Monacolin K is the major active component in red yeast rice (RYR) which is structurally identical to lovastatin and has the most powerful effect, in terms of reducing blood cholesterol levels. This review aimed to examine the effect and safety of different doses of monacolin K on blood cholesterol levels. PubMed and Cochrane were searched for articles published between 2012 and 2023 for clinical-trials and randomized-controlled-trials. Eligible studies included participants>18-years-old, of any gender and ethnicity. The intervention/exposure of interest was monacolin K. Hypercholesterolemia was considered the outcome of interest defined as the elevated total or low-density-lipoprotein (LDL) cholesterol levels. 12 randomized-controlled-trials were eligible for inclusion in the analysis including 769 participants>18-years-old. 11 out of 12 studies were assessed with high methodological quality and one study with low methodological quality. Monacolin K supplementation varied between 2mg and 10mg per day and the maximum period of supplementation was 12 weeks. All studies indicated a beneficial effect of monacolin supplementation on LDL and total cholesterol levels (p<0.05) regardless the dose and period of supplementation. Also, 3 of the included studies reported adverse side effects after treatment with monacolin K. Low doses of monacolin K equal to 3mg/day exert potential cholesterol-lowering effects although the number of relative studies is limited. Regarding the safety of monacolin K supplementation, findings seem to be more controversial and therefore, it is suggested for all patients treated with monacolin K to be routinely monitored regardless the dose of supplementation.
La monacolina K es el principal componente activo en el arroz de levadura roja (RYR) que es estructuralmente idéntico a la lovastatina y tiene el efecto más poderoso, en términos de reducción de los niveles de colesterol en la sangre. Esta revisión procuró examinar el efecto y la seguridad de diferentes dosis de monacolina K sobre los niveles de colesterol en sangre. Se realizaron búsquedas en PubMed y Cochrane de artículos publicados entre 2012 y 2023 para obtener ensayos clínicos y ensayos controlados aleatorios. Los estudios elegibles incluyeron participantes >de 18 años de edad, de cualquier sexo y etnia. La intervención/exposición de interés fue monacolina K. La hipercolesterolemia se consideró el resultado de interés definido como los niveles elevados de colesterol total o lipoproteína de baja densidad (LDL). Doce ensayos controlados aleatorios fueron elegibles para su inclusión en el análisis con 769 participantes >18 años de edad. Once de 12 estudios se evaluaron con alta calidad metodológica y un estudio con baja calidad metodológica. La suplementación con monacolina K varió entre 2mg y 10mg por día y el período máximo de suplementación fue de 12 semanas. Todos los estudios indicaron un efecto beneficioso de la suplementación con monacolina sobre los niveles de LDL y colesterol total (p<0,05) independientemente de la dosis y el período de suplementación. Además, 3 de los estudios incluidos informaron efectos secundarios adversos después del tratamiento con monacolina K. Las dosis bajas de monacolina K iguales a 3mg/día ejercen efectos potenciales reductores del colesterol, aunque el número de estudios relativos es limitado. Con respecto a la seguridad de la suplementación con monacolina K, los hallazgos parecen ser más controvertidos y, por lo tanto, se sugiere que todos los pacientes tratados con monacolina K sean monitoreados rutinariamente independientemente de la dosis de suplementación.
Recent decades have shown tremendous progress in the prevention of cardiovascular diseases as a result of several pharmaceuticals and dietary improvements. Modifications in diet have focused not only on the macronutrient components but also on some micronutrients with drug-like properties.1,2 In particular, there are some natural food components with potential properties related to health and disease including lipid and blood pressure-lowering effects.3,4 Therefore, based on scientific findings, many patients are more reluctant to use natural dietary products instead of conventional medicines causing several side-effects. Recently, health professionals are asking for more high-quality evidence-based recommendations relating to the efficacy of these natural products.4,5
RYR1 (RYR) is a new natural therapeutic agent, grown on white rice. It is a nutrient produced by the fermentation of rice with the fungus Monascus Purpureus and contains monacolins, pigments, organic acids and amino acids having anti-hyperlipidemic effects.6 RYR is recognized as a functional food, known for its cholesterol-lowering.1,6–9 It is a common dietary component of the Asian diet and it is widely used in the Chinese Medicine.10
Monacolin K is the major active component in RYR which is structurally identical to lovastatin and has the most powerful effect, in terms of reducing blood cholesterol levels.1,7 Under laboratory conditions, monacolin K is a transparent, white crystal with low solubility in water, but highly soluble in organic solvents such as methanol, ethanol, acetone, chloroform and benzene. Under low pH conditions, it adopts one of two forms, acidic or lactone. The acidic form plays a role in reducing blood lipid.11 Particularly, monacolin K acts as an inhibitor of HMG-CoA reductase preventing its conversion to mevalonic acid and thus reducing cholesterol biosynthesis in the liver.10,12
Researchers from around the world have conducted in-depth research on monacolin K to investigate its effects in cholesterol levels. A previous meta-analysis of 47 RCTs2 with studies published since 1997, aimed to examine the therapeutic effect of Chinese patented drugs containing RYR in people with hyperlipidemia and found that RYR supplements were more effective than simvastatin in regard to LDL3 and total cholesterol levels.13 A more recent meta-analysis of 30 RCTs aiming to examine the effect of RYR preparations in patients with Metabolic Syndrome found that RYR was associated with significant reductions in mortality, total cholesterol, triglycerides, fasting plasma glucose, and blood pressure without causing any adverse side effects compared to placebo.14 Moreover, another review of 22 CTs4 published in 2012 focused in evaluating the effect of RYR in dyslipidemia and showed that RYR supplementation had similar effects to statin, even in combination with other dietary supplements.15
RYR has been shown to be well-tolerated by patients at doses of monacolin K up to 10mg/day although previous studies reported mild side effects including myalgia.16 In 2011, EFSA,5 based on scientific evidence approved a health claim regarding the intake of RYR containing 10mg of monacolin K, as a dietary supplement for controlling blood cholesterol levels.17 Nevertheless, EFSA, in 2018, re-examined the safety of monacolin K and found that monacolin K, in lactone form, is identical to lovastatin and that the side effects of taking 10mg of monacolin K are similar to those caused by lovastatin supplementation, which are well.16,18 Thus, EFSA reviewed the recommended daily intake of monacolin K from 10mg to 3mg per day.18 Indeed, existing data regarding the efficacy and safety of 3mg supplementation of monacolin K in blood cholesterol levels is limited and requires further investigation. Therefore, the current systematic review aimed to examine the effect and safety of different doses of monacolin K on blood cholesterol levels.
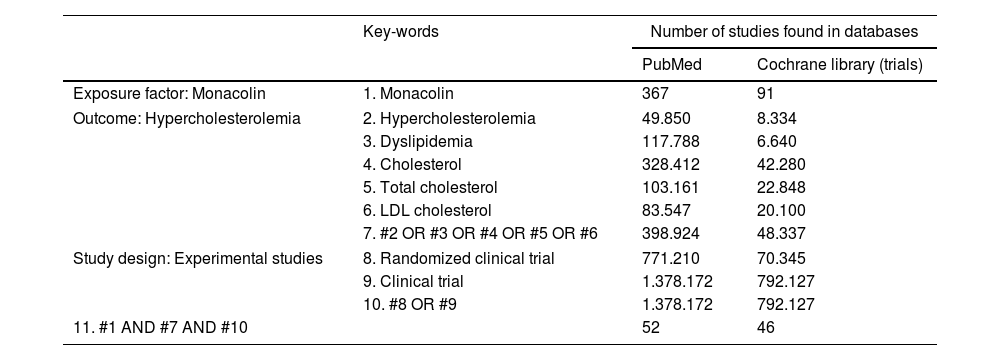
Materials and methodsSearch strategy and eligibility criteriaA systematic literature search was conducted through international databases, including PubMed and Cochrane Library using MeSH6 terms. The references cited in the selected articles were also searched manually. The literature search was conducted independently by two researchers from inception to September 2023. The search was limited to papers published in English; there was a date restriction on the studies included (2012–2023) in order to examine the most recent data. Moreover, the reference lists of the selected articles were searched to identify other eligible studies. Any discrepancies between reviewers were resolved in consultation with a third author. The search strategy used is presented in Table 1.
Strategic search and keywords used to find studies investigating the relationship between monacolin and hypercholesterolemia from PubMed and Cochrane Library (trials).
| Key-words | Number of studies found in databases | ||
|---|---|---|---|
| PubMed | Cochrane library (trials) | ||
| Exposure factor: Monacolin | 1. Monacolin | 367 | 91 |
| Outcome: Hypercholesterolemia | 2. Hypercholesterolemia | 49.850 | 8.334 |
| 3. Dyslipidemia | 117.788 | 6.640 | |
| 4. Cholesterol | 328.412 | 42.280 | |
| 5. Total cholesterol | 103.161 | 22.848 | |
| 6. LDL cholesterol | 83.547 | 20.100 | |
| 7. #2 OR #3 OR #4 OR #5 OR #6 | 398.924 | 48.337 | |
| Study design: Experimental studies | 8. Randomized clinical trial | 771.210 | 70.345 |
| 9. Clinical trial | 1.378.172 | 792.127 | |
| 10. #8 OR #9 | 1.378.172 | 792.127 | |
| 11. #1 AND #7 AND #10 | 52 | 46 | |
To identify included studies, we used specific inclusion criteria. Participants were ≥18-year-old adults of any gender and ethnicity. The intervention/exposure of interest was monacolin K. Hypercholesterolemia was considered the outcome of interest defined as the elevated total or LDL cholesterol levels. For the comparison, we used participants with hypercholesterolemia taking placebo. Only clinical trials and randomized controlled trials were eligible for inclusion. Published conference abstracts, dissertations, narrative reviews, and case reports were excluded.
Study selection and data extractionThe titles and abstracts from the electronic searches were examined independently by two reviewers and the full-text articles from the selected studies that were likely to meet the inclusion criteria were retrieved. Following the first stage, the same authors contacted a second evaluator in order to make the final decision. Any discrepancies between reviewers were resolved in consultation with a third author. From each study, we extracted the following variables: author, year, and country; study design, follow up time; population; main measures; intervention and main findings.
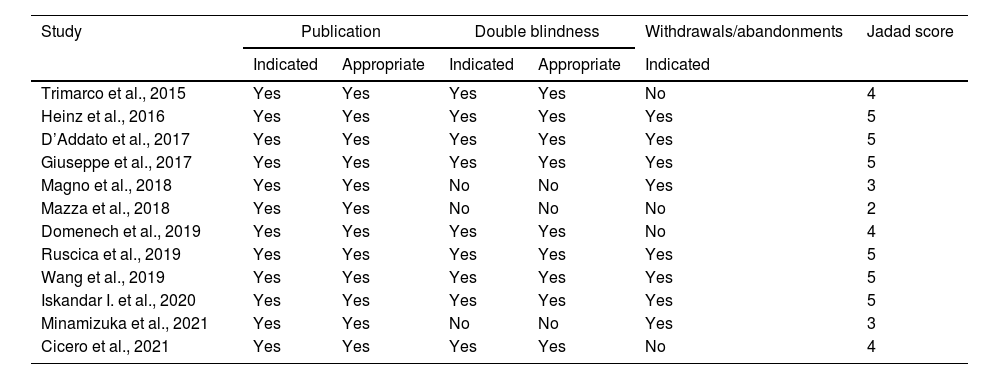
Quality assessmentThe assessment of the methodological quality of the included studies was performed by two independent reviewers. The Jadad score was used for assessing RCTs based on the randomization, the blinding and the participants’ dropout. The final score ranges from 0 to 5 points and is considered of “high quality” for Jadad score ≥3 and of “low” quality for Jadad score ≤2.19
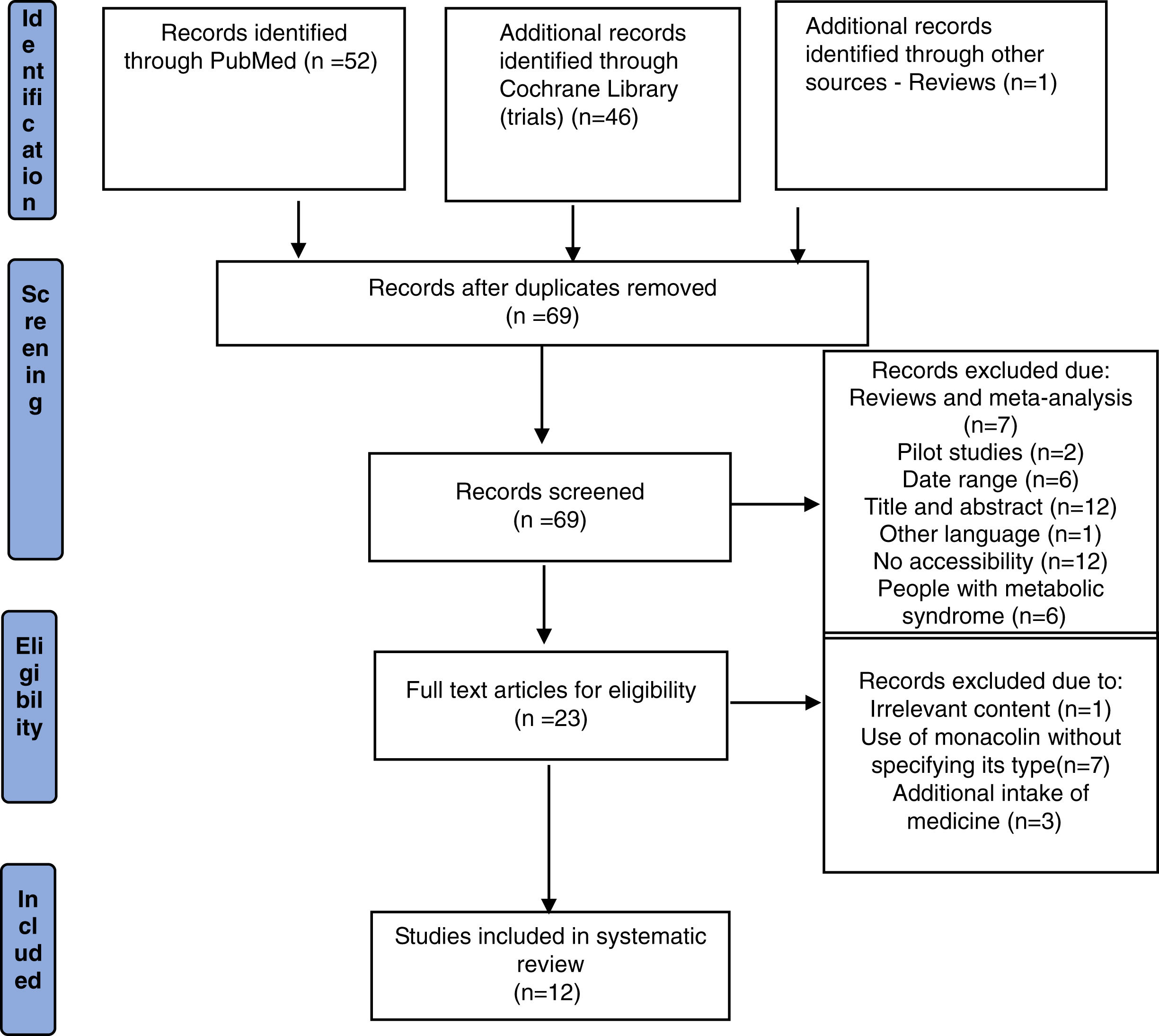
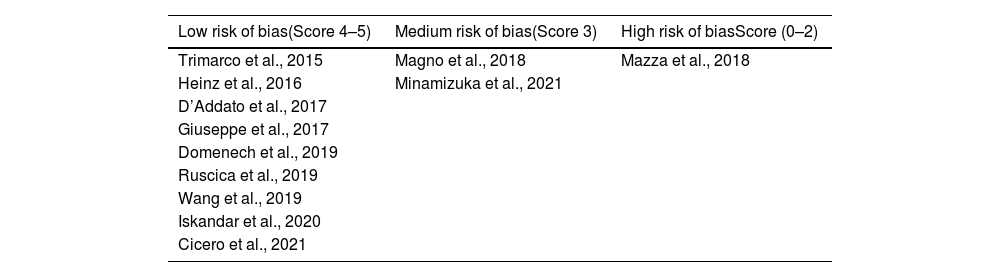
ResultsFrom the initial search, 99 articles were identified out of which 30 duplicate records were removed. Out of 69 articles that were screened based on their title and abstract, 46 articles were excluded as they did not meet the required inclusion criteria. From the remaining 23 articles, 11 articles were excluded after reading the whole text and the remaining 12 articles were included for qualitative analysis. The flow chart of selection studies regarding the systematic review is presented in Fig. 1. Regarding the methodological quality assessment, results showed that almost all studies (n=11) were assessed with high methodological quality (score≥3) and only one study was assessed with low methodological quality receiving a score equal to 2. Results of the methodological quality assessment are presented in Tables 2 and 3.
Assessment of methodological quality of studies based on the JADAD score (Jadad et al., 1996).
| Study | Publication | Double blindness | Withdrawals/abandonments | Jadad score | ||
|---|---|---|---|---|---|---|
| Indicated | Appropriate | Indicated | Appropriate | Indicated | ||
| Trimarco et al., 2015 | Yes | Yes | Yes | Yes | No | 4 |
| Heinz et al., 2016 | Yes | Yes | Yes | Yes | Yes | 5 |
| D’Addato et al., 2017 | Yes | Yes | Yes | Yes | Yes | 5 |
| Giuseppe et al., 2017 | Yes | Yes | Yes | Yes | Yes | 5 |
| Magno et al., 2018 | Yes | Yes | No | No | Yes | 3 |
| Mazza et al., 2018 | Yes | Yes | No | No | No | 2 |
| Domenech et al., 2019 | Yes | Yes | Yes | Yes | No | 4 |
| Ruscica et al., 2019 | Yes | Yes | Yes | Yes | Yes | 5 |
| Wang et al., 2019 | Yes | Yes | Yes | Yes | Yes | 5 |
| Iskandar I. et al., 2020 | Yes | Yes | Yes | Yes | Yes | 5 |
| Minamizuka et al., 2021 | Yes | Yes | No | No | Yes | 3 |
| Cicero et al., 2021 | Yes | Yes | Yes | Yes | No | 4 |
Distribution of clinical studies on the risk of bias (Jadad et al., 1996).
| Low risk of bias(Score 4–5) | Medium risk of bias(Score 3) | High risk of biasScore (0–2) |
|---|---|---|
| Trimarco et al., 2015 | Magno et al., 2018 | Mazza et al., 2018 |
| Heinz et al., 2016 | Minamizuka et al., 2021 | |
| D’Addato et al., 2017 | ||
| Giuseppe et al., 2017 | ||
| Domenech et al., 2019 | ||
| Ruscica et al., 2019 | ||
| Wang et al., 2019 | ||
| Iskandar et al., 2020 | ||
| Cicero et al., 2021 |
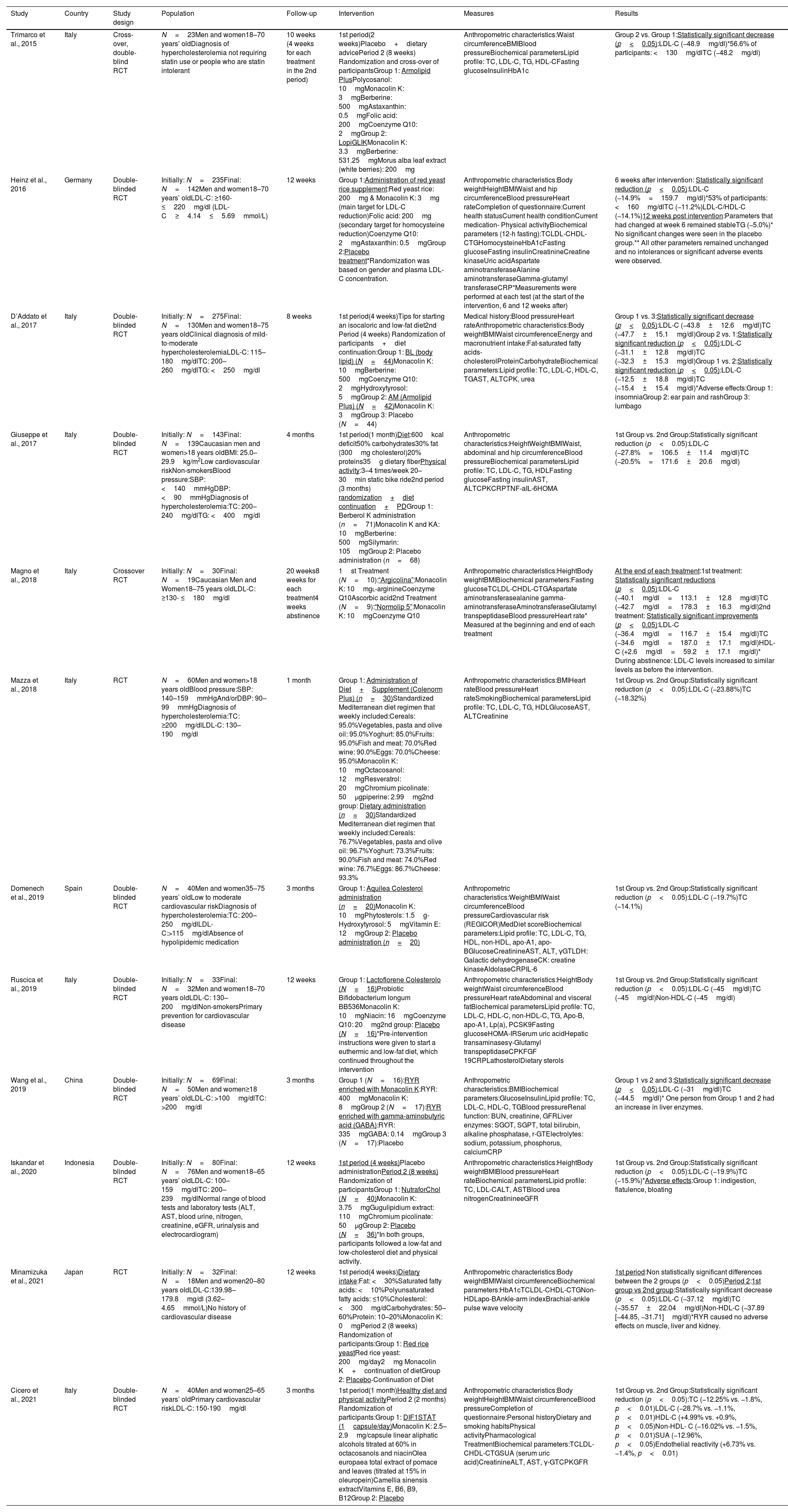
The current analysis included 769 participants>18 years of age in order to examine the potential effect that monacolin K has on hypercholesterolemia. All studies employed a randomized controlled design and were published between 2012 and 2021. Monacolin K dosage used in the studies ranged between 2mg and 10mg per day. In particular, 5 out of 12 studies examined the effect of lower doses of monacolin K ranged between 2mg and 3.75mg whereas 6 studies examined the effect higher doses of monacolin K ranged between 8mg and 10mg. Only one study compared the effectiveness of both doses of monacolin K in patients with hypercholesterolemia (10 vs 3mg/day). The main characteristics and findings of the included studies are presented in Table 4.
Characteristics and main findings of the included studies.
| Study | Country | Study design | Population | Follow-up | Intervention | Measures | Results |
|---|---|---|---|---|---|---|---|
| Trimarco et al., 2015 | Italy | Cross-over, double-blind RCT | N=23Men and women18–70 years’ oldDiagnosis of hypercholesterolemia not requiring statin use or people who are statin intolerant | 10 weeks (4 weeks for each treatment in the 2nd period) | 1st period(2 weeks)Placebo+dietary advicePeriod 2 (8 weeks) Randomization and cross-over of participantsGroup 1: Armolipid PlusPolycosanol: 10mgMonacolin K: 3mgBerberine: 500mgAstaxanthin: 0.5mgFolic acid: 200mgCoenzyme Q10: 2mgGroup 2: LopiGLIKMonacolin K: 3.3mgBerberine: 531.25mgMorus alba leaf extract (white berries): 200mg | Anthropometric characteristics:Waist circumferenceBMIBlood pressureBiochemical parametersLipid profile: TC, LDL-C, TG, HDL-CFasting glucoseInsulinHbA1c | Group 2 vs. Group 1:Statistically significant decrease (p<0.05):LDL-C (−48.9mg/dl)*56.6% of participants: <130mg/dlTC (−48.2mg/dl) |
| Heinz et al., 2016 | Germany | Double-blinded RCT | Initially: N=235Final: N=142Men and women18–70 years’ oldLDL-C: ≥160- ≤220mg/dl (LDL-C≥4.14≤5.69mmol/L) | 12 weeks | Group 1:Administration of red yeast rice supplement:Red yeast rice: 200mg & Monacolin K: 3mg (main target for LDL-C reduction)Folic acid: 200mg (secondary target for homocysteine reduction)Coenzyme Q10: 2mgAstaxanthin: 0.5mgGroup 2:Placebo treatment*Randomization was based on gender and plasma LDL-C concentration. | Anthropometric characteristics:Body weightHeightBMIWaist and hip circumferenceBlood pressureHeart rateCompletion of questionnaire:Current health statusCurrent health conditionCurrent medication- Physical activityBiochemical parameters (12-h fasting):TCLDL-CHDL-CTGHomocysteineHbA1cFasting glucoseFasting insulinCreatinineCreatine kinaseUric acidAspartate aminotransferaseAlanine aminotransferaseGamma-glutamyl transferaseCRP*Measurements were performed at each test (at the start of the intervention, 6 and 12 weeks after) | 6 weeks after intervention: Statistically significant reduction (p<0.05):LDL-C (−14.9%=159.7mg/dl)*53% of participants: <160mg/dlTC (−11.2%)LDL-C/HDL-C (−14.1%)12 weeks post intervention:Parameters that had changed at week 6 remained stableTG (−5.0%)* No significant changes were seen in the placebo group.** All other parameters remained unchanged and no intolerances or significant adverse events were observed. |
| D’Addato et al., 2017 | Italy | Double-blinded RCT | Initially: N=275Final: N=130Men and women18–75 years oldClinical diagnosis of mild-to-moderate hypercholesterolemiaLDL-C: 115–180mg/dlTC: 200–260mg/dlTG: <250mg/dl | 8 weeks | 1st period(4 weeks)Tips for starting an isocaloric and low-fat diet2nd Period (4 weeks) Randomization of participants+diet continuation:Group 1: BL (body lipid) (N=44)Monacolin K: 10mgBerberine: 500mgCoenzyme Q10: 2mgHydroxytyrosol: 5mgGroup 2: AM (Armolipid Plus) (N=42)Monacolin K: 3mgGroup 3: Placebo (N=44) | Medical history:Blood pressureHeart rateAnthropometric characteristics:Body weightBMIWaist circumferenceEnergy and macronutrient intake:Fat-saturated fatty acids-cholesterolProteinCarbohydrateBiochemical parameters:Lipid profile: TC, LDL-C, HDL-C, TGAST, ALTCPK, urea | Group 1 vs. 3:Statistically significant decrease (p<0.05):LDL-C (−43.8±12.6mg/dl)TC (−47.7±15.1mg/dl)Group 2 vs. 1:Statistically significant reduction (p<0.05):LDL-C (−31.1±12.8mg/dl)TC (−32.3±15.3mg/dl)Group 1 vs. 2:Statistically significant reduction (p<0.05):LDL-C (−12.5±18.8mg/dl)TC (−15.4±15.4mg/dl)*Adverse effects:Group 1: insomniaGroup 2: ear pain and rashGroup 3: lumbago |
| Giuseppe et al., 2017 | Italy | Double-blinded RCT | Initially: N=143Final: N=139Caucasian men and women>18 years oldBMI: 25.0–29.9kg/m2Low cardiovascular riskNon-smokersBlood pressure:SBP: <140mmHgDBP: <90mmHgDiagnosis of hypercholesterolemia:TC: 200–240mg/dlTG: <400mg/dl | 4 months | 1st period(1 month)Diet:600kcal deficit50% carbohydrates30% fat (300mg cholesterol)20% proteins35g dietary fiberPhysical activity:3–4 times/week 20–30min static bike ride2nd period (3 months) randomization+diet continuation+PDGroup 1: Berberol K administration (n=71)Monacolin K and KA: 10mgBerberine: 500mgSilymarin: 105mgGroup 2: Placebo administration (n=68) | Anthropometric characteristics:HeightWeightBMIWaist, abdominal and hip circumferenceBlood pressureBiochemical parametersLipid profile: TC, LDL-C, TG, HDLFasting glucoseFasting insulinAST, ALTCPKCRPTNF-aIL-6HOMA | 1st Group vs. 2nd Group:Statistically significant reduction (p<0.05):LDL-C (−27.8%=106.5±11.4mg/dl)TC (−20.5%=171.6±20.6mg/dl) |
| Magno et al., 2018 | Italy | Crossover RCT | Initially: N=30Final: N=19Caucasian Men and Women18–75 years oldLDL-C: ≥130- ≤180mg/dl | 20 weeks8 weeks for each treatment4 weeks abstinence | 1st Treatment (N=10):“Argicolina”:Monacolin K: 10mgl-arginineCoenzyme Q10Ascorbic acid2nd Treatment (N=9):“Normolip 5”:Monacolin K: 10mgCoenzyme Q10 | Anthropometric characteristics:HeightBody weightBMIBiochemical parameters:Fasting glucoseTCLDL-CHDL-CTGAspartate aminotransferasealanine gamma-aminotransferaseAminotransferaseGlutamyl transpeptidaseBlood pressureHeart rate* Measured at the beginning and end of each treatment | At the end of each treatment:1st treatment: Statistically significant reductions (p<0.05):LDL-C (−40.1mg/dl=113.1±12.8mg/dl)TC (−42.7mg/dl=178.3±16.3mg/dl)2nd treatment: Statistically significant improvements (p<0.05):LDL-C (−36.4mg/dl=116.7±15.4mg/dl)TC (−34.6mg/dl=187.0±17.1mg/dl)HDL-C (+2.6mg/dl=59.2±17.1mg/dl)* During abstinence: LDL-C levels increased to similar levels as before the intervention. |
| Mazza et al., 2018 | Italy | RCT | N=60Men and women>18 years oldBlood pressure:SBP: 140–159mmHgAnd/orDBP: 90–99mmHgDiagnosis of hypercholesterolemia:TC: ≥200mg/dlLDL-C: 130–190mg/dl | 1 month | Group 1: Administration of Diet+Supplement (Colenorm Plus) (n=30)Standardized Mediterranean diet regimen that weekly included:Cereals: 95.0%Vegetables, pasta and olive oil: 95.0%Yoghurt: 85.0%Fruits: 95.0%Fish and meat: 70.0%Red wine: 90.0%Eggs: 70.0%Cheese: 95.0%Monacolin K: 10mgOctacosanol: 12mgResveratrol: 20mgChromium picolinate: 50μgpiperine: 2.99mg2nd group: Dietary administration (n=30)Standardized Mediterranean diet regimen that weekly included:Cereals: 76.7%Vegetables, pasta and olive oil: 96.7%Yoghurt: 73.3%Fruits: 90.0%Fish and meat: 74.0%Red wine: 76.7%Eggs: 86.7%Cheese: 93.3% | Anthropometric characteristics:BMIHeart rateBlood pressureHeart rateSmokingBiochemical parametersLipid profile: TC, LDL-C, TG, HDLGlucoseAST, ALTCreatinine | 1st Group vs. 2nd Group:Statistically significant reduction (p<0.05):LDL-C (−23.88%)TC (−18.32%) |
| Domenech et al., 2019 | Spain | Double-blinded RCT | N=40Men and women35–75 years’ oldLow to moderate cardiovascular riskDiagnosis of hypercholesterolemia:TC: 200–250mg/dlLDL-C:>115mg/dlAbsence of hypolipidemic medication | 3 months | Group 1: Aquilea Colesterol administration (n=20)Monacolin K: 10mgPhytosterols: 1.5g- Hydroxytyrosol: 5mgVitamin E: 12mgGroup 2: Placebo administration (n=20) | Anthropometric characteristics:WeightBMIWaist circumferenceBlood pressureCardiovascular risk (REGICOR)MedDiet scoreBiochemical parameters:Lipid profile: TC, LDL-C, TG, HDL, non-HDL, apo-A1, apo-BGlucoseCreatinineAST, ALT, γGTLDH: Galactic dehydrogenaseCK: creatine kinaseAldolaseCRPIL-6 | 1st Group vs. 2nd Group:Statistically significant reduction (p<0.05):LDL-C (−19.7%)TC (−14.1%) |
| Ruscica et al., 2019 | Italy | Double-blinded RCT | Initially: N=33Final: N=32Men and women18–70 years oldLDL-C: 130–200mg/dlNon-smokersPrimary prevention for cardiovascular disease | 12 weeks | Group 1: Lactoflorene Colesterolo (N=16)Probiotic Bifidobacterium longum BB536Monacolin K: 10mgNiacin: 16mgCoenzyme Q10: 20mg2nd group: Placebo (N=16)*Pre-intervention instructions were given to start a euthermic and low-fat diet, which continued throughout the intervention | Anthropometric characteristics:HeightBody weightWaist circumferenceBlood pressureHeart rateAbdominal and visceral fatBiochemical parametersLipid profile: TC, LDL-C, HDL-C, non-HDL-C, TG, Apo-B, apo-A1, Lp(a), PCSK9Fasting glucoseHOMA-IRSerum uric acidHepatic transaminasesγ-Glutamyl transpeptidaseCPKFGF 19CRPLathosterolDietary sterols | 1st Group vs. 2nd Group:Statistically significant reduction (p<0.05):LDL-C (−45mg/dl)TC (−45mg/dl)Non-HDL-C (−45mg/dl) |
| Wang et al., 2019 | China | Double-blinded RCT | Initially: N=69Final: N=50Men and women≥18 years’ oldLDL-C: >100mg/dlTC: >200mg/dl | 3 months | Group 1 (N=16):RYR enriched with Monacolin K:RYR: 400mgMonacolin K: 8mgGroup 2 (N=17):RYR enriched with gamma-aminobutyric acid (GABA):RYR: 335mgGABA: 0.14mgGroup 3 (N=17):Placebo | Anthropometric characteristics:BMIBiochemical parameters:GlucoseInsulinLipid profile: TC, LDL-C, HDL-C, TGBlood pressureRenal function: BUN, creatinine, GFRLiver enzymes: SGOT, SGPT, total bilirubin, alkaline phosphatase, r-GTElectrolytes: sodium, potassium, phosphorus, calciumCRP | Group 1 vs 2 and 3:Statistically significant decrease (p<0.05):LDL-C (−31mg/dl)TC (−44.5mg/dl)* One person from Group 1 and 2 had an increase in liver enzymes. |
| Iskandar et al., 2020 | Indonesia | Double-blinded RCT | Initially: N=80Final: N=76Men and women18–65 years’ oldLDL-C: 100–159mg/dlTC: 200–239mg/dlNormal range of blood tests and laboratory tests (ALT, AST, blood urine, nitrogen, creatinine, eGFR, urinalysis and electrocardiogram) | 12 weeks | 1st period (4 weeks)Placebo administrationPeriod 2 (8 weeks) Randomization of participantsGroup 1: NutraforChol (N=40)Monacolin K: 3.75mgGugulipidium extract: 110mgChromium picolinate: 50μgGroup 2: Placebo (N=36)*In both groups, participants followed a low-fat and low-cholesterol diet and physical activity. | Anthropometric characteristics:HeightBody weightBMIBlood pressureHeart rateBiochemical parametersLipid profile: TC, LDL-CALT, ASTBlood urea nitrogenCreatinineeGFR | 1st Group vs. 2nd Group:Statistically significant reduction (p<0.05):LDL-C (−19.9%)TC (−15.9%)*Adverse effects:Group 1: indigestion, flatulence, bloating |
| Minamizuka et al., 2021 | Japan | RCT | Initially: N=32Final: N=18Men and women20–80 years oldLDL-C:139.98–179.8mg/dl (3.62–4.65mmol/L)No history of cardiovascular disease | 12 weeks | 1st period(4 weeks)Dietary intake:Fat: <30%Saturated fatty acids: <10%Polyunsaturated fatty acids: ≤10%Cholesterol: <300mg/dCarbohydrates: 50–60%Protein: 10–20%Monacolin K: 0mgPeriod 2 (8 weeks) Randomization of participants:Group 1: Red rice yeastRed rice yeast: 200mg/day2mg Monacolin K+continuation of dietGroup 2: Placebo-Continuation of Diet | Anthropometric characteristics:Body weightBMIWaist circumferenceBiochemical parameters:HbA1cTCLDL-CHDL-CTGNon-HDLapo-BAnkle-arm indexBrachial-ankle pulse wave velocity | 1st period:Non statistically significant differences between the 2 groups (p<0.05)Period 2:1st group vs 2nd group:Statistically significant decrease (p<0.05):LDL-C (−37.12mg/dl)TC (−35.57±22.04mg/dl)Non-HDL-C (−37.89 [−44.85, −31.71]mg/dl)*RYR caused no adverse effects on muscle, liver and kidney. |
| Cicero et al., 2021 | Italy | Double-blinded RCT | N=40Men and women25–65 years’ oldPrimary cardiovascular riskLDL-C: 150-190mg/dl | 3 months | 1st period(1 month)Healthy diet and physical activityPeriod 2 (2 months) Randomization of participants:Group 1: DIF1STAT (1capsule/day)Monacolin K: 2.5–2.9mg/capsule linear aliphatic alcohols titrated at 60% in octacosanols and niacinOlea europaea total extract of pomace and leaves (titrated at 15% in oleuropein)Camellia sinensis extractVitamins E, B6, B9, B12Group 2: Placebo | Anthropometric characteristics:Body weightHeightBMIWaist circumferenceBlood pressureCompletion of questionnaire:Personal historyDietary and smoking habitsPhysical activityPharmacological TreatmentBiochemical parameters:TCLDL-CHDL-CTGSUA (serum uric acid)CreatinineALT, AST, γ-GTCPKGFR | 1st Group vs. 2nd Group:Statistically significant reduction (p<0.05):TC (−12.25% vs. −1.8%, p<0.01)LDL-C (−28.7% vs. −1.1%, p<0.01)HDL-C (+4.99% vs. +0.9%, p<0.05)Non-HDL- C (−16.02% vs. −1.5%, p<0.01)SUA (−12.96%, p<0.05)Endothelial reactivity (+6.73% vs. −1.4%, p<0.01) |
Abbreviations: RCT; randomized clinical trial, RYR; red yeast rice, BMI; body mass index, TC; total cholesterol, LDL-C; low density lipoprotein cholesterol, HDL-C; high density lipoprotein cholesterol, TG; triglycerides, apo-A1; apolipoprotein-A, apo-B; apolipoprotein-B, SBP; systolic blood pressure, DBP; diastolic blood pressure, HbA1c; hemoglobin A1c, HOMA(-IR); Homeostatic Model Assessment for Insulin Resistance, CRP; C-reactive protein, AST; aspartate aminotransferase, ALT; alaline aminotransferase, γGT; gamma-glutamyl transferase, CPK; creatine phosphokinase, TNF-a; tumor necrosis factor alpha, IL-6; interleukin-6, FGF-19; fibroblast growth factor 19, BUN; blood urea nitrogen, SGOT; serum glutamic–oxaloacetic transaminase, SGPT; serum glutamic pyruvic transaminase, GFR; glomerular filtration rate, SUA; serum uric acid, MedDiet score; Mediterranean diet score.
Five of the included studies examined the effect of low doses of monacolin K (2–3.75mg/day) compared to either another supplemented group and a control group or just to a control group under placebo treatment. In the first cross-over clinical trial by Trimarco et al. (2015),20 the 1st group was administered with the dietary supplement “Armolipid Plus” containing 3mg of monacolin K, polycosanol, berberine, astaxanthin, folic acid and CoQ10 and the 2nd group was administered with the supplement “LopiGLIK” containing 3.3mg of monacolin K, berberine and Morus alba leaf extract for 10 weeks. The results showed a statistically significant reduction in LDL and total cholesterol in the 2nd group compared to the 1st (p<0.05). Moreover, the randomized double-blind clinical study by Heinz et al. (2016),21 used a RYR supplement containing 3mg monacolin K, folate and CoQ10 for 12 weeks and indicated a significant decrease in LDL, total cholesterol, LDL to HDL7 ratio compared to the control group (p<0.05). The study by Iskandar et al. (2020)22 used a dietary supplement containing 3.75mg of monacolin K, gugulipidium extract and chromium picolinate for 12 weeks. The study showed a significant reduction in LDL and total cholesterol compared to the control group (p<0.05). However, individuals in the intervention group experienced some adverse effects such as indigestion, flatulence and bloating. Also, the most recent study by Minamizuka et al. (2021)6 used RYR supplement containing 2mg of monacolin K for 12 weeks and showed a significant reduction in LDL, total and non-HDL cholesterol compared to the control group (p<0.05). Also, no severe treatment-related adverse effects were observed. Furthermore, the randomized double-blind clinical study by Cicero et al. (2021)23 used a supplement containing 2.5–2.9mg monacolin K along with linear aliphatic alcohols, niacin and Olea europaea for 8 weeks and indicated a significant decrease in LDL, total cholesterol, non-HDL cholesterol and a significant increase in HDL cholesterol compared to the control group (p<0.05).
On the other hand, 6 studies examined the efficacy of supplementation with higher doses of monacolin K compared either to another supplemented group or to a control group. In particular, the randomized double-blind clinical trial by Giuseppe et al. (2017)24 used a dietary supplement containing 10mg of monacolin K and KA (a type of monacolin), berberine and silymarin for 4 months and showed significant improvements in LDL and total cholesterol compared to the control group (p<0.05). The other study by Mazza et al. (2018)25 compared the dietary supplement “Colenorm Plus” containing 10mg of monacolin K, octocosanol, resveratrol, chromium pinolinate and piperine with a standardized Mediterranean diet for one month and showed significant reduction in LDL, total cholesterol in the monacolin K group compared to the control group (p<0.05). Moreover, the study by Domenech et al. (2019)26 used the dietary supplement “Aquilea Colesterol” containing 10mg of monacolin K, phytosterols, hydroxytyrosol and vitamin E for 3 months and showed significant reduction in LDL cholesterol and total cholesterol compared to the control group (p<0.05). In addition, the study by Ruscica et al. (2019)27 used the dietary supplement “Lactoflorene Colesterolo” containing 10mg of Monacolin K, niacin, CoQ10 and probiotic Bifidobacterium longum BB536 for 12 weeks and showed significant reduction in LDL, total cholesterol and non-HDL-cholesterol compared to the control group (p<0.05). Furthermore, the cross-over clinical trial by Magno et al. (2018)28 used two dietary supplements for 8 weeks each with 4 weeks of abstinence between them. The 1st group used the supplement “Argicolina” containing 10mg of monacolin K, l-arginine, CoQ10 and ascorbic acid and the 2nd group was supplemented with the supplement “Normolip 5” containing 10mg of monacolin K and CoQ10. Results showed significant reductions in LDL and total cholesterol in both groups (p<0.05). However, the reduction in LDL and total cholesterol levels in the 1st group was significantly higher compared to the 2nd group. The study by Wang et al. (2019)12 randomized participants in 3 different groups for 3 months. The first group was supplemented with a RYR containing 8mg of monacolin K, group 2 was supplemented with the same RYR enriched with gamma-aminobutyric acid, and group 3 was given a placebo. Results showed a significant reduction in LDL and total cholesterol levels in group 1 compared to the other groups (p<0.05). In addition, 2 participants reported elevated liver enzymes.
One study compared the effectiveness of two different doses of monacolin K on cholesterol levels. D’Addato et al. (2017)29 randomized participants in 3 groups for 8 weeks. Group 1 was given the dietary supplement “Body Lipid” containing 10mg of monacolin K, berberine, CoQ10 and hydroxytyrosol, group 2 was given the dietary supplement “Armolipid Plus” containing only 3mg of monacolin K and group 3 was given placebo. The results showed that LDL and total cholesterol levels were significantly reduced in group 1 compared to the other groups (p<0.05). In addition, a significant decrease in LDL and total cholesterol levels was shown in group 2 compared to group 3 (p<0.05). Adverse effects were observed in all 3 groups such as insomnia, ear pain, rashes and back pain.
DiscussionThe main purpose of this review was to examine the efficacy and safety of monacolin K supplementation in adult patients with hypercholesterolemia. All the included studies demonstrated a beneficial effect of monacolin K regardless the dose and duration of supplementation. Half of the included studies (N=6) used higher doses of monacolin K (8–10mg) and five studies examined the effect of lower doses of monacolin K (2–3.75mg) on hypercholesterolemia. The maximum duration of supplementation was 12 weeks for all studies. Regarding the safety of supplementation, 3 studies using both high and low doses of monacolin K reported side effects such as insomnia, indigestion, flatulence, bloating and increase in liver enzymes. Notably, the study by D’Addato et al. (2017)29 which used both high (10mg/day) and low doses (3mg/day) of monacolin K reported side effects in all groups. Therefore, it seems that patients treated with monacolin K should be monitored regardless the dose and duration of supplementation.
Our findings seem to be in line with previous reviews that examined the potential effect of monacolin K in people suffering from hypercholesterolemia. Particularly, the previous systematic review and meta-analysis by Gerards et al. (2015),8 included 20 randomized controlled trials in order to examine the effect monacolin K in patients with hypercholesterolemia. The dosage of monacolin K used ranged between 2.4mg and 24mg for a period of 6 weeks to 168 weeks. The meta-analysis indicated that monacolin K treatment had a significant effect on reducing LDL cholesterol levels. However, in contrast to the present review, the Gerards et al. (2015)8 study included several clinical trials assessed with low methodological quality. Another meta-analysis with similar findings is the one conducted by Li et al. (2014)30 which aimed to investigate the safety and efficacy of RYR and monacolin K in people with dyslipidemia. The analysis included 13 randomized controlled trials with 804 participants that were supplemented with monacolin K in dosages ranged between 2mg and 11.4mg for a period of 4 weeks and 24 weeks. The results demonstrated that RYR and monacolin K had a significant effect on reducing LDL, total cholesterol and TG8 levels. Notably, no serious adverse effects were reported. Moreover, our findings are also in line with those of a previous review by Yang et al. (2012)15 that aimed to examine the effect of RYR in various diseases including hypercholesterolemia. The review included 11 clinical trials using monacolin K in doses varied from 1.02mg to 5.1mg for a period between 8 weeks and 4.5 years. The results indicated that monacolin K significantly contributed to the reduction of LDL and total cholesterol. In addition, the authors supported that the supplementation of RYR has the same efficacy as statin, regardless of its combination with other dietary supplements. Nevertheless, based on our findings, it seems that any dose of monacolin K ranged between 3 and 10mg is effective in reducing cholesterol levels in people suffering from hypercholesterolemia. Overall, we could assume that RYR is an effective lipid-lowering dietary supplement regardless monacolin K content. However, since EFSA suggested using a lower dose of monacolin K (3mg/day) due to safety concerns an attention should be given when prescribing high doses RYR for statin-intolerant patients.
Regarding the safety, it seems that any dose may cause side effects and therefore all treated patients should be routinely monitored. Previous research support that the safety of RYR, generally is not related to the monacolin content, per se, but to variability in monacolin content and especially the presence of contaminants (particularly citrinin, a nephrotoxic product of rice fermentation). Therefore, it is very important to focus on the quality of production of RYR supplements together with monacolin K dose control.31 Recent concerns have been reported regarding the safety of RYR after publication of studies claiming toxicity.18 However, according to a recent meta-analysis of 53 RCTs with a total of 8.535 patients the supplementation of monacolin K is not associated with an increased risk of muscular adverse events.16 Also, the study demonstrated a reduced risk of nonmuscular adverse events and serious adverse events compared to the control group. Notably, our findings regarding the safety of monacolin K are more conflicting. Three studies using both low and high doses of monacolin K reported side effects indicating the need of monitoring patients treated with monacolin K regardless the dose of supplementation Indeed, in the study by D’Addato et al. (2017)29 all groups showed side effects after the treatment indicating that factors other than monacolin K dose may cause side effects and should be further investigated.
Strengths and limitationsThe key strength of the current review is the sole inclusion of RCTs, a study design, which is less likely to be biased and affected by confounding variables. The majority of the included studies were assessed as high quality (11/12) thus ascertaining reliability of the current review. Additionally, another strength is the fact that the current review included recent studies published during the last 10 years. Yet, our review has also some limitations. One limitation is the small number of studies included in the current analysis, while most of the studies have been conducted in the same country (Italy). Furthermore, all studies used monacolin k together with other dietary supplements that differed among the studies and therefore it was difficult to identify the effect of monacolin K as a single supplement. Moreover, another limitation is the different definition of hypercholesterolemia used in the included studies.
ConclusionsIn conclusion, this review has highlighted the potential effect of monacolin K together with other dietary components in people with hypercholesterolemia. It seems that even in lower doses equal to 3mg/day, monacolin K exerts potential cholesterol-lowering effects although the number of relative studies is very low. Regarding the safety of monacolin K supplementation, findings seem to be more controversial and therefore, it is suggested for all patients treated with monacolin K to be routinely monitored regardless the dose of supplementation. Interestingly, the quality of the RYR supplement seems to play a role on the efficacy of treatment and should be taken into consideration. Overall, the current review seems to support EFSA recommendation for lowering the recommended intake of monacolin K from 10 to 3mg per day although more RCTs are required to confirm the efficacy and safety of the lower dose of monacolin K supplementation.
Ethical considerationsNot applicable.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.
None.
Red yeast rice.
Randomized controlled trials.
Low density lipoprotein.