The efficacy and safety of acetylsalicylic acid (ASA) prophylaxis for the primary prevention of atherosclerotic cardiovascular disease (ACVD) remain controversial in people with diabetes (DM) without ACVD, because the possible increased risk of major bleeding could outweigh the potential reduction in the risk of mortality and of major adverse cardiovascular events (MACE) considered individually or together.
ObjectiveTo evaluate the overall risk-benefit of ASA prophylaxis in primary prevention in people with DM and to compare the recommendations of the guidelines with the results of the meta-analyses (MA) and systematic reviews (SR).
Material and methodsWe searched Medline, Google Scholar, Embase, and the Cochrane Library for SR and MA published from 2009 to 2020 which compared the effects of ASA prophylaxis versus placebo or control followed up for at least one year in people with DM without ACVD. Heterogeneity among the randomized clinical trials (RCT) included in the SR and MA was assessed. Cardiovascular outcomes of efficacy (all-cause mortality [ACM], cardiovascular mortality [CVM], myocardial infarction [MI], stroke and MACE) and of safety (major bleeding events [MBE], major gastrointestinal bleeding events [MGIBE], and intracranial and extracranial bleeding) were shown.
ResultsThe recommendations of 12 guidelines were evaluated. The results of 25 SR and MA that included a total of 20 RCT were assessed. None of the MA or SR showed that ASA prophylaxis decreased the risk of ACM, CVM or MI. Only two of the 19 SR and MA that evaluated ischemic stroke showed a decrease in the stroke risk (mean 20.0% [SD±5.7]), bordering on statistical significance. Almost half of the MA and SR showed, bordering on statistical significance, a risk reduction for the MACE composite endpoint (mean 10.5% [SD±3.3]). The significant increases in MGIBE risk ranged from 35% to 55%. The significant increases in the risk of MBE and extracraneal bleeding were 33.4% (SD±14.9) and 54.5% (SD±0.7) respectively.
ConclusionThe overall risk-benefit assessment of ASA prophylaxis in primary prevention suggests that it should not be applied in people with DM.
La eficacia y la seguridad de la profilaxis con ácido acetilsalicílico (AAS) para la prevención primaria de la enfermedad cardiovascular arteriosclerótica (ECVA) siguen siendo controvertidas en personas con diabetes (DM) sin ECVA, ya que el posible aumento del riesgo de hemorragias graves podría superar la posible disminución del riesgo de mortalidad y de los principales episodios adversos cardiovasculares (MACE) considerados individualmente o en conjunto.
ObjetivoEvaluar el riesgo-beneficio de la profilaxis con AAS en prevención primaria en personas con DM y comparar las recomendaciones de las guías de práctica clínica con los resultados de los metaanálisis (MA) y revisiones sistemáticas (RS).
Material y métodosSe realizaron búsquedas en Medline, Google Scholar, Embase y Biblioteca Cochrane de RS y MA publicados desde 2009 hasta 2020 que compararan los efectos de AAS versus placebo o control en seguimiento durante al menos un año en personas con DM sin ECVA. Se valoraron la heterogeneidad entre los ensayos clínicos aleatorizados (ECA) incluidos en las RS y MA. Se mostraron los resultados cardiovasculares de eficacia (muerte por cualquier causa [MCC], muerte cardiovascular [MCV], infarto de miocardio [IM], ictus y MACE) y de seguridad (episodios hemorrágicos importantes [EHI], episodios hemorrágicos gastrointestinales importantes [EHGI], hemorragias intracraneales y extracraneales).
ResultadosSe valoraron las recomendaciones de 12 guías de práctica clínica. Se evaluaron los resultados de 25 RS y MA que incluyeron un total de 20ECA. Ningún MA ni RS mostró que la profilaxis con AAS disminuyera el riesgo de MCC, MCV o IM. Solo dos de los 19 SR y MA que evaluaron el ictus isquémico mostraron una disminución en el riesgo de ictus (media 20,0% [DE±5,7]), rozando la significación estadística. Casi la mitad de los MA y SR mostraron una reducción del riesgo del criterio de valoración compuesto MACE (media 10,5% [DE±3,3]) al borde de la significación estadística. Los aumentos significativos en el riesgo de EHGI oscilaron entre el 35 y el 55%. Los aumentos significativos en el riesgo de EHI y hemorragia extracraneal fueron del 33,4% (DE±14,9) y del 54,5% (DE±0,7), respectivamente.
ConclusiónLa valoración global de riesgo-beneficio de la profilaxis con AAS en prevención primaria sugiere que esta no se debería aplicar en personas con DM.
The recommendation for the use of Aspirin® or acetylsalicylic acid (ASA) to reduce the risk of new cardiovascular events in patients with previous atherosclerotic cardiovascular disease (ACVD) is well established for both general population1–6 and people with diabetes (DM) in secondary prevention.7–12
However, its usefulness in primary prevention of ACVD is more controversial as regards people with DM. The guidelines have changed their recommendations in favor of or against this, based on extrapolation of data from other risk groups, on consensuses, or on scientific evidence which has sometimes been insufficient or of low quality.13,14
The scientific evidence on prophylaxis with ASA for the primary prevention of ACVD in patients with DM has been increasing with time and, consequently, the recommendations of the panels of experts and of clinical practice guidelines have also changed; and their conclusions on many occasions do not coincide.
The risk of bleeding associated with the use of ASA is 5 times higher in patients with high cardiovascular risk (CVR) compared to patients with a lower CVR.15 The most relevant latest studies on this subject, ARRIVE,16 ASCEND17 and ASPREE,18 have found increased risks of major bleeding events (MBE), without showing benefits in the reduction of the risk of primary objectives16,18 and even showing an increase of 14% in the risk of all-cause mortality (ACM).18
Despite the fact that there are many randomized clinical trials (RCT), systematic reviews (SR) and meta-analyses (MA) evaluating the risks and/or benefits of the use of ASA in primary prevention, there is a high level of uncertainty about the indication of ASA prophylaxis in people with DM without ACVD. Because this issue has major implications in clinical practice, a scope review was carried out.19 This article assesses the current state of the scientific evidence available on the safety of prophylaxis with ASA associated with increased risk of MBE, and on its efficacy in reducing the risk of ACM, cardiovascular mortality (CVM), myocardial infarction (MI), and stroke, considered either individually or as composite endpoint of major adverse cardiovascular events (MACE).
Material and methodsWe reviewed the recommendations of 12 evidence-based clinical practice guidelines on the use of ASA in primary prevention in people with DM. The recommendations were accompanied by class of recommendation (COR) and level of evidence (LOE) (Table S1).
We conducted a comprehensive search for SR and MA published from 2009 to 2020, which included RCT that evaluated during a follow-up period of at least one year in people with DM, the use of ASA as a primary prevention strategy versus placebo or no ASA. The search was made on Medline, Google Scholar, Embase, and the Cochrane Library, using the terms aspirin, acetylsalicylic acid, diabetes, human adults, cardiovascular events, and primary prevention. The search strategy followed is specified in the supplement. Twenty-five RS and MA were selected, which evaluated a total of 20 RCT (Table 1). Twenty-three RS or MA assessed the effects of prophylaxis with ASA in people with DM in primary prevention on the risk of cardiovascular events, 20 on the risk of bleeding events, and 18 on both risks.
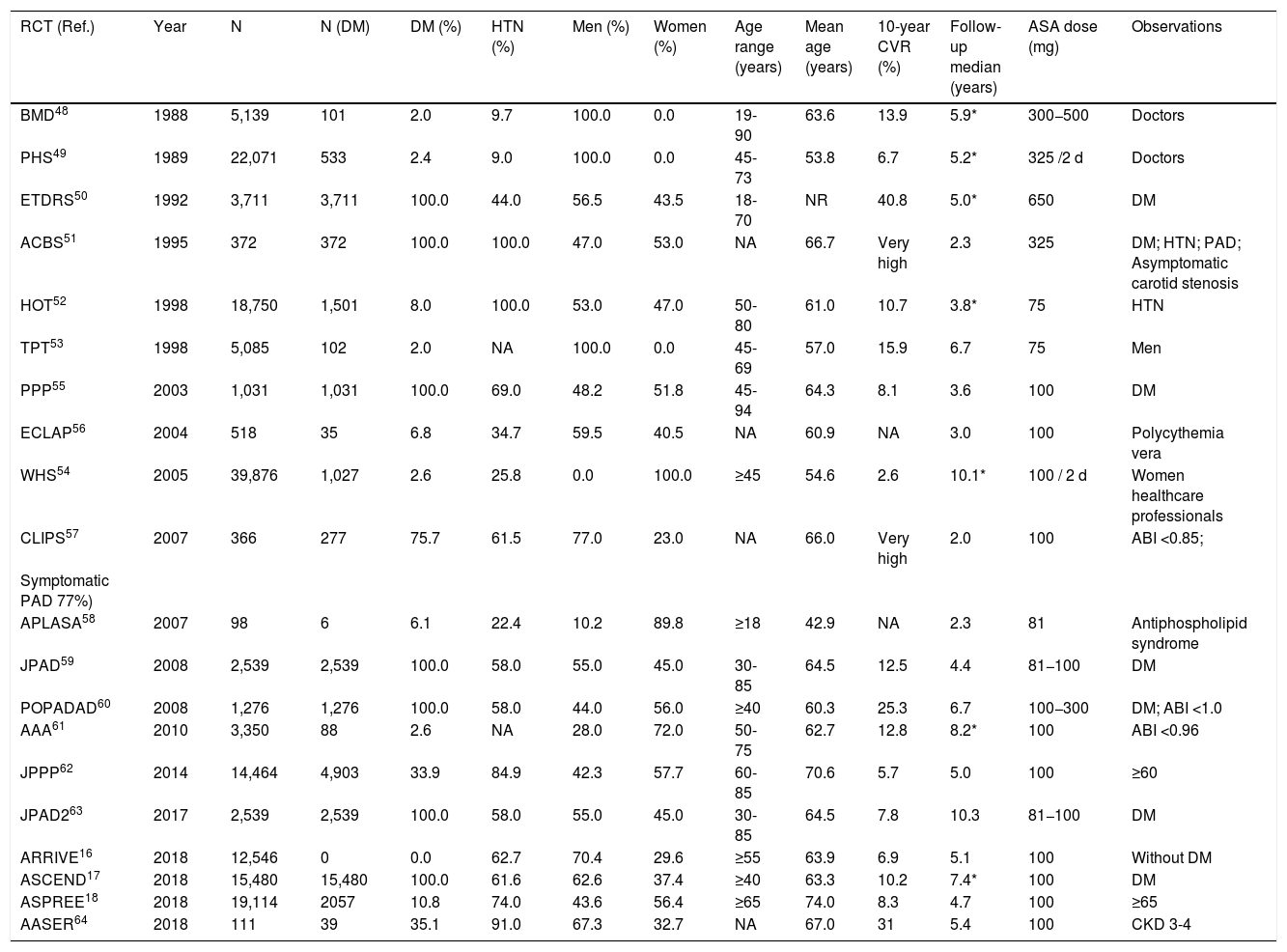
Different criteria of cardiovascular risk in people with DM.
| CVR | AACE/ACE 2019 (8) | ACC/AHA 2019 (2)a | ADA 2021 (9) | NICE (7) | SIGN (11) | Primary Care Diabetes Europe 2020 (6) | ESC/EAS 2019 (4) | ESC/EASD 2019 (12) | ESC 2021 (5) |
|---|---|---|---|---|---|---|---|---|---|
| Low | <5% | ||||||||
| Moderate | Borderline: ≥5% to <7.5% | QRISK2 | Younger people (<35 years old [T1DM]; <50 years old [T2DM]); <10 years from onset | Short duration from onset (<10 years), well-controlled DM and without TOD or CVRF | |||||
| Intermediate: ≥7.5% to <20% | |||||||||
| High | Without very high or extreme CVR | ≥20% | ≥40 years old; <40 years old and ≥1 CVRF, ≥20 years from onset, or TOD | Without very high CVR | ≥10 years from onset, or ≥1 CVRF, without TOD or ACVD | Without moderate or very high CVR | |||
| Very high | ≥1 CVRF | <40 years old, ACVD, eGFR <60, albuminuria, or multiple CVRF | ACVD, TOD or ≥3 CVRF; T1DM ≥20 years from onset | ACVD, severe TODb | |||||
| Extreme | ACVD | ||||||||
CVR: cardiovascular risk; (%) 10-year risk; DM: diabetes mellitus; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; ACVD: atherosclerotic cardiovascular disease. CVRF: major cardiovascular risk factor (family history of premature ACVD, smoking, hypertension, hypercholesterolemia); TOD: albuminuria, neuropathy, retinopathy; eGFR: estimated glomerular filtration rate (mL/min/1.73m2); ABI: ankle-brachial index.
The criteria of clinical efficacy assessed were ACM, CVM, MI, stroke, and MACE. MACE was defined as the composite endpoint of CVM (fatal IM or fatal stroke), non-fatal MI, and non-fatal stroke. The safety outcomes considered were intracranial bleeding (including hemorrhagic stroke), major gastrointestinal bleeding events (MGIBE), and MBI including intracranial hemorrhagic or other bleedings that required transfusion and/or surgery, or that caused hospitalization, surgery or death.
The analyzed studies assessed the risk of cardiovascular or bleeding events by determining the odds ratio (OR), the relative risk (RR), or the hazard ratio in survival analysis (HR). The confidence intervals of estimates were determined at 95% (95%CI). The two-tailed p values<0.05 were considered statistically significant. Some MA or SR reported on the number of patients under treatment required to avoid a cardiovascular event (number needed to treat [NNT]), or the number of patients under treatment required to have an adverse bleeding event (number needed to harm [NNH]). The interpretation of the NNT and NNH should take into account the baseline CVR of the patients included, and the mean follow-up time of each SR or MA. Information was provided on the heterogeneity between the RCT analyzed by means of Higgins’ I2 statistic20 when it was moderate (I2: 25–50%) or high (I2>50%).
ResultsEvaluation of the guidelines on cardiovascular risk and prophylaxis with ASA in primary prevention in people with DMDM confers a risk equivalent to 15-year aging, and a higher risk of premature cardiovascular morbidity and mortality than people without DM.21 The study by Haffner et al.22 revealed that patients with DM without previous MI had a risk of MI as high as patients without DM with previous MI, which justifies the management of CVR factors (CVRF) in patients with DM as intensively as in patients with MI. The MA of the Emerging Risk Factor Collaboration23 showed that patients with DM had twice the risk of MACE, independently of having other CVRF.
The guidelines show important differences both in the classification of CVR (Table 1) and in their recommendations on prophylaxis with ASA in primary prevention in patients with DM.
The guidelines of the American cardiology associations (ACC/AHA 2019)2 assess the 10-year risk of having a first MACE in people aged 40–79, establishing 4 groups of CVR (low, borderline, intermediate, and high) (COR I, LOE B) (Table 1). They consider DM as another CVRF, so the assessment of CVR in people with DM is similar to general population. The guidelines indicate that the use of ASA could be considered for primary prevention of ACVD in people aged 40–70 who have a higher risk of ACVD but not a higher risk of bleeding (COR IIb, LOE A), and that it should not be given to patients older than 70 (COR III, LOE B) or to adults of any age with a higher risk of bleeding (COR III, LOE C).
The American endocrinology societies (AACE/ACE 2019) give patients with DM a high, very high or extreme CVR (Table 1).8
The American Diabetes Association (ADA 2021) recommends not giving prophylaxis with ASA to people with DM at low CVR (younger than 50 without CVRF).9 In the context of a decision shared with the patient, the guideline recommends applying clinical judgment in people with DM and intermediate CVR (younger than 50 with CVRF or older than 50 without CVRF). It also points out that the use of ASA can be considered in people aged 50–70 with increased CVR, with at least one major CVRF and without increased risk of bleeding. In people older than 70, the risk is greater than the benefit (LOE A).
The 2018 Canadian guideline on diabetes recommends that ASA should not be used routinely for primary prevention of MACE in people with DM (COR I, LOE A), and that ASA could be used when additional CVRF are present (COR IIb, LOE C).10
The guideline of the National Institute for Health and Care Excellence (NICE)7 recommends not using ASA in patients with DM without ACVD (LOE C). The guidelines of the Scottish Intercollegiate Network (SIGN)3,11 consider that people with DM have an increased CVR (Table 1), and recommend not using ASA in primary prevention in people with DM (COR I, LOE A).
The position paper of the Primary Care Diabetes Europe 2021 states that patients with type 2 DM (T2DM) have a high or very high CVR (Table 1).24
The European societies of Cardiology, of Atherosclerosis (ESC/EAS 2019)4 and the European Association for the Study of Diabetes (ESC/EASD 2019)12 recommend not using the CVR assessment tables in people with DM (COR III, LOE C). All the above has been endorsed by the new European SCORE2 algorithms, where DM is not included as a risk predictor, and people with DM are considered to have at least a high CVR.24
The guidelines ESC/EAS 2019,4 ESC/EASD 2019,12 and the recent 2021 ESC guidelines on cardiovascular disease prevention in clinical practice (ESC 2021)5 indicate that people with DM have a moderate, high or very high CVR (Table 1). The guidelines ESC/EASD 201912 and ESC 20215 recommend not using ASA in primary prevention in people with DM at moderate CVR (COR III, LOE B); they also say that the use of ASA can be considered in primary prevention in patients with DM and high or very high CVR if there is no clear contraindication (COR IIb, LOE A). However, the 2020 update of the Association of Preventive Cardiology of the ESC on cardiovascular prevention in clinical practice6 pointed out that the lack of net clinical benefit of ASA in primary prevention was evident not only in general population but also in patients with high CVR or with DM, because it increases the risk of bleeding without reducing the risk of MACE (COR I, LOE A).
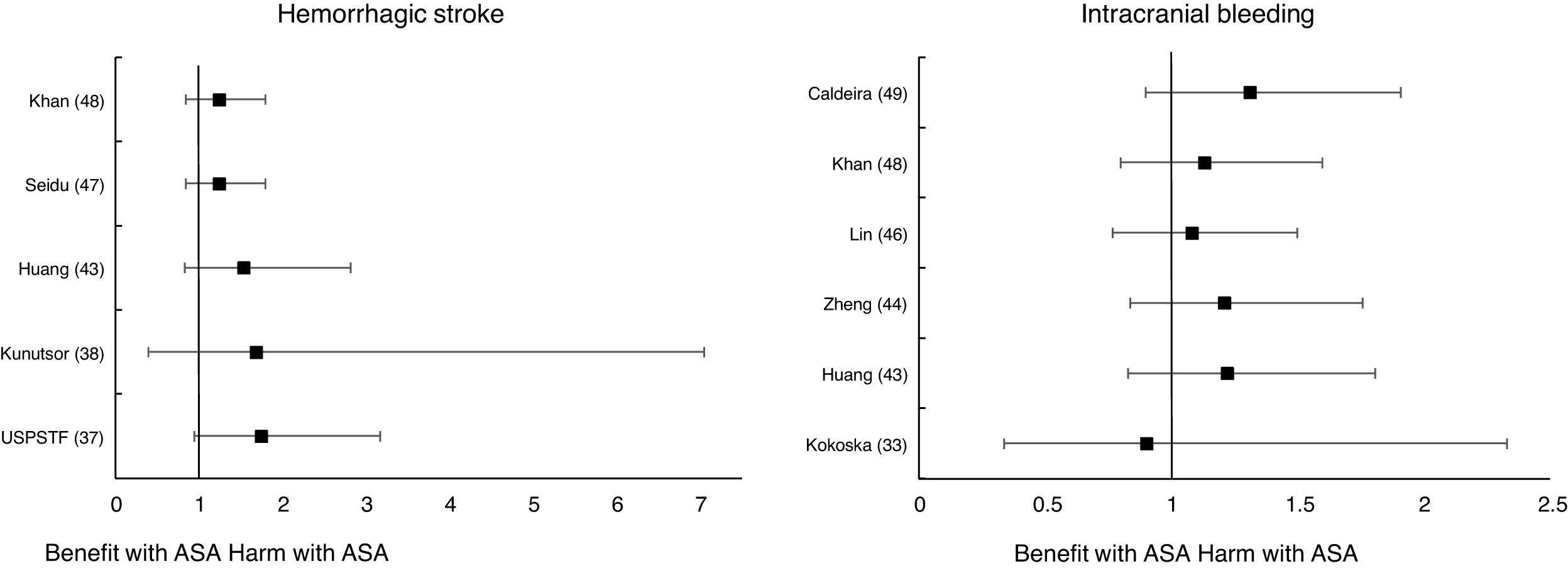
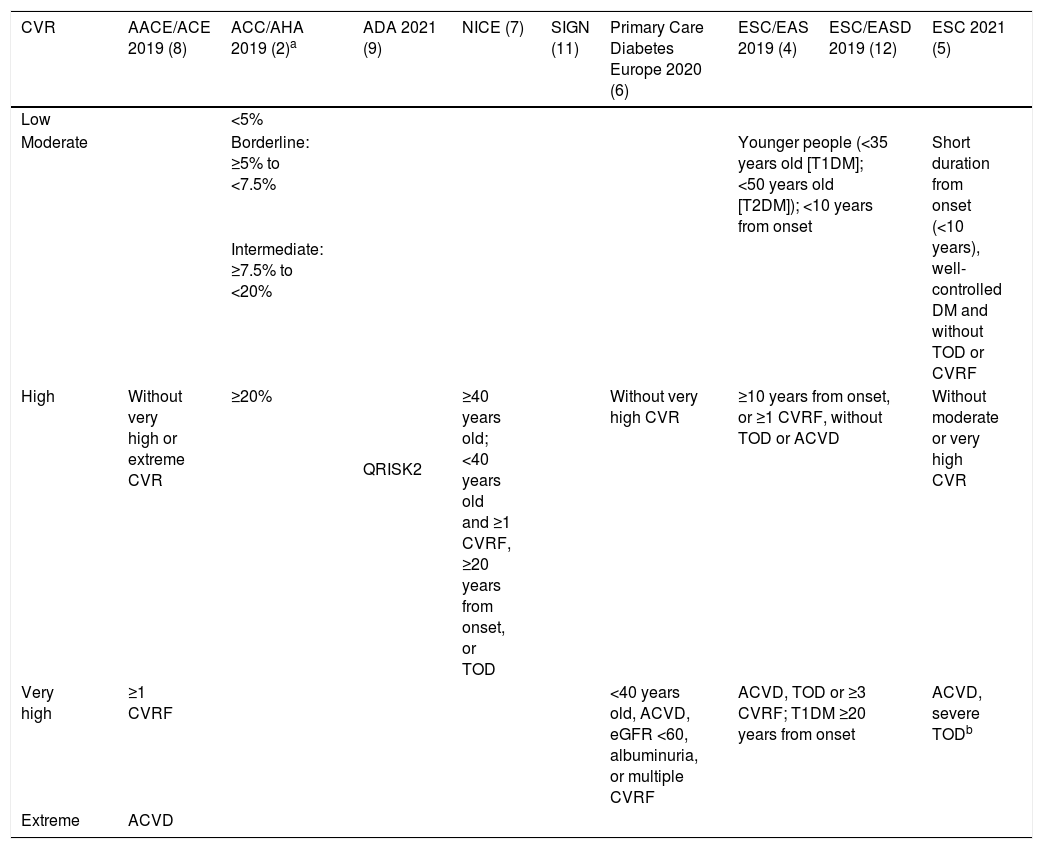
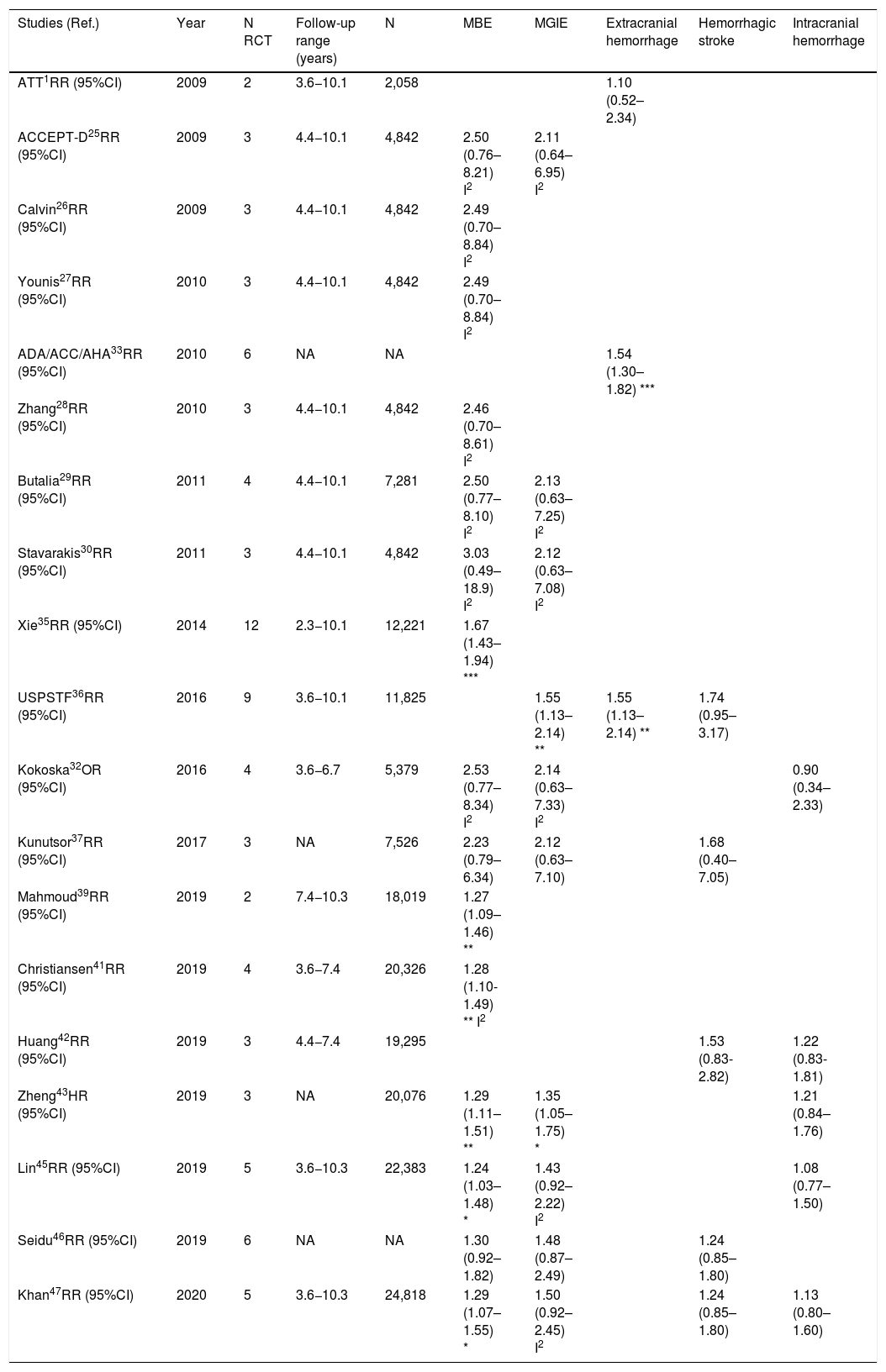
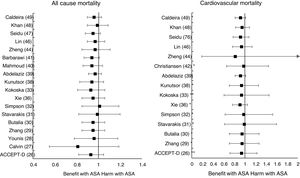
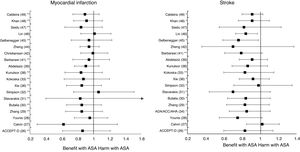
Meta-analyses and systematic reviews on prophylaxis with ASA in primary prevention in people with DMThe main characteristics of the study subjects of the RCT included in the MA or SR,1,26–49 the medians of the follow-up periods, and the daily doses of ASA used in each RCT50–66 are shown in Table 2. The RCT included in the MA and SR evaluated,1,26–66 and the respective years of publication are shown in Table 3 (ACM and cardiovascular events) and Table 4 (bleeding events). The graphics of the MA and SR of efficacy on ACM and cardiovascular events are shown in figures 1–3. The graphics of the MA and SR of safety on bleeding events are shown in Figs. 4a, 4b and 5. The results of efficacy and safety are shown in Tables S2 and S3, respectively, indicating the number of RCT evaluated in each SR or MA, the range of the medians of the follow-up periods, and the number of persons with DM included in the analysis. The most relevant results of the MA or SR that evaluated prophylaxis with ASA in primary prevention in people with DM are the following:
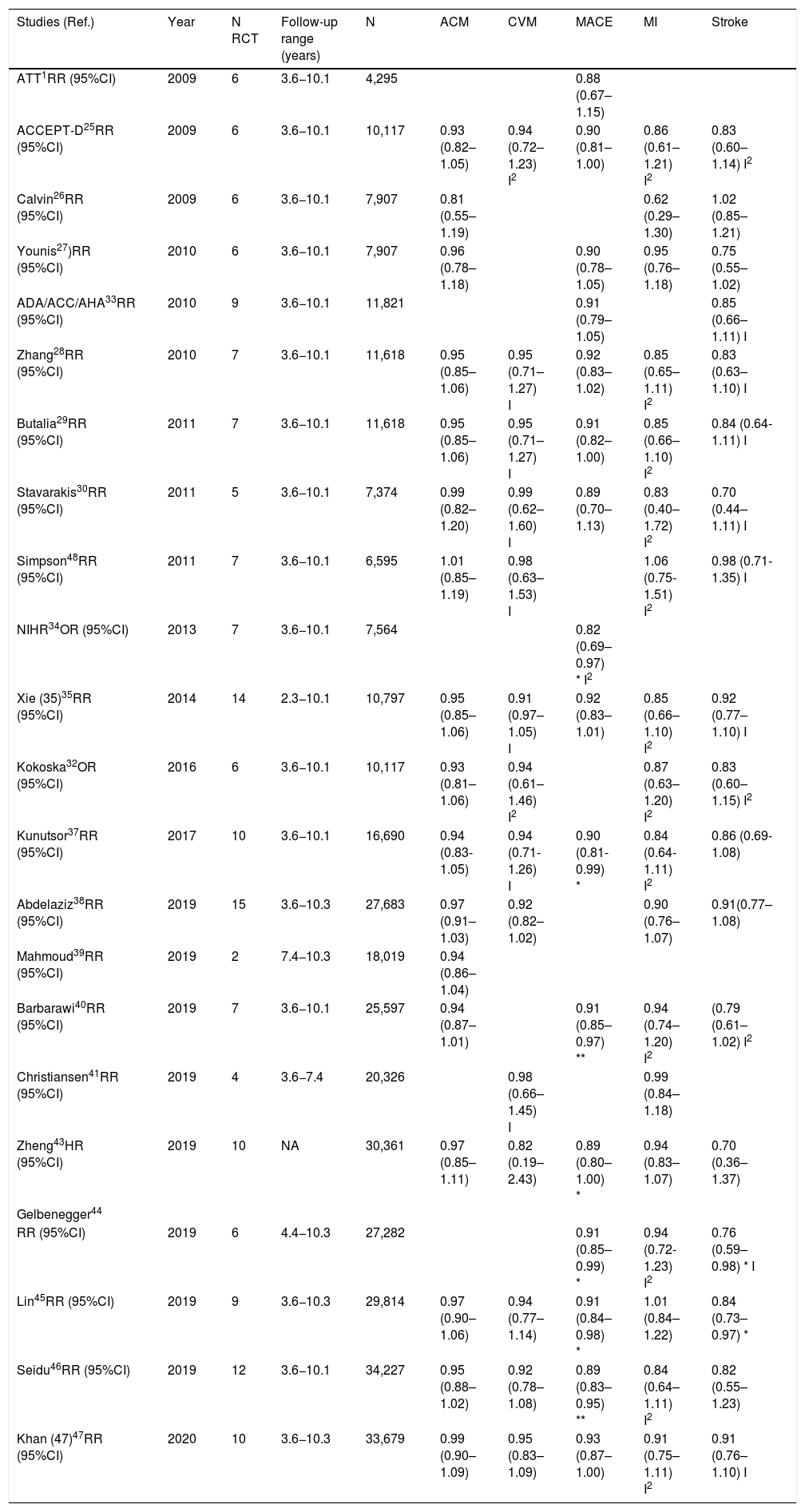
Randomized clinical trials on prophylaxis with ASA in primary prevention.
| RCT (Ref.) | Year | N | N (DM) | DM (%) | HTN (%) | Men (%) | Women (%) | Age range (years) | Mean age (years) | 10-year CVR (%) | Follow-up median (years) | ASA dose (mg) | Observations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMD48 | 1988 | 5,139 | 101 | 2.0 | 9.7 | 100.0 | 0.0 | 19-90 | 63.6 | 13.9 | 5.9* | 300−500 | Doctors |
| PHS49 | 1989 | 22,071 | 533 | 2.4 | 9.0 | 100.0 | 0.0 | 45-73 | 53.8 | 6.7 | 5.2* | 325 /2 d | Doctors |
| ETDRS50 | 1992 | 3,711 | 3,711 | 100.0 | 44.0 | 56.5 | 43.5 | 18-70 | NR | 40.8 | 5.0* | 650 | DM |
| ACBS51 | 1995 | 372 | 372 | 100.0 | 100.0 | 47.0 | 53.0 | NA | 66.7 | Very high | 2.3 | 325 | DM; HTN; PAD; Asymptomatic carotid stenosis |
| HOT52 | 1998 | 18,750 | 1,501 | 8.0 | 100.0 | 53.0 | 47.0 | 50-80 | 61.0 | 10.7 | 3.8* | 75 | HTN |
| TPT53 | 1998 | 5,085 | 102 | 2.0 | NA | 100.0 | 0.0 | 45-69 | 57.0 | 15.9 | 6.7 | 75 | Men |
| PPP55 | 2003 | 1,031 | 1,031 | 100.0 | 69.0 | 48.2 | 51.8 | 45-94 | 64.3 | 8.1 | 3.6 | 100 | DM |
| ECLAP56 | 2004 | 518 | 35 | 6.8 | 34.7 | 59.5 | 40.5 | NA | 60.9 | NA | 3.0 | 100 | Polycythemia vera |
| WHS54 | 2005 | 39,876 | 1,027 | 2.6 | 25.8 | 0.0 | 100.0 | ≥45 | 54.6 | 2.6 | 10.1* | 100 / 2 d | Women healthcare professionals |
| CLIPS57 | 2007 | 366 | 277 | 75.7 | 61.5 | 77.0 | 23.0 | NA | 66.0 | Very high | 2.0 | 100 | ABI <0.85; |
| Symptomatic PAD 77%) | |||||||||||||
| APLASA58 | 2007 | 98 | 6 | 6.1 | 22.4 | 10.2 | 89.8 | ≥18 | 42.9 | NA | 2.3 | 81 | Antiphospholipid syndrome |
| JPAD59 | 2008 | 2,539 | 2,539 | 100.0 | 58.0 | 55.0 | 45.0 | 30-85 | 64.5 | 12.5 | 4.4 | 81−100 | DM |
| POPADAD60 | 2008 | 1,276 | 1,276 | 100.0 | 58.0 | 44.0 | 56.0 | ≥40 | 60.3 | 25.3 | 6.7 | 100−300 | DM; ABI <1.0 |
| AAA61 | 2010 | 3,350 | 88 | 2.6 | NA | 28.0 | 72.0 | 50-75 | 62.7 | 12.8 | 8.2* | 100 | ABI <0.96 |
| JPPP62 | 2014 | 14,464 | 4,903 | 33.9 | 84.9 | 42.3 | 57.7 | 60-85 | 70.6 | 5.7 | 5.0 | 100 | ≥60 |
| JPAD263 | 2017 | 2,539 | 2,539 | 100.0 | 58.0 | 55.0 | 45.0 | 30-85 | 64.5 | 7.8 | 10.3 | 81−100 | DM |
| ARRIVE16 | 2018 | 12,546 | 0 | 0.0 | 62.7 | 70.4 | 29.6 | ≥55 | 63.9 | 6.9 | 5.1 | 100 | Without DM |
| ASCEND17 | 2018 | 15,480 | 15,480 | 100.0 | 61.6 | 62.6 | 37.4 | ≥40 | 63.3 | 10.2 | 7.4* | 100 | DM |
| ASPREE18 | 2018 | 19,114 | 2057 | 10.8 | 74.0 | 43.6 | 56.4 | ≥65 | 74.0 | 8.3 | 4.7 | 100 | ≥65 |
| AASER64 | 2018 | 111 | 39 | 35.1 | 91.0 | 67.3 | 32.7 | NA | 67.0 | 31 | 5.4 | 100 | CKD 3-4 |
RCT: Randomized clinical trials; ASA: Acetylsalicylic acid; N: Number of study subjects, DM: Diabetes mellitus; HTN: Hypertension; CVR: 10-year cardiovascular risk. BMD: British Male Doctors; PHS: Physicians’ Health Study; ETDRS: Early Treatment Diabetic Retinopathy Study; ACBS: Asymptomatic Cervical Bruit Study Group; HOT: Hypertension Optimal Treatment; TPT: Thrombosis Prevention Trial; PPP: Primary Prevention Project; ECLAP: European Collaboration on Low-Dose Aspirin in Polycythemia Vera; WHS: Women's’ Health Study; CLIPS: Critical Leg Ischaemia Prevention Study; APLASA: Antiphospholipid Antibody Acetyl-salicylic Acid; JPAD: Japanese Primoary Prevention of Atherosclerosis with Aspirin for Diabetes; POPADAD: Prevention and Progression of Arterial Disease and Diabetes; AAA: Aspirin for Asymptomatic Atherosclerosis; ACBS: Asymptomatic Cervical Bruit Study, JPPP: Japanese Primary Prevention Project; JPAD2: Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes 2; ARRIVE: Aspirin to Reduce Risk of Initial Vascular Events; ASCEND: A Study of Cardiovascular Events in Diabetes; ASPREE: Aspirin in Reducing Events in the Elderly; AASER: Ácido Acetil Salicílico en la Enfermedad Renal (Acetylsalicylic acid in kidney disease; PAD: Peripheral artery disease; ABI: Ankle-brachial index; CKD: Chronic kidney disease; NA: Not available.
MA and SR which assessed the efficacy of prophylaxis with ASA in people with DM in primary prevention.
| Studies (Ref.) | Year | N RCT | Follow-up range (years) | N | ACM | CVM | MACE | MI | Stroke |
|---|---|---|---|---|---|---|---|---|---|
| ATT1RR (95%CI) | 2009 | 6 | 3.6−10.1 | 4,295 | 0.88 (0.67–1.15) | ||||
| ACCEPT-D25RR (95%CI) | 2009 | 6 | 3.6−10.1 | 10,117 | 0.93 (0.82–1.05) | 0.94 (0.72–1.23) I2 | 0.90 (0.81–1.00) | 0.86 (0.61–1.21) I2 | 0.83 (0.60–1.14) I2 |
| Calvin26RR (95%CI) | 2009 | 6 | 3.6−10.1 | 7,907 | 0.81 (0.55–1.19) | 0.62 (0.29–1.30) | 1.02 (0.85–1.21) | ||
| Younis27)RR (95%CI) | 2010 | 6 | 3.6−10.1 | 7,907 | 0.96 (0.78–1.18) | 0.90 (0.78–1.05) | 0.95 (0.76–1.18) | 0.75 (0.55–1.02) | |
| ADA/ACC/AHA33RR (95%CI) | 2010 | 9 | 3.6−10.1 | 11,821 | 0.91 (0.79–1.05) | 0.85 (0.66–1.11) I | |||
| Zhang28RR (95%CI) | 2010 | 7 | 3.6−10.1 | 11,618 | 0.95 (0.85–1.06) | 0.95 (0.71–1.27) I | 0.92 (0.83–1.02) | 0.85 (0.65–1.11) I2 | 0.83 (0.63–1.10) I |
| Butalia29RR (95%CI) | 2011 | 7 | 3.6−10.1 | 11,618 | 0.95 (0.85–1.06) | 0.95 (0.71–1.27) I | 0.91 (0.82–1.00) | 0.85 (0.66–1.10) I2 | 0.84 (0.64-1.11) I |
| Stavarakis30RR (95%CI) | 2011 | 5 | 3.6−10.1 | 7,374 | 0.99 (0.82–1.20) | 0.99 (0.62–1.60) I | 0.89 (0.70–1.13) | 0.83 (0.40–1.72) I2 | 0.70 (0.44–1.11) I |
| Simpson48RR (95%CI) | 2011 | 7 | 3.6−10.1 | 6,595 | 1.01 (0.85–1.19) | 0.98 (0.63–1.53) I | 1.06 (0.75-1.51) I2 | 0.98 (0.71-1.35) I | |
| NIHR34OR (95%CI) | 2013 | 7 | 3.6−10.1 | 7,564 | 0.82 (0.69–0.97) * I2 | ||||
| Xie (35)35RR (95%CI) | 2014 | 14 | 2.3−10.1 | 10,797 | 0.95 (0.85–1.06) | 0.91 (0.97–1.05) I | 0.92 (0.83–1.01) | 0.85 (0.66–1.10) I2 | 0.92 (0.77–1.10) I |
| Kokoska32OR (95%CI) | 2016 | 6 | 3.6−10.1 | 10,117 | 0.93 (0.81–1.06) | 0.94 (0.61–1.46) I2 | 0.87 (0.63–1.20) I2 | 0.83 (0.60–1.15) I2 | |
| Kunutsor37RR (95%CI) | 2017 | 10 | 3.6−10.1 | 16,690 | 0.94 (0.83-1.05) | 0.94 (0.71-1.26) I | 0.90 (0.81-0.99) * | 0.84 (0.64-1.11) I2 | 0.86 (0.69-1.08) |
| Abdelaziz38RR (95%CI) | 2019 | 15 | 3.6−10.3 | 27,683 | 0.97 (0.91–1.03) | 0.92 (0.82–1.02) | 0.90 (0.76–1.07) | 0.91(0.77–1.08) | |
| Mahmoud39RR (95%CI) | 2019 | 2 | 7.4−10.3 | 18,019 | 0.94 (0.86–1.04) | ||||
| Barbarawi40RR (95%CI) | 2019 | 7 | 3.6−10.1 | 25,597 | 0.94 (0.87–1.01) | 0.91 (0.85–0.97) ** | 0.94 (0.74–1.20) I2 | (0.79 (0.61–1.02) I2 | |
| Christiansen41RR (95%CI) | 2019 | 4 | 3.6−7.4 | 20,326 | 0.98 (0.66–1.45) I | 0.99 (0.84–1.18) | |||
| Zheng43HR (95%CI) | 2019 | 10 | NA | 30,361 | 0.97 (0.85–1.11) | 0.82 (0.19–2.43) | 0.89 (0.80–1.00) * | 0.94 (0.83–1.07) | 0.70 (0.36–1.37) |
| Gelbenegger44 | |||||||||
| RR (95%CI) | 2019 | 6 | 4.4−10.3 | 27,282 | 0.91 (0.85–0.99) * | 0.94 (0.72-1.23) I2 | 0.76 (0.59–0.98) * I | ||
| Lin45RR (95%CI) | 2019 | 9 | 3.6−10.3 | 29,814 | 0.97 (0.90–1.06) | 0.94 (0.77–1.14) | 0.91 (0.84–0.98) * | 1.01 (0.84–1.22) | 0.84 (0.73–0.97) * |
| Seidu46RR (95%CI) | 2019 | 12 | 3.6−10.1 | 34,227 | 0.95 (0.88–1.02) | 0.92 (0.78–1.08) | 0.89 (0.83–0.95) ** | 0.84 (0.64–1.11) I2 | 0.82 (0.55–1.23) |
| Khan (47)47RR (95%CI) | 2020 | 10 | 3.6−10.3 | 33,679 | 0.99 (0.90–1.09) | 0.95 (0.83–1.09) | 0.93 (0.87–1.00) | 0.91 (0.75–1.11) I2 | 0.91 (0.76–1.10) I |
MA: Meta-analysis; SR: Systematic reviews; ASA: Acetylsalicylic acid; DM: Diabetes mellitus; RCT: randomized clinical trials; N: Number of study subjects with DM. ACM: all-cause mortality; CVM: cardiovascular mortality; MACE: major adverse cardiovascular events; MI: myocardial infarction; Stroke: ischemic stroke; RR: Relative risk; OR: Odds ratio; HR: Hazard ratio; 95%CI: 95% confidence interval; I2: high degree of heterogeneity between RCT; I: moderate degree of heterogeneity between RCT; NA: Not available.
Bold type: significant risk reduction (* p <0.05; ** p <0.01; *** p <0.001).
MA and SR which assessed safety of prophylaxis with ASA in people with DM in primary prevention.
| Studies (Ref.) | Year | N RCT | Follow-up range (years) | N | MBE | MGIE | Extracranial hemorrhage | Hemorrhagic stroke | Intracranial hemorrhage |
|---|---|---|---|---|---|---|---|---|---|
| ATT1RR (95%CI) | 2009 | 2 | 3.6−10.1 | 2,058 | 1.10 (0.52–2.34) | ||||
| ACCEPT-D25RR (95%CI) | 2009 | 3 | 4.4−10.1 | 4,842 | 2.50 (0.76–8.21) I2 | 2.11 (0.64–6.95) I2 | |||
| Calvin26RR (95%CI) | 2009 | 3 | 4.4−10.1 | 4,842 | 2.49 (0.70–8.84) I2 | ||||
| Younis27RR (95%CI) | 2010 | 3 | 4.4−10.1 | 4,842 | 2.49 (0.70–8.84) I2 | ||||
| ADA/ACC/AHA33RR (95%CI) | 2010 | 6 | NA | NA | 1.54 (1.30–1.82) *** | ||||
| Zhang28RR (95%CI) | 2010 | 3 | 4.4−10.1 | 4,842 | 2.46 (0.70–8.61) I2 | ||||
| Butalia29RR (95%CI) | 2011 | 4 | 4.4−10.1 | 7,281 | 2.50 (0.77–8.10) I2 | 2.13 (0.63–7.25) I2 | |||
| Stavarakis30RR (95%CI) | 2011 | 3 | 4.4−10.1 | 4,842 | 3.03 (0.49–18.9) I2 | 2.12 (0.63–7.08) I2 | |||
| Xie35RR (95%CI) | 2014 | 12 | 2.3−10.1 | 12,221 | 1.67 (1.43–1.94) *** | ||||
| USPSTF36RR (95%CI) | 2016 | 9 | 3.6−10.1 | 11,825 | 1.55 (1.13–2.14) ** | 1.55 (1.13–2.14) ** | 1.74 (0.95–3.17) | ||
| Kokoska32OR (95%CI) | 2016 | 4 | 3.6−6.7 | 5,379 | 2.53 (0.77–8.34) I2 | 2.14 (0.63–7.33) I2 | 0.90 (0.34–2.33) | ||
| Kunutsor37RR (95%CI) | 2017 | 3 | NA | 7,526 | 2.23 (0.79–6.34) | 2.12 (0.63–7.10) | 1.68 (0.40–7.05) | ||
| Mahmoud39RR (95%CI) | 2019 | 2 | 7.4−10.3 | 18,019 | 1.27 (1.09–1.46) ** | ||||
| Christiansen41RR (95%CI) | 2019 | 4 | 3.6−7.4 | 20,326 | 1.28 (1.10-1.49) ** I2 | ||||
| Huang42RR (95%CI) | 2019 | 3 | 4.4−7.4 | 19,295 | 1.53 (0.83-2.82) | 1.22 (0.83-1.81) | |||
| Zheng43HR (95%CI) | 2019 | 3 | NA | 20,076 | 1.29 (1.11–1.51) ** | 1.35 (1.05–1.75) * | 1.21 (0.84–1.76) | ||
| Lin45RR (95%CI) | 2019 | 5 | 3.6−10.3 | 22,383 | 1.24 (1.03–1.48) * | 1.43 (0.92–2.22) I2 | 1.08 (0.77–1.50) | ||
| Seidu46RR (95%CI) | 2019 | 6 | NA | NA | 1.30 (0.92–1.82) | 1.48 (0.87–2.49) | 1.24 (0.85–1.80) | ||
| Khan47RR (95%CI) | 2020 | 5 | 3.6−10.3 | 24,818 | 1.29 (1.07–1.55) * | 1.50 (0.92–2.45) I2 | 1.24 (0.85–1.80) | 1.13 (0.80–1.60) |
MA: Meta-analysis; SR: Systematic reviews; ASA: Acetylsalicylic acid; DM: Diabetes mellitus; RCT: randomized clinical trials; N: Number of study subjects with DM. MBE: Major bleeding events; MGIBE: Major gastrointestinal bleeding events; RR: Relative risk; OR: Odds ratio; HR: Hazard ratio; 95%CI: 95% confidence interval; I2: high degree of heterogeneity between RCT; I: moderate degree of heterogeneity between RCT; Bold type: significant risk increase (# p= 0.05; * p <0.05; ** p <0.01; *** p <0.001). NA: Not available.type: significant risk increase (# p= 0.05; * p <0.05; ** p <0.01; *** p <0.001). NA: Not available.
BMD: British Male Doctors; PHS: Physicians’ Health Study; ETDRS: Early Treatment Diabetic Retinopathy Study; HOT: Hypertension Optimal Treatment; TPT: Thrombosis Prevention Trial; PPP: Primary Prevention Project; WHS: Women's’ Health Study; JPAD: Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes; POPADAD: Prevention and Progression of Arterial Disease and Diabetes; AAA: Aspirin for Asymptomatic Atherosclerosis; ECLAP: European Collaboration on Low-Dose Aspirin in Polycythemia Vera; CLIPS: Critical Leg Ischaemia Prevention Study. JPPP: Japanese Primary Prevention Project; JPAD2: Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes 2; ARRIVE: Aspirin to Reduce Risk of Initial Vascular Events; ASCEND: A Study of Cardiovascular Events in Diabetes; ASPREE: Aspirin in Reducing Events in the Elderly. ATT: Antithrombotic Trialists’ Collaboration; ACCEPT-D: Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes; ADA/ACC/AHA: American Diabetes Association / American College of Cardiology / American Heart Association; USPSTF: U.S. Preventive Services Task Force.
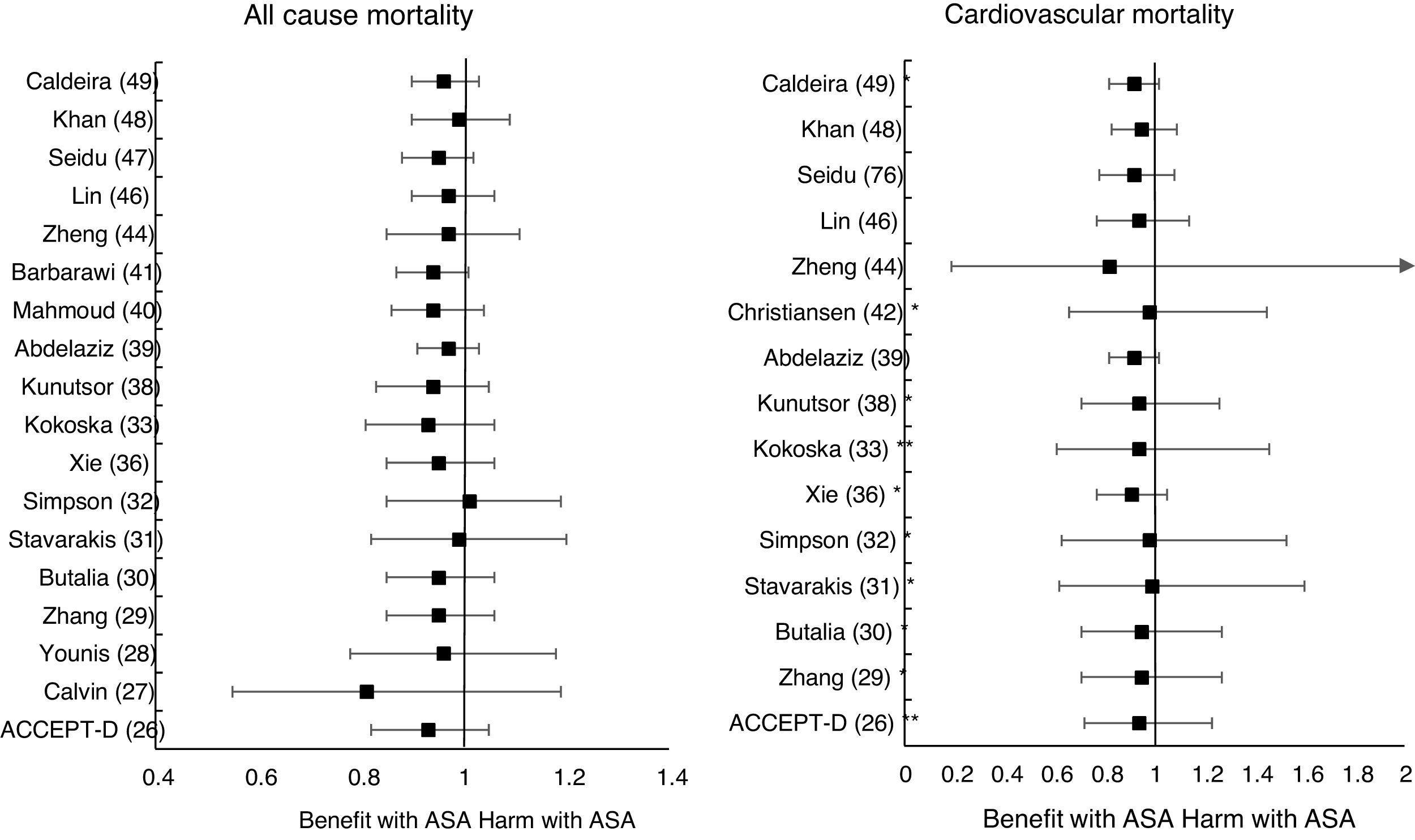
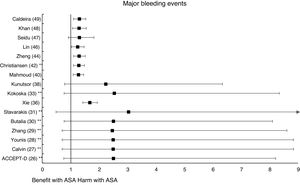
The MA/SR of the Antithrombotic Trialists’ Collaboration (ATT),1 ACCEPT-D,26 Calvin et al.,27 Younis et al.,28 Zhang et al.,29 de Butalia et al.,30 Stavrakis et al.,31 Simpson et al.,32 and Kokoska et al.33 showed that prophylaxis with ASA was not associated with a reduced risk of ACM, CVM or cardiovascular events, or with a significantly increased risk of MBE.
The MA of the position paper of the American associations of Diabetes and of Cardiology (ADA/ACC/AHA)34 showed that prophylaxis with ASA was not associated with reduced risks of MACE or stroke. On the contrary, it did show an important increase in the risk of extracranial bleeding events (RR 1.54 [95%CI 1.30–1.82] p<0.001).
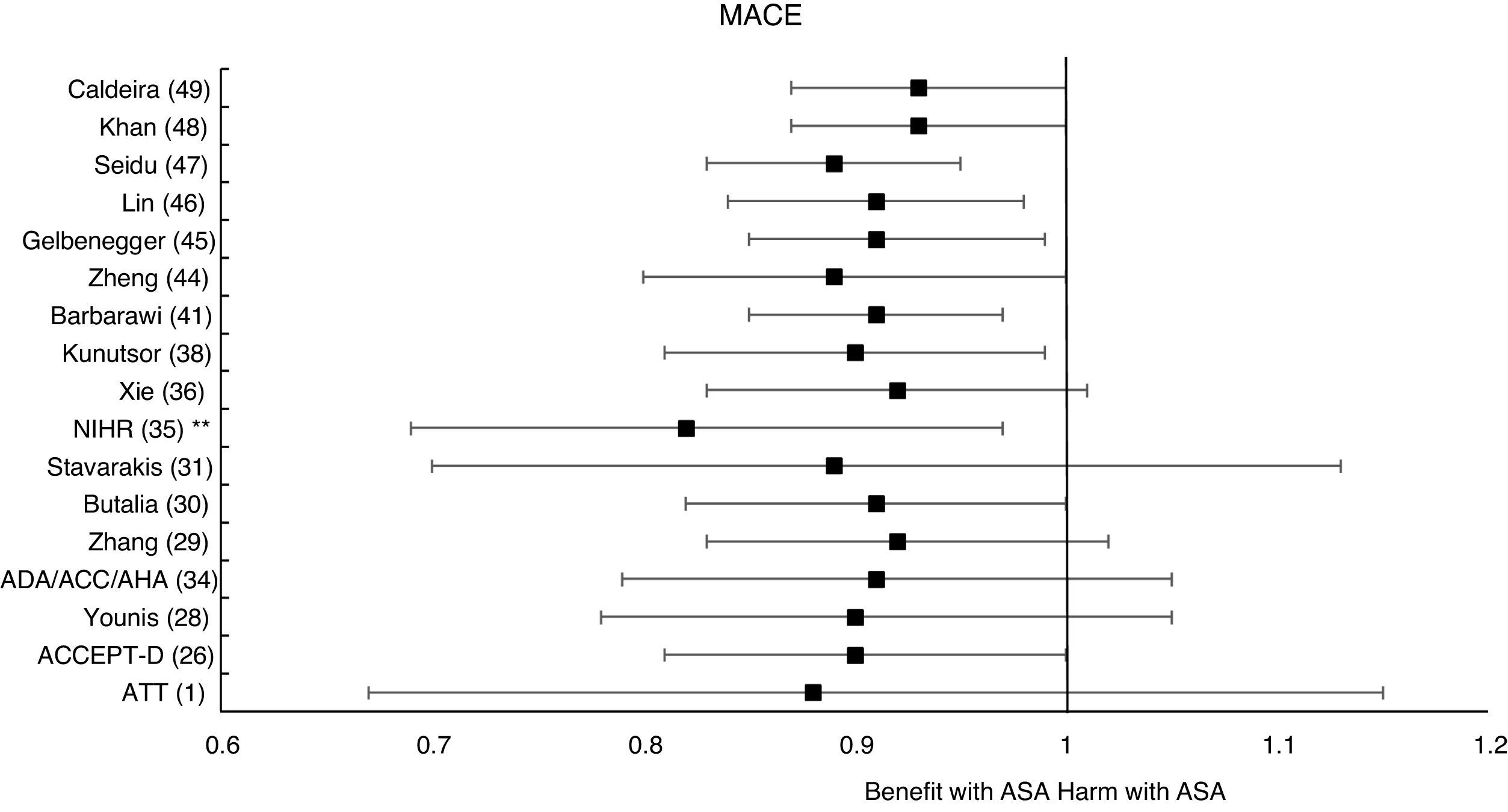
These previous results contrast with the SR with MA of the National Institute for Health Research (NIHR)35 which showed, bordering on statistical significance, that prophylaxis with ASA was associated with a reduced risk of the MACE composite criteria (RR: 0.82 [95%CI 0.69–0.97] p=0.04]).
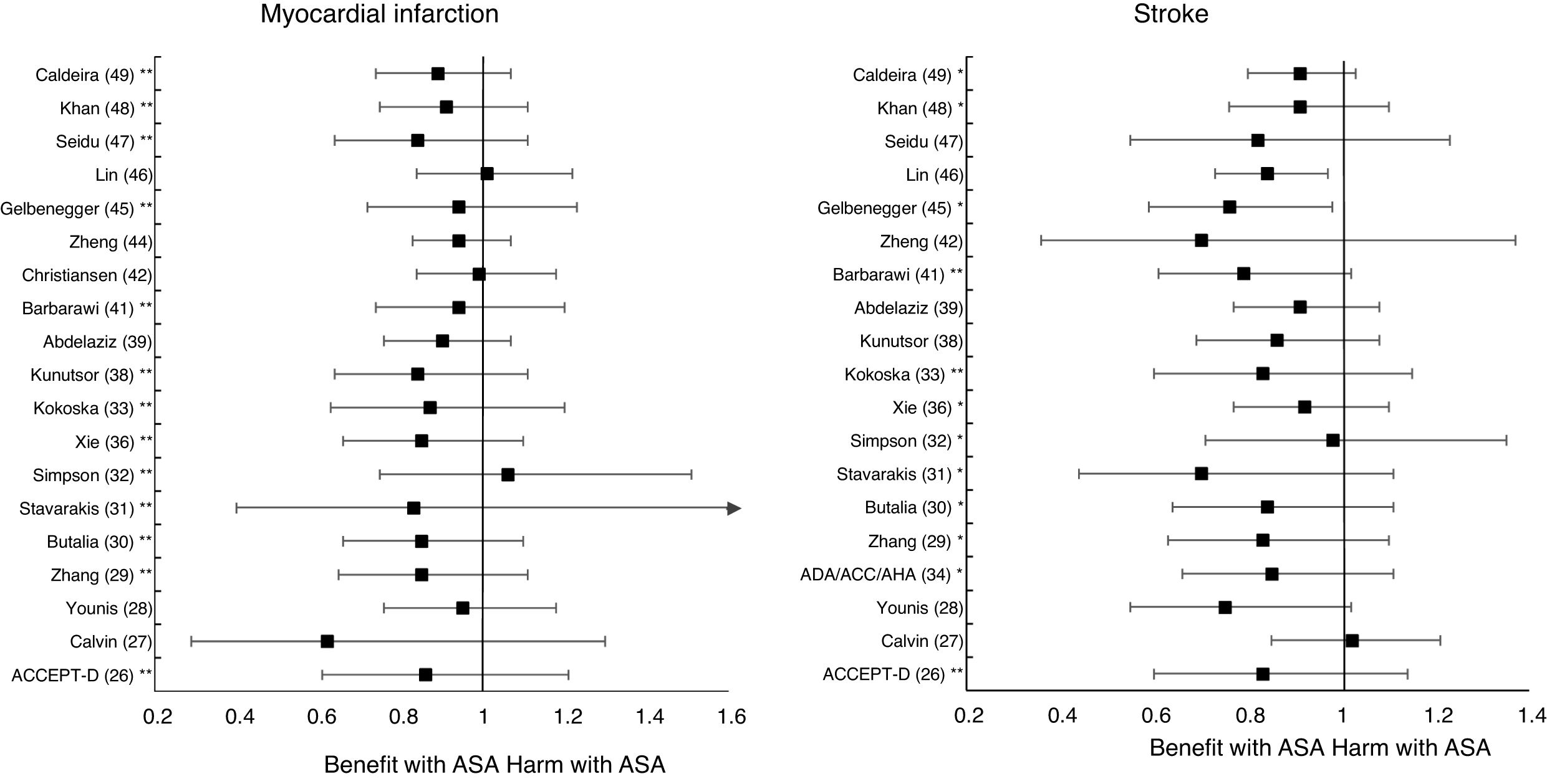
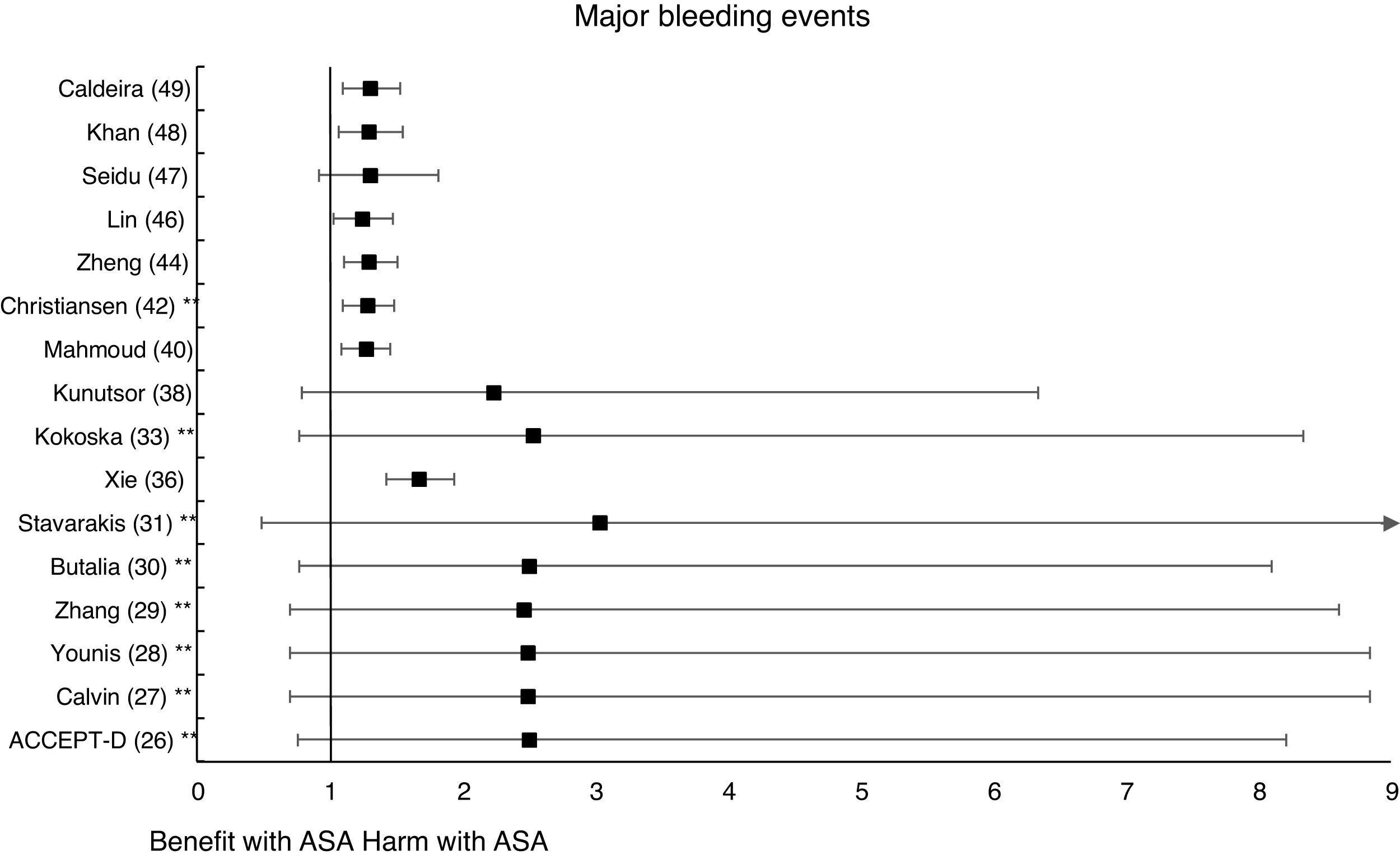
In the MA by Xie et al.,36 the prophylaxis with ASA was not associated with reduced risk of cardiovascular events, but it did show an important increase in the risk of MBE (RR: 1.67 [95%CI 1.43–1.94] p<0.01).
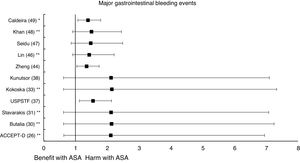
A subanalysis of the SR carried out by the United States Preventive Services Taskforce (USPSTF)37 showed that prophylaxis with ASA was associated with an important increase in the incidence of extracranial hemorrhage or MGIBE (RR: 1.55 [95%CI 1.13–2.14] p<0.01).
The MA by Kunutsor et al.38 showed, bordering on statistical significance, that prophylaxis with ASA was associated with a reduced risk of MACE (RR:.0.90 [95%CI 0.81–0.99] p=0.031).
A subanalysis of the SR by Abdelaziz et al.39 showed that prophylaxis with ASA was not associated with decrease in the risk of cardiovascular events in patients with DM in primary prevention.
The subanalysis of the MA by Mahmoud et al.40 showed that prophylaxis with ASA was not associated with a decreased risk of ACM in patients with DM in primary prevention, but it was associated with a 27% increase in the incidence of MBE (RR:1.27 [95%CI 1.09–1.46] p=0.002).
A subanalysis of the MA by Barbarawi et al.41 showed that prophylaxis with ASA in primary prevention in patients with DM was associated with a reduced risk of MACE (RR: 0.91 [95%CI 0.85–0.97] p=0.006) and was not associated with a decreased risk of ACM, MI, or stroke.
A subanalysis of the MA/RS by Christiansen et al.,42 which included patients with DM in primary prevention, showed that prophylaxis with ASA was associated with a significantly increased risk of MBE (RR: 1.28 [95%CI 1.10–1.49] p=0.001), and was not associated with a reduced risk of MI or CVM.
A subanalysis of the MA/RS by Huang et al.43 showed that prophylaxis with ASA was not associated with an increased risk of intracranial hemorrhage in diabetic patients in primary prevention.
A subanalysis of the MA/RS by Zheng and Roddick,44 with people with DM in primary prevention during a follow-up median of 5.0 years, showed a reduced risk of MACE (RR: 0.89 [95%CI 0.80–1.00] p<0.05 [NNT: 153]), bordering on statistical significance. On the other hand, it showed important increases in the risk of MBE (RR: 1.29 [95%CI 1.11–1.51] p<0.01 [NNH: 121]), and of MGIBE (RR: 1.35 [95%CI 1.05–1.75] p<0.05 [NNH: 243]).
A subanalysis of the MA by Gelbenegger et al.45 showed a reduced risk of MACE (RR: 0.91 [95%CI 0.85–0.99] p=0.02) and stroke (RR: 0.76 [95%CI 0.59–0.98] p=0.03) in diabetic patients in primary prevention.
The MA by Lin et al.46 did not show significant reductions in the risk of ACM, CVM, or MI, or significant increases in the risk of intracranial hemorrhagic events or MGIBE. However, it did show a reduced risk of MACE (RR: 0.91 [95%CI 0.84–0.98] p=0.018) and of stroke (RR: 0.84 [95%CI 0.73–0.97] p=0.017), and an increased risk of MBE (RR: 1.24 [95%CI 1.03–1.48] p=0.022).
In the MA of Seidu et al.,47 the prophylaxis with ASA in people with DM in primary prevention during a follow-up median of 5 years was associated with a reduction in the risk of MACE (RR: 0.89 [95%CI 0.83–0.95] p=0.005 [NNT: 95]), without association with reduced risk of ACM, CVM, MI or ischemic stroke, or with increased risk of hemorrhagic stroke, MBE or MGIBE.
In the MA by Khan et al.,48 the prophylaxis with ASA was associated with a significant increase in the risk of MBE (RR: 1.29 [95%CI 1.07–1.55] p=0.01), without association with reduced risk of ACM, CVM, MACE, MI, or stroke.
The MA/RS by Caldeira et al.49 showed, bordering on statistical significance, that prophylaxis with ASA was associated with a reduced risk of MACE (RR: 0.92 [95%CI 0.84–1.00] p=0.049), without association with reduced risk of ACM, CVM, MI or stroke. Likewise, it showed that prophylaxis with ASA was associated with a significantly increased risk of MBE (RR: 1.30 [95%CI 1.10–1.53] p=0.002), and of MGBIE (RR: 1.39 [95%CI 1.08–1.80] p=0.001).
Only two MA45,46 of the 19 MA/SR that assessed the risk of ischemic stroke, showed a slightly significant decrease in the stroke risk (mean 20.0% [SD±5.7]), with a moderate heterogeneity between the RCT evaluated.
The 47% of the 17 MA/SR that assessed the MACE composite endpoint showed a slight reduction in risk (mean 10.5% [SD±3.3]), all of them bordering on statistical significance.
In the MA with statistically significant results, the means (SD) of risk reduction of MBE, MGIBE and extracranial bleeding were 33.4% (±14.9), 43.0% (±10.6), and 54.5% (±0.7) respectively.
DiscussionIn routine clinical practice, decisions about preventive pharmacological interventions are usually justified by scientific evidence. This paper summarizes the current state of available evidence on the efficacy and safety of prophylaxis with ASA in patients with DM without ACVD. MA and SR lead the available scientific evidence, although they may ignore the fact that unfavorable findings or non-significant results are not highlighted as eminently as significant findings. The hierarchy of the grade of evidence continues with RCT, analytical studies, observational studies, and expert opinion, consensuses which may have biases if arguments are justified after a limited literature review that may miss key publications, especially if results are negative.13,14
After assessing the risk-benefit balance of prophylaxis with ASA in people with DM in primary prevention, some guidelines2,5,9,12 recommend considering the use of ASA in people with DM and increased CVR. However, this work shows that the clinical practice guidelines offer different considerations about CVR in people with DM, and that their recommendations do not always agree. Some recommendations appear to be based on data extrapolations from other groups with high CVR, rather than a comprehensive review of all available evidence. The guidelines assess the CVR of people with DM in different ways, even within the same country. For example, in the United States, while the ACC/AHA2 and the ADA9 distinguish four CVR groups (low, borderline, intermediate, and high), the AACE/ACE consider that people with DM can have a high, very high, or extreme CVR.8 Additionally, the European ESC, EAS and EASD guidelines consider that it can be moderate, high or very high.4,5,12 The different criteria about CVR assessment in people with DM are also apparent in RCT. The variability of the 10-year risk between the RCT which include only study subjects with DM in primary prevention ranges between 7.8% and 40.8%17,52,53,56,61,62,65 (Table 2). It is likely that the RCT show CVR results that differ from the reality of population in Primary Care, where the age-adjusted prevalence of moderate CVR is only 5.5% in the people with DM.67
In addition to the different considerations about CVR, the guidelines also differ in their recommendations on prophylaxis with ASA in primary prevention. Some guidelines justify their recommendations on the basis of the findings of few RCT, SR, or MA, so they can also have a certain degree of bias. The American ACC/AHA2 guidelines recommend considering its use in population aged between 40 and 70 or with additional CVRF regardless of age, without distinction between persons with and without DM, based on one SR of RCT,68 one review of observational studies,69 and two RCT.18,64 The ADA9 recommends considering prophylaxis with ASA in people with DM aged 50–70, with increased CVR and without increased risk of bleeding, based on the results of an initial MA1 published in 2009, which evaluated six RCT including people with DM,17,18 and on another RCT which included subjects without DM.66 Additionally, the ADA considers the chronic kidney disease as a factor to contemplate prophylaxis, and as a risk factor for bleeding.
On the contrary, the 2020 update of the ESC cardiovascular prevention guideline6 and the NICE7 and SIGN3,11 guidelines recommend not using ASA in patients in primary prevention, regardless of whether they have DM or not. The 2020 update of the ESC guideline6 maintains that the risk of bleeding outweighs the potential benefit of the prophylaxis with ASA, and argues that it should be avoided in primary prevention, on the basis of the study by Ikeda et al.,64 and three MA1,39,40 which show that its use has no impact on the reduction of ACM or CVM. Similarly, the guideline refers specifically to people with DM without ACVD with high CVR and, based on the MA/SR by Khan et al.,48 recommends avoiding the use of ASA in these patients because it increases the risk of MBE without reducing the risk of MACE. Similary, the NICE7 and SIGN3,11 guidelines made their conclusions on the NIHR report,35 a more thorough evaluation which included a report of their own as well as 9 more MA/SR, of which six1,26,28–31 assessed its efficacy and safety in people with DM, concluding with the recommendation against the use of ASA in patients with DM without ACVD.
This review contrasts these recommendations, showing several strengths and limitations, compared to previous reviews. It is the most comprehensive and complete evaluation to date on the clinical efficacy and safety of prophylaxis with ASA in patients with DM in primary prevention. The results of 25 MA/SR published from 2009 to 2020, without omitting any relevant one, and which together analyzed a total of 20 RCT (Table S2).
Most of the MA/SR included three old RCT50,51,56 whose study subjects were healthcare professionals; this could limit the generalizability of the findings.
The most recent studies ARRIVE,16 ASCEND,17 and ASPREE18 should be mentioned due to their influence on the latest MA. The ARRIVE study16 did not include persons with DM, and was included in the MA by Abdelaziz et al.39 The ASCEND study17 analyzed 15,480 people with DM, four times more than the MA of the ATT,1 considered very important and frequently cited by the guidelines,3,4,7,9,11,24–26 which it only included 3818 people with DM. The ASCEND study17 reported a 12% reduction of MACE and a 29% increase of first MBE. However, the ASPREE study,18 which included 2057 patients with DM aged 65 or older, showed a 14% increase of ACM, without reduction of the cardiovascular primary endpoints, and a 38% increase of MBE.
The number of RCT which analyzed the risk of MBE was small. In the four MA that evaluated this risk,34,38,44,48 no information was found about which RCT were included.
The MA by Xie et al.36 assessed the ACBS study,53 which included patients with DM and asymptomatic carotid stenosis (≥50%), and the CLIPS study,59 which included 366 subjects (76% with DM) who had an ABI<0.85 (mean 0.64), of which 77% had symptomatic peripheral artery disease (PAD). Numerous MA and SR26,28–35,38,39,41–46,48,49 included the POPADAD study,62 which assessed 1276 patients with DM and asymptomatic PAD defined by an ABI≤0.99. The AAA study63 was included in 5 MA32,35–37,39 and evaluated 3350 subjects with an ABI≤0.95 (median 0.86). Although this review assesses people with DM without ACVD, it should be noted that many MA analyzed include the POPADAD62 and AAA63 studies, and that asymptomatic PAD can be considered as the beginning of ACVD.
The ASA dosages administered to the RCT subjects were not analyzed in this review. These ranged from 75 to 650mg/day, although the daily dose analyzed in most of the RCT included in the SR and MA was 100mg/day (Table S2). Nonetheless, in a recent RCT with 15,076 study subjects,70 no significant differences have been found in ACM, or in hospitalization as a result of MI, stroke, or MBE, when comparing the 81–325mg/day doses.
It is important to emphasize that none of the MA/SR proved that prophylaxis with ASA in people with DM reduced the risk of ACM, CVM, or MI taken individually, and yet 47% of the MA/SR that assessed the MACE composite endpoint showed, bordering on statistical significance, a modest risk reduction of approximately 10%. This could be explained by the fact that the risk assessment criteria for MACE offers better statistical power because it is a composite endpoint of CVM, MI, and stroke.
On the other hand, the risks of MBE associated with prophylaxis with ASA in people with DM in primary prevention are supported by very solid evidence and occur more frequently. The use of ASA was associated with important increases in MBE (between 24% and 67%), in MGIBE (between 35% and 55%), and in extracranial bleeding (between 54% and 55%).
In general, the analyzed MA seem to be up to date according to the time when they were conducted. However, the inferences and conclusions differ from one study to another. This could be due to the existence of important differences in the management of the preventive treatment used in the earliest RCT, and therefore in the earliest MA, with respect to the treatment used during the most current RCT; for instance, a higher intensity of lipid lowering or hypertension treatments in people with DM.
ConclusionsThe main clinical practice guidelines differ from one another in their assessment of CVR in people with DM. While the American guidelines of the ACC/AHA and the ADA classify the CVR of patients with DM in four groups (low, borderline, intermediate, and high), the Endocrinology guidelines (AACE/ACE) consider that these can have a high, very high, or extreme CVR. On the other hand, the European guidelines ESC, EAS, and EASD consider that the CVR of people with DM can be moderate, high, or very high.
The guidelines also differ about recommendations on the use of ASA in people with DM in primary prevention. The ACC/AHA guidelines consider that the use of ASA could be considered in patients aged 40–70 with a higher CVR and without risk of bleeding. The ADA points out that the use of ASA can be considered in people with DM between 50 and 70 years old with increased CVR. The 2021 ESC guideline on cardiovascular prevention maintain with a COR IIb, that the use of ASA can be contemplated in primary prevention in patients with DM and high or very high CVR if there are no clear contraindications. This contrasts with the 2020 ESC update, which considered with a COR I, that the lack of net clinical benefit of ASA in primary prevention was evident not only in general population but also in patients with high CVR or with DM, because it increases the risk of bleeding without reducing the risk of MACE. Additionally, the NICE and SIGN guidelines recommend not using ASA in any patient in primary prevention, even with high CVR or with DM.
The MA/SR consistently show that prophylaxis with ASA in people with DM in primary prevention does not reduce the risk of ACM, CVM, or MI, taken individually. Non-significant results are not highlighted as prominently as significant findings. Most studies on the possible reduction of ischemic stroke remain inconclusive, since only two MA showed a slightly significant risk reduction. The results on the MACE composite endpoint are also inconclusive, because less than half of the MA/SR showed, bordering on statistical significance, a slight risk reduction. However, prophylaxis with ASA in this population group was associated with important increases in the risk of MBE, MGIBE, and extracranial bleeding.
In conclusion, the risk-benefit assessment of the available evidence about results on efficacy and safety of prophylaxis with ASA in patients with DM for primary prevention of ACVD suggests that the use of ASA is associated with net harm due to a greater potential for MBE. Therefore, the prophylaxis with ASA in primary prevention should not be used in people with DM.
Authors’ contributionARG, VPC: Conception, drafting, analysis, literature search, final review and validation.
ASC, CEC, VB: Drafting, final review and validation.
ABG, JADG, MTY, MAPD, SCS, FJAM, PBF, LGM, DRA, EWMR, AMA, RCS, APC, JCRV, ECC, SVZ, MCSV, JLGT, JPG: review and validation.
All authors have read and approved this research.
FundingThe authors declare that they have received no financial support for this research.
Conflicts of interestNone to declare.
Ajenjo González, M.; Alonso Moreno, F.J.; Araujo Márquez, L.; Arina Cordeu, C.; Artigao Rodenas, L.M.; Barquilla García, A.; Barrios Alonso, V.; Beato Fernández, P.; Caballero Pajares, V.; Cabezudo Moreno, F.; Calderon Montero, A.; Carrasco Carrasco, E.; Carrasco Martín, J.L.; Carrizo Sánchez, J.; Castillo Moraga, M.J.; Cinza Sanjurjo, S.; Crespo Sibaris, R.; de las Cuevas Miguel, M.P.; Divisón Garrote, J.A.; Eguía, H.; Escobar Cervantes, C.; Esteban Rojas, M.B.; Fernández Toro, J.M.; Frías Vargas, M.; Fuentes Martínez, D.; García Criado, E.; García Lerín, A.; García Matarín, L.; García Vallejo, O.; Genique Martínez, R.; Gil Gil, I.; González Casado, I.; González Lillo, I.; Górriz Teruel, J.L.; Jiménez Baena, E.; Juan Gaceo, J.; Martí Canales, J.C.; Martín Rioboó, E.; Mediavilla Bravo, J.J.; Molina Escribano, F.; Morales Quintero,S.; Moyá Amengual, A. (secretary); Nieto Barco, G.; Ochoa Linares, A.; Pallarés Carratalá, V. (coordinator); Pérez Vázquez, E.; Piera Carbonell, A.; Polo García, J.; Prieto Díaz, M.A.; Rama Martínez, T.; Redondo Prieto, M.; Rey Aldana, D.; Riesgo Escudero, B.E.; Rodríguez Roca, G.C.; Rojas Martelo, G.A.; Romero Sencin, A.; Romero Vigara, J.C.; Ruiz García, A.; Sánchez Rodríguez, R.; Sánchez Ruíz, T.; Santos Altozano, C.; Sanz García, F.J.; Seoane Vicente, M.C.; Serrano Cumplido, A.; Turégano Yedro, M.; Valls Roca, F.; Velilla Zancada, S.M.; Vicente Molinero, A.