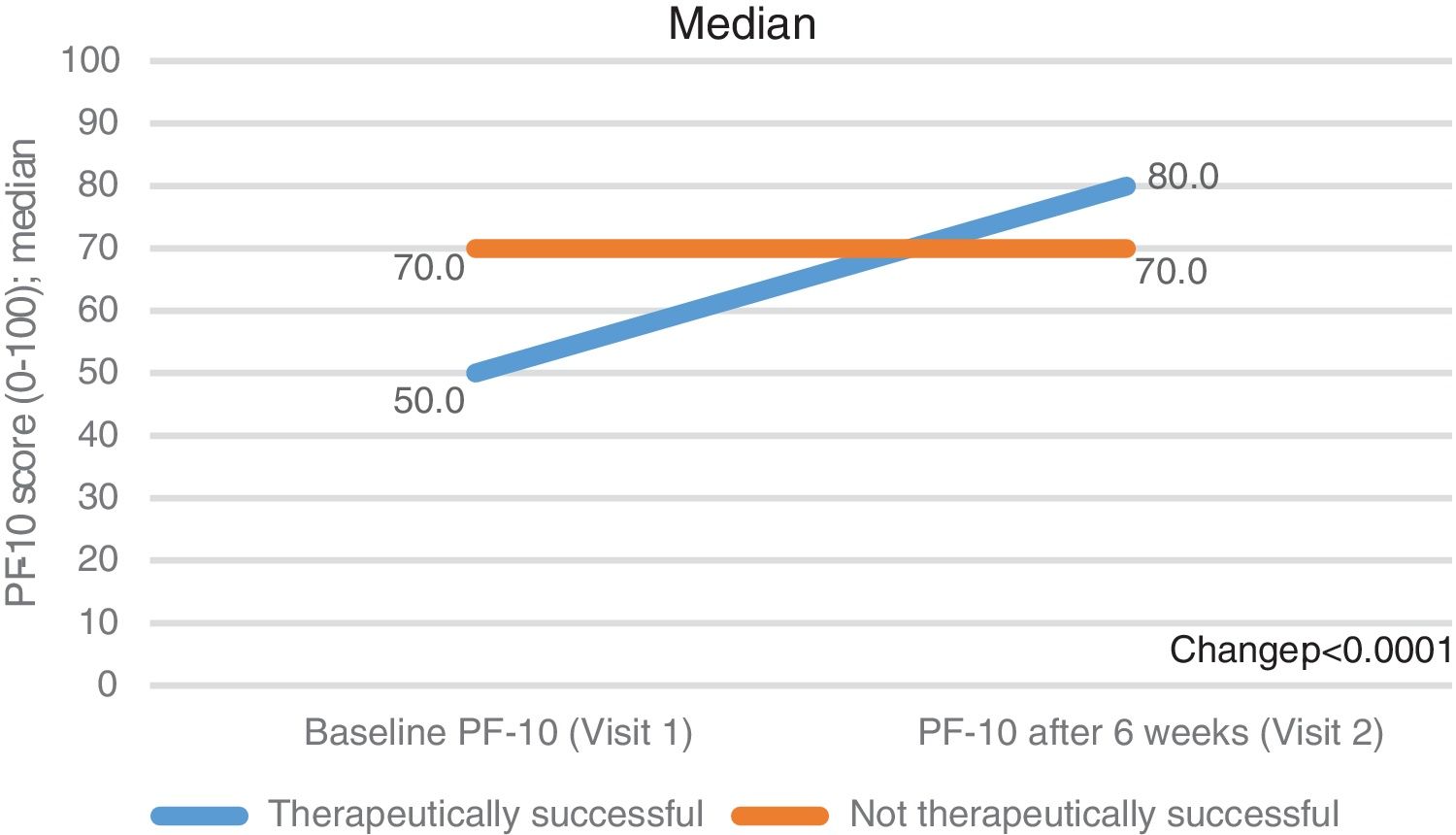
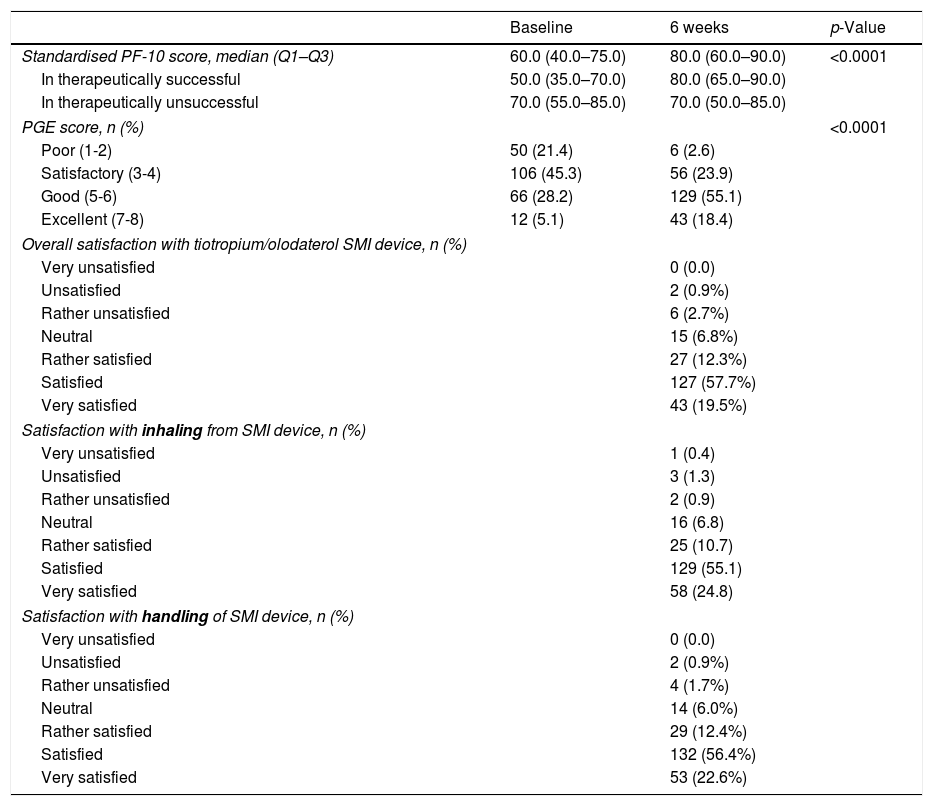
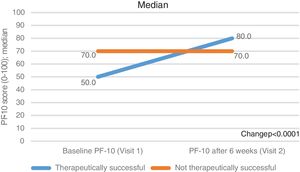
Secondary outcomesThe standardised PF-10 score changed from a median (Q1-Q3) of 60.0 (40.0–75.0) at baseline to 80.0 (60.0–90.0) at the 6th week of FU (p<0.0001, Table 2). The increase was more evident in the subgroup of therapeutic success (from 50.0 [35.0–70.0] to 80.0 [65.0–90.0]) than in the therapeutically unsuccessful subgroup (from 70.0 [55.0–85.0] to 70.0 [50.0–85.0]). Of note, the therapeutically unsuccessful subgroup had better physical functioning at baseline than the therapeutically successful group (p<0.001) (Fig. 2, Table 2).
Results for secondary outcomes (n=234).
| Baseline | 6 weeks | p-Value | |
|---|---|---|---|
| Standardised PF-10 score, median (Q1–Q3) | 60.0 (40.0–75.0) | 80.0 (60.0–90.0) | <0.0001 |
| In therapeutically successful | 50.0 (35.0–70.0) | 80.0 (65.0–90.0) | |
| In therapeutically unsuccessful | 70.0 (55.0–85.0) | 70.0 (50.0–85.0) | |
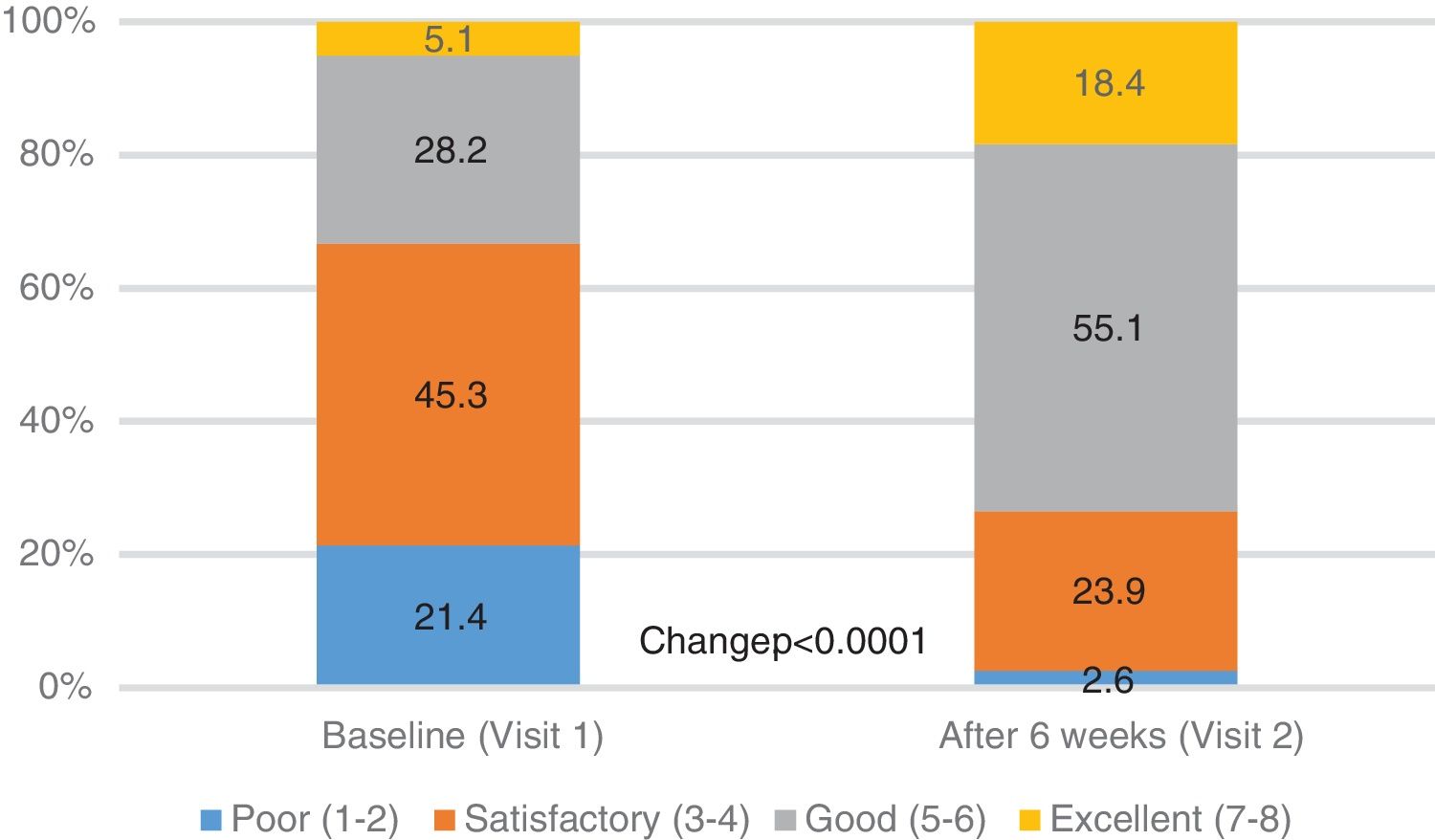
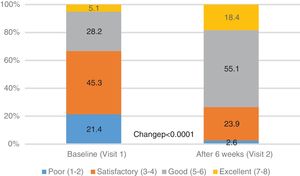
| PGE score, n (%) | <0.0001 | ||
| Poor (1-2) | 50 (21.4) | 6 (2.6) | |
| Satisfactory (3-4) | 106 (45.3) | 56 (23.9) | |
| Good (5-6) | 66 (28.2) | 129 (55.1) | |
| Excellent (7-8) | 12 (5.1) | 43 (18.4) | |
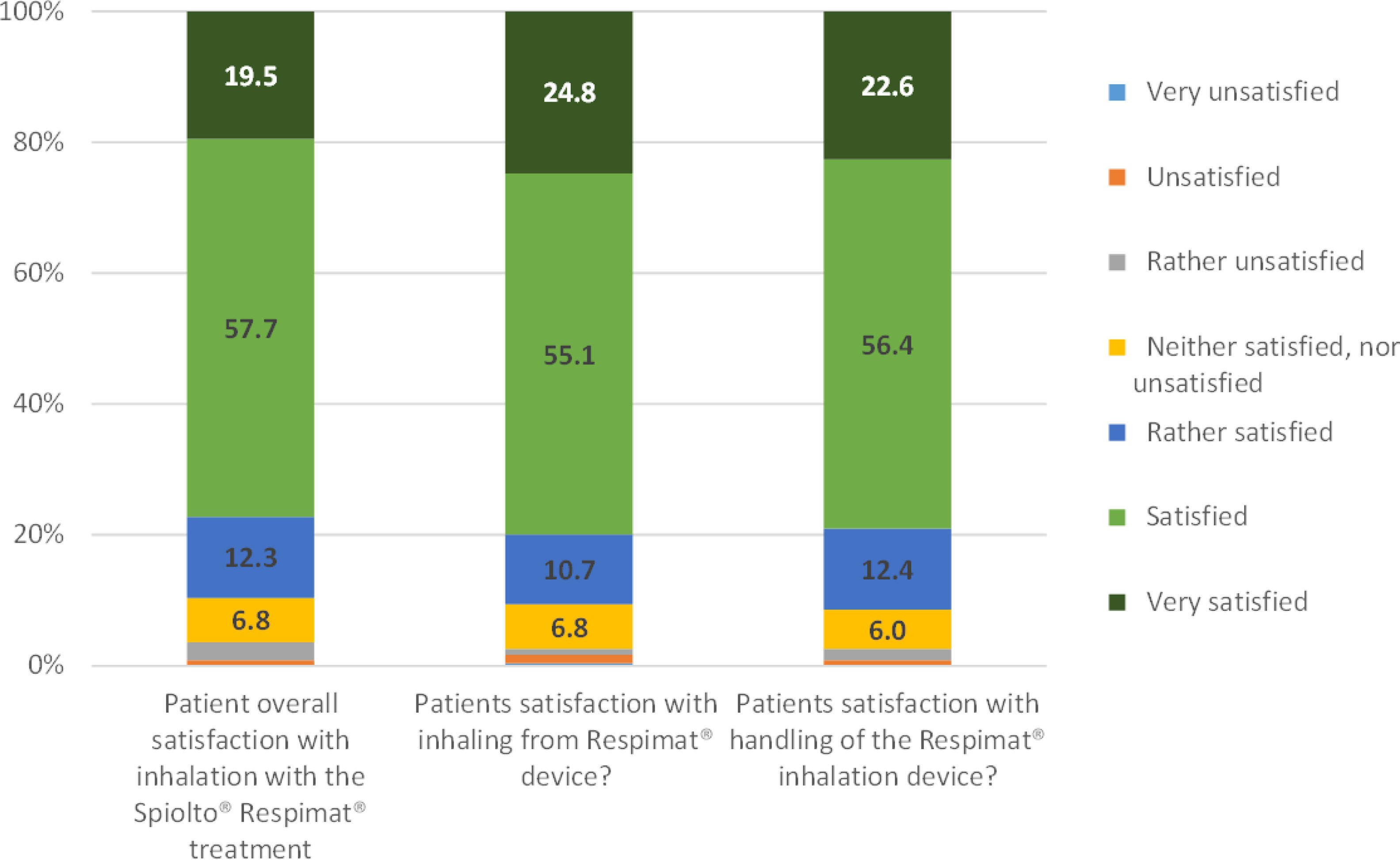
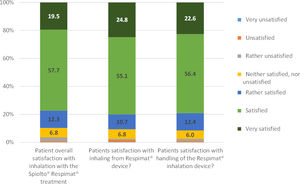
| Overall satisfaction with tiotropium/olodaterol SMI device, n (%) | |||
| Very unsatisfied | 0 (0.0) | ||
| Unsatisfied | 2 (0.9%) | ||
| Rather unsatisfied | 6 (2.7%) | ||
| Neutral | 15 (6.8%) | ||
| Rather satisfied | 27 (12.3%) | ||
| Satisfied | 127 (57.7%) | ||
| Very satisfied | 43 (19.5%) | ||
| Satisfaction with inhaling from SMI device, n (%) | |||
| Very unsatisfied | 1 (0.4) | ||
| Unsatisfied | 3 (1.3) | ||
| Rather unsatisfied | 2 (0.9) | ||
| Neutral | 16 (6.8) | ||
| Rather satisfied | 25 (10.7) | ||
| Satisfied | 129 (55.1) | ||
| Very satisfied | 58 (24.8) | ||
| Satisfaction with handling of SMI device, n (%) | |||
| Very unsatisfied | 0 (0.0) | ||
| Unsatisfied | 2 (0.9%) | ||
| Rather unsatisfied | 4 (1.7%) | ||
| Neutral | 14 (6.0%) | ||
| Rather satisfied | 29 (12.4%) | ||
| Satisfied | 132 (56.4%) | ||
| Very satisfied | 53 (22.6%) | ||
PGE: Physician's General Evaluation.
The general condition of the patient (PGE score), as assessed by the physician, improved significantly from baseline to 6th week FU: the percentage of patients with PGE score ≥5 (good or excellent) increased from 33.3% at baseline to 73.5% after 6 weeks (p<0.0001, Table 2, Fig. 3).
The patient's overall satisfaction with the treatment using a FDC of tiotropium/olodaterol SMI was excellent, with 77.2% reporting to be satisfied or very satisfied. Similar satisfaction was observed with inhaling from the SMI device (79.9% satisfied or very satisfied) and with the handling of SMI device (79.0%), (Table 2, Fig. 4). There was a direct correlation between the changes in PF-10 score and the overall satisfaction with the treatment, when unsatisfied patients were excluded (Fig. 2S, supplementary information). Likewise, therapeutic success was associated with both the overall satisfaction (p=0.0131) and the satisfaction with the handling of the SMI device (p=0.0117).
Pre-specified subgroup analysis did not render any significant trend toward a differential effect in the following subgroups: treatment-naïve patients vs. patients already treated with long-acting bronchodilators; 2014 GOLD severity classification (2 vs. 3/4), 2014 GOLD classification (B vs. C/D), previous treatment with inhaled corticosteroids, age, number of exacerbations in the last 12 months and hospital-related exacerbations in the last 12 months. However, a non-significant trend to higher proportion of therapeutic success was observed in the patients with more exacerbations or hospitalisations for exacerbations in the preceding 12 months (Table 1S, supplementary information).
Safety resultsAt the 6th week of FU, 4 patients (1.6%) experienced AEs: 1 serious AE (fatal ischemic stroke, non-related to the study medication, 0.4%) and 3 (1.2%) minor drug-related adverse reactions (cough=2, oropharyngeal pain=1).
DiscussionThe main findings of this study can be summarised as follows: (1) After 6 weeks of treatment with a FDC of tiotropium/olodaterol SMI, 66.2% of COPD patients improve ≥10 points in the standardised PF-10 score of physical functioning, a surrogate for physical activity, which is a key prognostic factor in the evolution of the disease; (2) this improvement in physical functioning is more pronounced in those patients with worse PF-10 score at baseline; (3) the general condition of the patient, as assessed by the physician, also improves after 6 weeks of treatment with a FDC of tiotropium/olodaterol SMI; (4) the treatment with a FDC of tiotropium/olodaterol SMI is perceived as satisfactory by most of the patients; (5) The treatment is safe and well tolerated, with minimal incidence of AE and adverse drug reactions.
Clinical studies of both LAMA tiotropium and LABA olodaterol separately21,22 and as a FDC23,24 in COPD patients have demonstrated significant improvement in exercise capacity.21–24 There is evidence that tiotropium/olodaterol combination therapy significantly reduced sedentary time and improved physical activity compared with tiotropium monotherapy.24 Besides, recent studies found significant improvement in physical functioning, as surrogate for exercise capacity, in German17 and Central/Eastern European20 COPD patients treated with a tiotropium/olodaterol FDC in real-world conditions. Nonetheless, there is scarce evidence of the therapeutic effects in a South-European Mediterranean population. A recent study in COPD patients showed that low levels of PA were associated with higher risk of comorbidities, irrespective of COPD treatment, and therefore an increase in PA levels might be recommendable for COPD patients.25
As expected, the combination of LAMA+LABA significantly improved the score in physical functioning, considered as a surrogate for physical activity, in practically two thirds of the patients in the current study. This proportion with therapeutic success is higher than in the German study (51.5%) and very similar to the Central/Eastern European study (67.8%), both performed in similar conditions and design. Differences in baseline characteristics of these populations could have influenced these results. The patients in the German study were at lower risk and had less symptoms (41% GOLD A and 34% GOLD B) than the patients in the Central/Eastern European and in the Spanish studies, who had lower proportion of GOLD A (21% and 29%, respectively) and higher proportion of GOLD B (48% and 42%, respectively). These worse baseline characteristics may have led to a better perception of the treatment and therefore to better PF-10 scores.
Physical activity is believed to play a relevant role in the development of extra-pulmonary complications of COPD, such as deconditioning, skeletal muscle atrophy, weakness, osteoporosis and cardiovascular disease.2 This in turn feeds back to form a vicious circle that reduces physical activity even more and results in a steeper decline in lung function.1 A therapy breaking down this harmful spiral on physical activity might thereby improve the prognosis of COPD.
Interestingly, in the subgroup analysis, comparing the subgroup with therapeutic success (≥10 points in the PF-10 score) vs. the subgroup without success (<10 points in the PF-10 score), we found that the subgroup without therapeutic success showing less improvement in physical functioning after 6 weeks have higher PF-10 scores at baseline, thus suggesting that the subgroup of patients who would perceive the most benefit from the FDC of tiotropium/olodaterol would be those patients with lower physical functioning at baseline. This analysis was not performed in the German study, which found however that GOLD classification was a better predictor of the therapeutic success.17 Although the subgroup analysis in the current study did not confirm the association with GOLD classification, the findings of both studies were consistent: the more severely diseased the patients, the higher the likelihood to perceive improvement. Additionally, the non-significant finding of higher therapeutic success in patients with historically more exacerbations/hospitalisations also follows the same trend and might have reached statistical significance with a larger sample size.
Paradoxically, although the therapeutic success was higher in the current Spanish study than in the German study (66.2% vs. 51.5%, respectively), the satisfaction with the treatment was lower in the Spanish study than in the German study: proportion of patients reporting to be satisfied or very satisfied were 77.2% vs. 82.5% for overall satisfaction, 79.9% vs. 87.5% for satisfaction with SMI device inhaling and 79.0% vs. 85.2% for satisfaction with the SMI device handling. On the other hand, in the study of Central and Eastern Europe the therapeutic success was high (67.8%) according to high levels of satisfaction with the treatment (81%; 85% and 84%, respectively).
Finally, the number of patients reporting an AE in this study was low (1.6%) and consistent with the results observed in other similar studies (1.3% and 1.0%).17,20
LimitationsThe study was prematurely interrupted before achieving the calculated sample size due to logistic recruitment problems. This circumstance might have jeopardised the intended level of accuracy for the estimation of the primary and secondary objectives. Nonetheless, notwithstanding the difference between the planned and the obtained sample size, the results are still representative of the target population and have been estimated with acceptable accuracy (confidence interval±6.1). Furthermore, the results of the primary objective were better than expected (therapeutic success was achieved in 66.2% of patients, whilst sample size was calculated on an expected success of 50%).17
This was an observational and descriptive study. This study design is unable to discern the true effect of the treatment and to isolate it from a placebo effect or the natural evolution of the disease. Likewise, observational studies are prone to bias, although selection bias has been partially counterbalanced in this study by means of the prospective design and the consecutive enrolment of the patients. As such the results are hypothesis-generating and instrumental to plan future comparative studies.
Patients could be included with a previous diagnosis of COPD, irrespective whether this diagnosis stemmed from a specialised hospital or a primary care setting. Spirometric data are indeed available for just 53% of the patients, thus assuming a considerable proportion of diagnosis made at primary care. This might have resulted in some methodological heterogeneity, as previous studies have pointed out substantial disagreement between the COPD diagnosis at different care levels.26
Non-pharmacological actions were left at the treating physician's criterion. This might have increased the variability of the therapeutic action and eventually introduced some random factor impacting at some extent on the final result.
The satisfaction with the treatment was evaluated by means of a questionnaire, designed by the study promotor. Although not a validated instrument, the same questionnaire has been widely used in other COPD studies17,20 showing consistent results (81 - 82.5% satisfied of very satisfied, vs. 77.2% in the current study).17,20
AE and adverse drug reactions were adjudicated and reported by local investigators onsite. Under these conditions, a trend to under-report AE has been consistently described,27 so the safety data must be carefully interpreted. This important limitation could have been circumvented with an external AE Committee for the adjudication of events.
A strength of this study is that therapeutic decision was made on a strictly clinical basis in a real-life setting with a large COPD population and therefore results can be considered generalizable to the real population. Importantly, the patients included in the current study present similar demographic characteristics and severity of symptoms to other COPD studies performed on a Spanish population,28 thus strongly suggesting that they are representative for their target population.
ConclusionTreatment of COPD patients with a FDC of inhaled tiotropium/olodaterol SMI for 6 weeks resulted in significant improvements in the patients’ condition as assessed by patients and physicians with no new safety findings. Two thirds of patients experienced an increase ≥10 points in the PF-10 score of physical functioning, a surrogate for physical activity, hence potentially helping patients to remain physically active and thus to improve their prognosis.
FundingThis study was funded by Boehringer-Ingelheim.
Assessment of physical functioning in patients with chronic obstructive pulmonary disease (COPD) requiring long-acting dual bronchodilation in routine clinical practice
Conflict of interestHelena Aguilar and Xavier Ribera are employees of Boehringer-Ingelheim Spain.
Medical Writing and editorial support was provided by Content Ed Net (Madrid, Spain) and supported by Boehringer-Ingelheim Spain.
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE).