Diet and exposure to certain chemical compounds related to industrial development could be responsible for the increase in the incidence of human reproductive diseases and the consequent decrease in reproductive function worldwide. The role of diet and environment in fertility has received more attention in recent years because nutrition and exposure to contaminants can affect fecundity in women of reproductive age. Here, we review how diet could affect reproduction. Furthermore, we will review how the most ubiquitous contaminants could disrupt endometrial receptivity and implantation, focusing also on clinical studies performed in the last years.
La dieta y la exposición a determinados compuestos químicos relacionados con el desarrollo industrial podrían ser responsables del aumento de la incidencia de las enfermedades reproductivas humanas y de la consiguiente disminución de la función reproductiva en todo el mundo. El papel de la dieta y el medio ambiente en la fertilidad ha recibido más atención en los últimos años porque la nutrición y la exposición a contaminantes pueden afectar a la fecundidad de las mujeres en edad reproductiva. Aquí revisamos cómo la dieta puede afectar a la reproducción. Además, revisaremos cómo los contaminantes más ubicuos podrían alterar la receptividad endometrial y la implantación, centrándonos también en los estudios clínicos realizados en los últimos años.
Since the mid-twentieth century, an increase in the incidence of human reproductive diseases and the consequent decrease in reproductive function worldwide have been reported. This fact can hardly be explained by genetic changes. It is thought that changes in diet and exposures to certain compounds related to industrial development could be responsible for these trends. A very high number of chemical compounds used in food and chemical industries to which we are exposed daily and the uncertainty about their clinical consequences have generated a reasonable concern in the scientific community and in the society. In this review, we will go over the relationships between diet and environmental factors affecting the main reproductive clinical parameters studied so far.
Diet and female fertilityThe role of diet in fertility has received more attention in recent years because diet can affect fecundity in women of reproductive age. However, nutrition is difficult to measure and human fertility is difficult to assess directly. Therefore, the epidemiological literature focuses on findings suggesting an association between nutrition and proxy measures such as embryo quality, ovulation rates, premature ovarian failure, successful implantation probability of blastocyst formation, and clinical outcomes in patients undergoing in vitro fertilization (IVF) or assisted reproductive technologies (ART). In this review, relevant dietary factors include minerals and vitamins, folic acid, whole grain products, vegetables, fruits, fatty acids, dairy products, red meat, soy products, and fish (Fig. 1).
In a randomized, double-blind, placebo-controlled trial, Modarres et al. (2018) found that selenium supplementation for 8 weeks in lymphocytes from 40 infertile women with polycystic ovary syndrome (PCOS) who were eligible for IVF significantly increased gene expression of PPAR-γ, which contributes to the regulation of ovarian function.
Vitamin B6 improves reproductive performance because it increases conception rate, reduces the risk of miscarriage in early pregnancy, and contributes to the success of IVF/intracytoplasmic sperm injection (ICSI) treatment in subfertile women (Vujkovic et al., 2010). However, other authors found little evidence of an association between pregnancy rates after IVF and dietary intake of calcium, potassium, magnesium or vitamin D (Wise et al., 2017).
Folic acidThe association between folate supplementation, infertility, and ART outcomes has been investigated in prospective cohort studies and small randomized controlled trials (RCT). In these studies, folate concentrations higher than those currently recommended for the prevention of neural tube defects (NTDs) showed a lower risk of ovarian infertility, a lower incidence of sporadic anovulation, positive effects on fertility, increased adherence in preconception, better oocytes quality and a higher number of mature oocytes, and a shorter time to pregnancy among users compared to non-users (Gaskins et al., 2012; Cueto et al., 2016; Louis et al., 2016).
Whole grainsConsumption of cereals positively influenced embryo quality at the cleavage stage (Braga et al., 2015). A prospective study demonstrated that higher preconceptional intake of whole grains (due to lignin, the hormonally active compound in whole grains) showed higher likelihood of live birth (Gaskins et al., 2016).
Vegetables and fruitsConsumption of vegetables in the months before conception significantly improved preimplantation quality of embryos (Braga et al., 2015).
Periconceptional consumption of fruit had a positive effect on cleavage stage embryo quality (Braga et al., 2015). The same authors observed an increase in formation and hatched blastocysts in patients who consumed fruit.
Vegetables and fruits are associated with lower antioxidant stress and higher antioxidant status, both necessities for normal fertilization and preimplantation development of the embryo (Braga et al., 2015).
Fatty acidsFatty acids are important substrates in oocyte maturation and embryo implantation. Moran et al. (2016) confirmed that a change in preconceptional fatty acid intake was associated with improved pregnancy rates in overweight and obese women (study of 38 women) receiving IVF treatment. Polyunsaturated fatty acids (PUFAs), particularly omega-3 long-chain fatty acids (omega-3 PUFAs), may influence oocyte quality, endometrial receptivity, and embryo implantation. The effect of omega-6 PUFAs on fertility is less clear. While trans fatty acids (TFA) increase insulin resistance, which negatively affects the ovulation process (Vujkovic et al., 2010; Jungheim et al., 2013; Wise et al., 2017). Furthermore, Jungheim et al. (2013) demonstrated that a higher serum linoleic acid to α-linolenic acid ratio was associated with a significantly increased chance of pregnancy and embryo implantation in women undergoing IVF.
Dairy productsHuman studies that examined the relationship between dairy consumption and fertility yielded conflicting results. Afeiche et al. (2016) showed in a prospective cohort of 232 women undergoing infertility treatment with ART that dairy consumption was associated with live birth in women≥35 years. However, dairy consumption was not associated with ovarian response to stimulation, embryology, implantation, or clinical pregnancy outcomes. Similarly, Greenlee et al. (2003) concluded in a prospective cohort study that women who consumed three or more glasses of milk/day had a 70% lower risk of infertility than women who did not consume milk. Wise et al. (2017) conducted 2 preconception cohort studies, one in the USA (1300 women) and one in Denmark (11,126 women). They found that total dairy and milk (low-fat) consumption was associated with increased fertility only among women aged 30 years in the Danish cohort but not in the USA cohort. In contrast, Chavarro et al. (2007) found no association between total dairy consumption and the risk of infertility due to anovulation.
Read meatsRed meat can be a good source of protein and other essential nutrients; however, it also contains high levels of saturated fat. Braga et al. (2015) demonstrated a negative impact on the likelihood of blastocyst formation in women from an infertility cohort who consumed red meat prior to IVF treatment. These authors suggested that the negative effect of red meat consumption on embryonic development and likelihood of pregnancy may be due to increased absorption and accumulation of advanced glycation end products, which are correlated with poor follicular and embryonic development and lower pregnancy. In the same vein, Chavarro et al. (2008) conducted a cohort study of pregnancy follow-up in 18,555 women. They found that animal protein consumption was associated with an increased risk of ovarian infertility.
Soy-based productsPhyotoestrogens from soy are isoflavones with estrogenic effects that could improve ovulation. In a prospective cohort of women undergoing IVF in the USA, intake of 12mg phytoestrogen/day increased live births by 77% during ART compared to women who did not consume soy products (Vanegas et al., 2015). In addition, higher isoflavone concentrations (120mg/day) increased live birth rates in a RCT in couples undergoing infertility treatment with clomiphene citrate (Shahin et al., 2008), or increased endometrial thickness and sustained pregnancy rates (1500mg/day) in women undergoing intrauterine insemination and IVF (Unfer et al., 2004). Similar results were obtained in a cohort study conducted by Chavarro et al. (2008) during an 8-year follow-up period in 18,555 women. They demonstrated that consumption of protein from vegetables sources (e.g., soy) was associated with a significantly lower risk of ovarian infertility in women older than 32 years. Although there is no clear explanation for this association.
FishAccording to European Food Safety Authority (EFSA), fish has a good lipid profile and provides long-chain omega-3 PUFA, a component of the dietary pattern associated with good health, and low saturated fat. Similarly, seafood is a good source of long-chain omega-3 PUFA. The EFSA has linked normal fish consumption during pregnancy with positive neurodevelopmental effects in children (EFSA, 2015). In addition, high fish intake has been linked to a higher likelihood of blastocyst formation and hatching (Braga et al., 2015).
Healthy eating habitsThe purpose of this section is to examine the relationship between dietary quality and female fertility outcomes. There is no association between adherence to healthy dietary patterns (high intakes of vegetables, fruits, fish, and whole grains) before pregnancy and the risk of pregnancy loss. However, high adherence to the “Mediterranean” dietary pattern (high intake of olive oil, vegetables, fruits, nuts, fish, and legumes) by the couple may improve the chance of pregnancy after IVF/ICSI treatment (Vujkovic et al., 2010). In addition, a healthy dietary pattern combined with body weight control and increased physical activity may have beneficial effects on fertility in healthy women to prevent infertility due to problems with ovulation (Toledo et al., 2011).
In summary, embryo developmental potential and clinical outcomes can be influenced by dietary intake. Thus, a healthy diet and lifestyle for the women in the periconceptional months can significantly improve fertility or preimplantation quality of embryos after a ART.
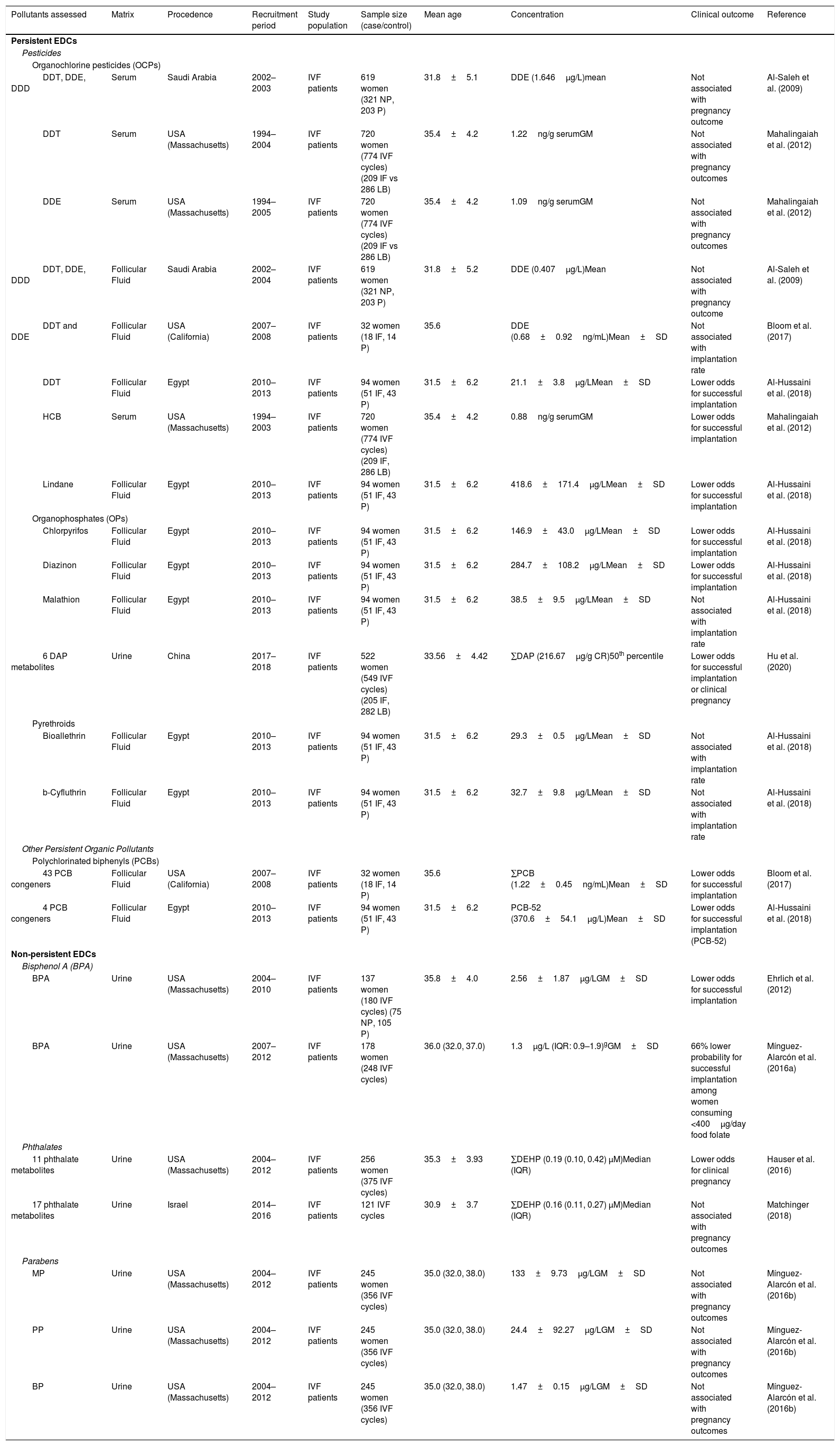
Environmental factors affecting implantation and pregnancyEmbryo implantation is a tightly coordinated process in which a competent blastocyst-stage embryo adheres to and invades a receptive endometrium to enable placentation and initiation of pregnancy. This process can only occur during a limited period in which the endometrium is receptive, known as the window of implantation. Therefore, it is essential that there is synchrony between the acquisition of competence for implantation of the blastocyst and the receptive state of the endometrium. Both events are finely orchestrated by maternal hormones, particularly the steroid hormones estrogen and progesterone (Zhang et al., 2013). Foundational evidence on the relationship between exposures to environmental pollutants and the ability to initiate and achieve a pregnancy has come from epidemiological studies on populations undergoing in vitro fertilization (IVF treatments) and animal models with controlled exposure. Foundational evidence on the relationship between exposures to environmental pollutants and the ability to initiate and achieve a pregnancy has come from epidemiological studies on populations undergoing in vitro fertilization and animal models with controlled exposure (Table 1).
Epidemiological studies assessing the relationship between environmental pollutant exposure and implantation or pregnancy outcome.
| Pollutants assessed | Matrix | Procedence | Recruitment period | Study population | Sample size (case/control) | Mean age | Concentration | Clinical outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Persistent EDCs | |||||||||
| Pesticides | |||||||||
| Organochlorine pesticides (OCPs) | |||||||||
| DDT, DDE, DDD | Serum | Saudi Arabia | 2002–2003 | IVF patients | 619 women (321 NP, 203 P) | 31.8±5.1 | DDE (1.646μg/L)mean | Not associated with pregnancy outcome | Al-Saleh et al. (2009) |
| DDT | Serum | USA (Massachusetts) | 1994–2004 | IVF patients | 720 women (774 IVF cycles) (209 IF vs 286 LB) | 35.4±4.2 | 1.22ng/g serumGM | Not associated with pregnancy outcomes | Mahalingaiah et al. (2012) |
| DDE | Serum | USA (Massachusetts) | 1994–2005 | IVF patients | 720 women (774 IVF cycles) (209 IF vs 286 LB) | 35.4±4.2 | 1.09ng/g serumGM | Not associated with pregnancy outcomes | Mahalingaiah et al. (2012) |
| DDT, DDE, DDD | Follicular Fluid | Saudi Arabia | 2002–2004 | IVF patients | 619 women (321 NP, 203 P) | 31.8±5.2 | DDE (0.407μg/L)Mean | Not associated with pregnancy outcome | Al-Saleh et al. (2009) |
| DDT and DDE | Follicular Fluid | USA (California) | 2007–2008 | IVF patients | 32 women (18 IF, 14 P) | 35.6 | DDE (0.68±0.92ng/mL)Mean±SD | Not associated with implantation rate | Bloom et al. (2017) |
| DDT | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | 21.1±3.8μg/LMean±SD | Lower odds for successful implantation | Al-Hussaini et al. (2018) |
| HCB | Serum | USA (Massachusetts) | 1994–2003 | IVF patients | 720 women (774 IVF cycles) (209 IF, 286 LB) | 35.4±4.2 | 0.88ng/g serumGM | Lower odds for successful implantation | Mahalingaiah et al. (2012) |
| Lindane | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | 418.6±171.4μg/LMean±SD | Lower odds for successful implantation | Al-Hussaini et al. (2018) |
| Organophosphates (OPs) | |||||||||
| Chlorpyrifos | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | 146.9±43.0μg/LMean±SD | Lower odds for successful implantation | Al-Hussaini et al. (2018) |
| Diazinon | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | 284.7±108.2μg/LMean±SD | Lower odds for successful implantation | Al-Hussaini et al. (2018) |
| Malathion | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | 38.5±9.5μg/LMean±SD | Not associated with implantation rate | Al-Hussaini et al. (2018) |
| 6 DAP metabolites | Urine | China | 2017–2018 | IVF patients | 522 women (549 IVF cycles) (205 IF, 282 LB) | 33.56±4.42 | ∑DAP (216.67μg/g CR)50th percentile | Lower odds for successful implantation or clinical pregnancy | Hu et al. (2020) |
| Pyrethroids | |||||||||
| Bioallethrin | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | 29.3±0.5μg/LMean±SD | Not associated with implantation rate | Al-Hussaini et al. (2018) |
| b-Cyfluthrin | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | 32.7±9.8μg/LMean±SD | Not associated with implantation rate | Al-Hussaini et al. (2018) |
| Other Persistent Organic Pollutants | |||||||||
| Polychlorinated biphenyls (PCBs) | |||||||||
| 43 PCB congeners | Follicular Fluid | USA (California) | 2007–2008 | IVF patients | 32 women (18 IF, 14 P) | 35.6 | ∑PCB (1.22±0.45ng/mL)Mean±SD | Lower odds for successful implantation | Bloom et al. (2017) |
| 4 PCB congeners | Follicular Fluid | Egypt | 2010–2013 | IVF patients | 94 women (51 IF, 43 P) | 31.5±6.2 | PCB-52 (370.6±54.1μg/L)Mean±SD | Lower odds for successful implantation (PCB-52) | Al-Hussaini et al. (2018) |
| Non-persistent EDCs | |||||||||
| Bisphenol A (BPA) | |||||||||
| BPA | Urine | USA (Massachusetts) | 2004–2010 | IVF patients | 137 women (180 IVF cycles) (75 NP, 105 P) | 35.8±4.0 | 2.56±1.87μg/LGM±SD | Lower odds for successful implantation | Ehrlich et al. (2012) |
| BPA | Urine | USA (Massachusetts) | 2007–2012 | IVF patients | 178 women (248 IVF cycles) | 36.0 (32.0, 37.0) | 1.3μg/L (IQR: 0.9–1.9)gGM±SD | 66% lower probability for successful implantation among women consuming <400μg/day food folate | Mínguez-Alarcón et al. (2016a) |
| Phthalates | |||||||||
| 11 phthalate metabolites | Urine | USA (Massachusetts) | 2004–2012 | IVF patients | 256 women (375 IVF cycles) | 35.3±3.93 | ∑DEHP (0.19 (0.10, 0.42) μM)Median (IQR) | Lower odds for clinical pregnancy | Hauser et al. (2016) |
| 17 phthalate metabolites | Urine | Israel | 2014–2016 | IVF patients | 121 IVF cycles | 30.9±3.7 | ∑DEHP (0.16 (0.11, 0.27) μM)Median (IQR) | Not associated with pregnancy outcomes | Matchinger (2018) |
| Parabens | |||||||||
| MP | Urine | USA (Massachusetts) | 2004–2012 | IVF patients | 245 women (356 IVF cycles) | 35.0 (32.0, 38.0) | 133±9.73μg/LGM±SD | Not associated with pregnancy outcomes | Mínguez-Alarcón et al. (2016b) |
| PP | Urine | USA (Massachusetts) | 2004–2012 | IVF patients | 245 women (356 IVF cycles) | 35.0 (32.0, 38.0) | 24.4±92.27μg/LGM±SD | Not associated with pregnancy outcomes | Mínguez-Alarcón et al. (2016b) |
| BP | Urine | USA (Massachusetts) | 2004–2012 | IVF patients | 245 women (356 IVF cycles) | 35.0 (32.0, 38.0) | 1.47±0.15μg/LGM±SD | Not associated with pregnancy outcomes | Mínguez-Alarcón et al. (2016b) |
Abbreviations: BP, butylparaben; BPA, Bisphenol A; CR, creatinine; DAP, dialkilphosphate; DDD, dichlorodiphenyldichloroethane; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichlorethane; DEHP, di-(2-ethyl-hexyl) phthalate; GM, geometric mean; HCB, hexachlorobenzene; IF, implantation failure; IQR, interquartile range; IVF, in vitro fertilization; LB, live birth; MP, methylparaben; NP, non-pregnancy; P, pregnancy; PCB, polychlorinated biphenyl; PP, propylparaben, SD, standard deviation.
Dialkilphosphate metabolites were diethylthiophosphate (DETP), diethylphosphate (DEP), diethyldithiophosphate (DEDTP), dimethylthiophosphate (DMTP), dimethyl-phosphate (DMP), dimethyldithiophosphate (DMDTP).
OCPs are characterized by having at least one chlorine in their chemical structure. The most abundant OCPs in the environment are dichlorodiphenyltrichlorethane (DDT) and its degradation products, hexachlorocyclohexanes such as lindane and hexachlorobenzene, methoxychlor or endosulfan. So far, only three epidemiological studies evaluated the impact of OCPs in biological fluids, specifically DDT and its metabolites, lindane and hexachlorobenzene, on embryo implantation and achievement of clinical pregnancy in women undergoing IVF treatments (Al-Saleh et al., 2009; Mahalingaiah et al., 2012; Al-Hussaini et al., 2018). Exposure to DDT and its metabolites, measured in serum or follicular fluid, were not associated with lower pregnancy rates in studies conducted in Saudi Arabia (Al-Saleh et al., 2009) or the US (Mahalingaiah et al., 2012). However, in an Egyptian population, high concentrations of DDT in follicular fluid were significantly associated with a lower implantation rate (Al-Hussaini et al., 2018). Curiously, the geometric mean of DDT levels is 20 times higher in the Egyptian population (Al-Hussaini et al., 2018) than in the population of Saudi Arabia (Al-Saleh et al., 2009). The blockage of embryo implantation may be mediated by the effect of OCPs on embryo transport or development or by a direct effect on preimplantation uterine development. Hall et al. separately analyzed development and embryo transport in mice exposed to methoxychlor during the preimplantation period. They observed a delay in embryonic development and a slowing of embryo transport through the oviduct following exposure (Hall et al., 1997). Therefore, endometrial function seems to be compromised after acute exposure to OPCs. This finding aligns with the observations of Al-Hussaini et al. of a negative correlation between the levels of lindane and DDT in follicular fluid and the endometrial thickness of patients undergoing IVF (Al-Hussaini et al., 2018).
Organophosphate pesticides (OPs)OPs are compounds characterized by having at least one phosphorus in their chemical structure. They were used as alternative pesticides to OCPs, although they continue to bioaccumulate in the environment and have adverse effects on human health. The most used OPs are glyphosate, chlorpyros, diazinon and malathion. Two epidemiological studies, one on an Egyptian population and one on a Chinese population, evaluated the association between organophosphate exposure in the pre-conception period and embryo implantation in women undergoing IVF treatments. The study of the Egyptian population observed a lower implantation rate associated with higher concentrations of chlorpyros and diazinon, but not with malathion levels (Al-Hussaini et al., 2018). In the Chinese population study, women with high levels of dialkilphosphate metabolites had lower odds of successful implantation (Hu et al., 2020) [Table 46.1]. Studies in animal models evaluated the effect of exposure to another OP, glyphosate, on endometrial function and embryonic implantation. Rats exposed to glyphosate alone or in combination with endosulfan (an organochlorine pesticide) during the neonatal period exhibit an increase in post-implantation losses (Ingaramo et al., 2016, 2017). These deficiencies in maintaining early pregnancy seem to be due to an altered decidualization response, since morphological alterations at implantation sites have been described, and are associated with lower expression of estrogen and progesterone receptors, increased proliferation and alterations in uterine signaling (Ingaramo et al., 2016, 2017). In addition to these long-term disturbances following neonatal exposure to glyphosate, short-term disturbances also occur. Importantly, there are multiple windows of susceptibility and even transgenerational effects related to alterations in endometrial physiology after exposure to different environmental pollutants. For example, in animal models, exposure to glyphosate during embryonic development increases the rate of pre-implantation losses in the F1 generation (Milesi et al., 2018). This disruption of molecular targets essential for endometrial receptivity seems to be caused by long-term epigenetic alterations of estradiol signaling (Lorenz et al., 2019), which could be maintained throughout the individual's life and even subsequent generations.
PyrethroidsPyrethroids are a group of artificial insecticides developed to emulate the effects of pyrethrins isolated from chrysanthemum. Due to their biodegradability, today they are the most-used insecticides. Among the more than 1000 existing pyrethroids, the most commonly used are bioallethrin, B-cyfluthrin, and cypermentrin. In an epidemiological study on pyrethroids, Al-Hussaini et al. did not observe any relationship between bioallethrin and B-cyfluthrin and alterations in implantation rates (Al-Hussaini et al., 2018). However, in animal models, exposure to another commonly used pyrethroid, cypermentrin, is detrimental to embryo implantation (Zhou et al., 2018; Singh et al., 2020). Adult exposure to cypermentrin appears to decrease embryo implantation, disrupting estradiol and progesterone signaling and leading to lower expression of Hoxa10, a downstream effector essential for endometrial receptivity (Zhou et al., 2018). These effects of cypermentrin exposure are not exclusive to adults; after exposure during embryonic development, increased preimplantation and post-implantation losses were observed in rodents. These alterations in embryo implantation also appear to be caused by alterations in the expression of essential molecules for uterine integrity and function, such as Hoxa10 and α-SMA (Singh et al., 2020).
Polychlorinated biphenyls (PCBs)PCBs represent a complex mixture of 209 compounds used in heat exchange fluids, electrical transformers, as additives in paints or in the manufacture of plastics.
Their estimated half-life is approximately 10 years. Non-occupational exposure to PCBs occurs mainly through diet (particularly via contaminated fish and meat) but also through inhalation and dermal contact with contaminated soils and sediment (Fernández-González et al., 2015). Epidemiological studies suggest that exposure to PCBs negatively affects embryo implantation. Specifically, in a population of Egyptian women undergoing IVF treatment, embryo implantation negatively correlated with follicular fluid levels of PCB-52, but not with the three other PCBs analyzed (Al-Hussaini et al., 2018). At least in part, the anti-implantation effects of PCBs seem to be due to insufficient endometrial preparation to initiate or maintain pregnancy, since women with higher levels of PCBs reportedly have decreased endometrial thickness (Bloom et al., 2017).
Bisphenol A (BPA)BPA is a synthetic chemical used in the manufacture of materials present in numerous common consumer products, such as plastic bottles, food and beverage can liners, water pipes, dental sealants and dental composites. Continued exposure of products containing BPA to light and heat, contact with cleaning agents and product aging can result in increased leaching of BPA into food and drink. Human exposure to BPA can occur by ingestion, inhalation and dermal absorption. The impact of BPA exposure on human embryonic implantation was evaluated only in the EARTH study cohort, which includes women undergoing IVF treatments in the US. Investigators observed increased probability of implantation failure in women with higher levels of BPA in their urine (Ehrlich et al., 2012). However, this relationship did not appear in a two-year expansion of the study cohort (with almost twice as many participants evaluated) (Mínguez-Alarcón et al., 2016a). In the same cohort, Minguez-Alarcón et al. observed that consumption of 400μg/day of dietary folate protected against the anti-implantation effects of BPA; women with lower folate consumption and higher levels of BPA in their urine had a 66% lower probability of embryo implantation. These data highlight the importance of considering the set of factors to which the individual is exposed when analyzing exposure outcomes in human populations. In addition, studies must consider the possibility of modifying the reproductive effects of excessive exposure to different EDCs by reducing compound exposure or increasing consumption of substances that reverse the biological effects of these compounds.
PhthalatesPhthalates are a family of synthetic chemicals used in many consumer products to provide flexibility and durability to plastic or to provide a matrix in cosmetics. They can be found in a wide variety of products from vinyl slabs and tiling to adhesives, detergents, lubricants, medical devices, pharmaceuticals (such as the coating of certain oral drugs), clothing, food packaging and toys. Phthalates are not covalently bound to plastic, allowing them to be released to the environment, which, along with their widespread use, makes them ubiquitous contaminants (Wittassek et al., 2011). The main sources of human exposure are food and consumer products, such as cosmetics. Once ingested, inhaled or absorbed, phthalates have a low half-life in the body. They are rapidly hydrolyzed in their bioactive monoesters, some of which can subsequently be metabolized by oxidation or phase II conjugation. Metabolites are excreted as glucuronides, mainly through urine and feces (Wittassek et al., 2011). Interestingly, young women are more exposed than men of the same age, possibly due to increased use of cosmetics (Wittassek et al., 2011), which highlights the need to identify possible adverse effects of these compounds on female reproductive health.
Two epidemiological studies evaluated the relationship between urinary levels of phthalate metabolites in cohorts of women undergoing IVF treatments in the US and Israel. In the US-based EARTH study cohort, after multivariate adjustment, women with higher urinary concentrations of di-(2-ethyl-hexyl) phthalate (DEHP) metabolites were less likely to have a clinical pregnancy (Hauser et al., 2016). However, this association was not observed in the Israeli cohort (Machtinger et al., 2018). These differences could be due to other exposures that were not considered or to the fact that the Israeli population are younger and had slightly lower phthalate levels than those of the US cohort.
ParabensParabens are a group of chemicals commonly used as antimicrobial preservatives in personal care products, drugs and food. Human exposure to parabens can occur by ingestion, inhalation, or dermal absorption. After absorption, parabens do not accumulate in the body, but are metabolized by esterases, conjugated, and excreted through the urine, bile and feces (Nowak et al., 2018). Parabens are suspected to have estrogenic activity, which increases with increasing length and derivation of the alkyl chain (Nowak et al., 2018). However, significant associations between urinary paraben concentrations and reproductive clinical outcomes such as implantation or clinical pregnancy rates were not observed in the EARTH study cohort (Mínguez-Alarcón et al., 2016b).
Mechanism of action of environmental pollutants on embryo implantationThe simultaneous presence of two components, a competent blastocyst and an appropriately receptive endometrium, is crucial for successful embryo implantation. Both milestones rely primarily on steroid hormone signaling, and any defect or excess of these hormones can prevent embryo implantation (Zhang et al., 2013). Since xenobiotics have both a direct and indirect effect on the signaling of these steroid hormones, embryonic development, endometrial receptivity, and their interaction during the embryo implantation process are potential targets of their effect. Next, we present the common effects of different xenobiotics with EDC activity on key processes for proper embryo implantation. Some of the anti-implantation effects of xenobiotics may be due to deleterious effects on pre-implantation embryo development and transport along the oviduct. Levels of various EDCs (polychlorinated biphenyls, polybrominated diphenyl ethers, organochlorine pesticides or trace metals) in follicular fluid of women undergoing IVF treatment were associated with a lower probability of embryo fertilization and lower embryo quality during development (Bloom et al., 2011). This is in line with the delayed embryonic development observed in mice exposed to various organochlorine pesticides and BPA during the first days of pregnancy (Xiao et al., 2011). These rodents also had slower embryo transport along the oviduct (Xiao et al., 2011), which could increase asynchrony between the competent embryo and the receptive endometrium, thus hindering embryo implantation.
In addition, in vitro exposure models using embryos from different animal species indicated that numerous EDCs, such as pesticides, PCBs, BPA, triclosan or phthalates, at concentrations assumed to have no negative consequences for human health, have embryotoxic effects. Notably, the embryotoxicity of commercial pesticide products is not usually due to the active compound, which is typically measured in human epidemiological studies, but is probably attributable to the presence of secondary ingredients. This observation highlights the need for non-targeted studies to identify excipients or toxic by-products that may be common and responsible for negative effects on fertility. The effect of EDCs on preimplantation embryonic development appears to be due to lower proliferation, deregulated autophagy and increased apoptosis in the exposed embryos (Amstislavksy et al., 2003), leading to fewer embryonic cells and reduced ability to develop to the blastocyst stage. The embryos that do develop to the blastocyst stage are of poor quality. These alterations appear to be due, among other reasons, to increased ROS production (Chu et al., 2013). Although other xenobiotics, such as dioxins, do not have embryo-toxic effects a priori, exposure to them can also negatively affect the development potential of embryos. For example, after exposure to TCDD, PCBs or phthalates, epigenetic alterations occur that affect the expression of necessary genes for differentiation during these early stages of development and/or may affect fetal development (Kalo et al., 2019).
Impact of environmental pollutant exposure on endometrial receptivityThe endometrium is the inner mucous layer of the uterus, and its main function is to coordinate with the myometrium, the muscular layer of the uterus, to receive the embryo during implantation, maintain its growth and allow its delivery when it is sufficiently mature (Zhang et al., 2013). Histologically, the endometrium is composed of epithelial cells, stromal cells, endothelial cells forming the vasculature and resident immune cells. Uterine epithelial cells may be subdivided into luminal cells, located on the surface of the uterus, and glandular cells, which radiate in the glandular stroma to the base of the myometrium and form the uterine gland. In response to cyclical changes in steroid hormones, the endometrium undergoes cyclic remodeling during each menstrual cycle that can be subdivided into three phases. The first phase is called proestrus in rodents or the follicular/proliferative phase in humans, during which estradiol levels are very high and the cells have a high proliferative and regenerative pattern after menstruation. In the second phase, ovulation occurs and levels of estradiol and progesterone decrease. Finally, in the diestrum phase in rodents or luteal/secretory phase in humans, high levels of estradiol and progesterone are secreted by the corpus luteum and the endometrium acquires a secretory pattern. It is during this phase that endometrial receptivity is reached and implantation may occur (Zhang et al., 2013). Sub-optimal or asynchronous endometrial development may be responsible for implantation failure.
Given the relevance of steroid hormones, specifically progesterone, in maintaining uterine function and receptivity, decreases in circulating progesterone levels or difficulties in responding to it by target cells, known as progesterone resistance, are possible mechanisms of implantation failure. The anti-progesterone and/or pro-estrogenic effect of EDCs may alter this balance. For example, in animal models, exposure to different xenobiotics decreases serum levels of progesterone (Martínez-Peña et al., 2017) or inhibits progesterone signaling in the endometrium (Berger et al., 2010). This effect may be underestimated in epidemiological studies comparing a population subjected to IVF treatment to the general population, since women undergoing IVF usually have an artificial luteal preparation. However, in many women undergoing IVF treatments with higher levels of xenobiotic exposure, the response of the endometrium to hormones was decreased, indicated by a reduced endometrial thickness (Mínguez-Alarcón et al., 2016a; Bloom et al., 2017; Al-Hussaini et al., 2018). In addition, expression of various endometrial morphogenic genes essential for endometrial differentiation and acquisition of endometrial receptivity, such as Hoxa10, α-SMA, Bmp-2, FOXO-1, Wnt7a, Wnt5a, and β-catenin (Zhang et al., 2013), is deregulated in the endometrium of rodents exposed to BPA, DEHP, BaP, PCBs, OCPs and OPs during uterine development, the perinatal period or the peri-gestational period (Guerrero Schimpf et al., 2017; Li et al., 2017).
Concluding remarksA healthy diet in the periconceptional months with whole grain products, fruits, vegetables, fish (omega-3 PUFAs) and olive oil in women undergoing IVF or ART treatment may significantly improve fertility or preimplantation quality of embryos. And may also contribute to the success of achieving pregnancy.
Further, embryo implantation is an essential process for achieving a viable pregnancy. This process is particularly sensitive to the effects of numerous environmental pollutants, and any disturbance at this early stage of pregnancy can contribute to difficulties in conceiving, appearance of complications during pregnancy or, even pregnancy loss. In studies on human populations, the association between certain environmental pollutants and alterations in embryo implantation is not so evident. it is interesting to note the temporary decrease in the levels of non-persistent environmental pollutants in the EARTH study population, accompanied by a decrease in their deleterious effect on embryo implantation. This underlines the importance of policies and awareness-raising aimed at lowering the levels of these compounds in the environment.
FundingFrancisco Dominguez is supported by Instituto de Salud Carlos III trough a Miguel Servet II contract (CPII18/00002).
Conflict of interestsThe authors declare that they have no conflict of interest.