The aim of this systematic review and meta-analysis is to study the efficacy of Dehydroepiandrosterone in patients with normal ovarian reserve undergoing in vitro fertilization treatment.
DesignSystematic review and meta-analysis.
SettingCenters for reproductive care.
PatientsPatients with normal ovarian reserve (NOR) undergoing in vitro fertilization treatment and previously supplemented or not with DHEA.
InterventionA comprehensive electronic literature search was conducted in Pubmed, the Cochrane Library and Web of Science up to March 2021. Randomized controlled trials studying the effect of DHEA supplementation on reproductive outcomes in patients with normal ovarian reserve were included.
Main outcome measuresThe outcomes of interest were miscarriage rate, clinical pregnancy rate and live birth rate per embryo transfer.
ResultsDHEA supplementation, compared with placebo or no treatment, was associated with a significant decrease in miscarriage rate (OR = 0.30, 95% CI: 0.10–0.93; p = 0.04) in DHEA group. In contrast, there were no statistically significant differences in live birth rate (OR = 1.52, 95% CI: 0.8–2.89; p = 0.2) or clinical pregnancy rate (OR = 1.19, 95% CI: 0.73–1.95; p = 0.47) per embryo transfer. There were also no statistically significant differences in the number of oocytes (MD = 0.66 95% CI: - 0.04–1.36; p = 0.07) or metaphase II oocytes retrieved (MD = 0.32 95% CI: −0.12–0.76; p = 0.16) in one IVF cycle between the two groups.
ConclusionsOur study suggested that DHEA supplementation could improve the miscarriage rate in NOR patients who underwent IVF treatment. However, no increase in clinical pregnancy rate, live birth rate or number of oocytes retrieved per IVF cycle could be demonstrated.
El objetivo de esta revisión sistemática y metanálisis es estudiar la eficacia de la Dehidroepiandrosterona en pacientes con reserva ovárica normal sometidas a tratamiento de fecundación in vitro.
DiseñoRevisión sistemática y metanálisis.
EntornoCentros de atención reproductiva.
PacientesPacientes con reserva ovárica normal (RON) a las que se les realiza tratamiento de fecundación in vitro y previamente suplementadas o no con DHEA.
IntervenciónSe realizó una búsqueda exhaustiva de la literatura electrónica en Pubmed, Biblioteca Cochrane y Web of Science hasta marzo de 2021. Se incluyeron ensayos controlados aleatorizados que estudiaron el efecto de la suplementación con DHEA en los resultados reproductivos en pacientes con reserva ovárica normal.
Resultados principalesLos resultados de interés fueron la tasa de aborto espontáneo, la tasa de embarazo clínico y la tasa de nacidos vivos por transferencia de embriones.
ResultadosLa suplementación con DHEA, en comparación con el placebo o ningún tratamiento, se asoció con una disminución significativa en la tasa de aborto espontáneo (OR = 0,30, IC del 95%: 0,10-0,93; p = 0,04) en el grupo de DHEA. En cambio, no hubo diferencias estadísticamente significativas en la tasa de recién nacido vivo (OR = 1,52, IC 95%: 0,8-2,89; p = 0,2) ni en la tasa de embarazo clínico (OR = 1,19, IC 95%: 0,73-1,95; p = 0,47) por transferencia de embriones. Tampoco hubo diferencias estadísticamente significativas en el número total de ovocitos (DM = 0,66 IC 95%: - 0,04-1,36; p = 0,07) u ovocitos recuperados en metafase II (DM = 0,32 IC 95%: −0,12-0,76; p = 0,16) en un ciclo de FIV entre los dos grupos.
ConclusionesNuestro estudio sugirió que la suplementación con DHEA podría mejorar la tasa de aborto espontáneo en pacientes con RON que se sometieron a un tratamiento de FIV. Sin embargo, no se pudo demostrar un aumento en la tasa de embarazo clínico, la tasa de nacidos vivos o la cantidad de ovocitos recuperados por ciclo de FIV.
Infertility is a common condition with important psychological, economic, demographic and medical implications. The demand for assisted reproductive techniques has increased in recent years, one of the main causes has been delayed childbearing. The growing role of women in the labor market, a greater access to university and post-university studies and also to reliable contraceptive methods have contributed to this (West, 1987). Its management is often a challenge, especially in those patients in whom a low number of oocytes is obtained after adequate ovarian stimulation.
Recently, the possible effect of dehydroepiandrosterone (DHEA) in infertility patients and especially in those with poor ovarian reserve1 (POR) has been studied for its potential effect on oocyte quantity and quality, but its effectiveness and mechanism of action are not yet known exactly. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAs) are precursors for intracellular production of estrogens and androgens; they are synthesized mainly in the reticular zone of the cortex of the adrenal glands but also in the ovarian theca cells.
It has been postulated that DHEA is able to modify early follicular maturation by regulating androgen receptor transcription, increasing FSH receptor expression and modulating FSH activity in granulosa cells. In this way, it is able to increase the number of growing preantral follicles and small antral follicles (Hu et al., 2017). In addition, it has been proposed that DHEA and DHEAs may increase oocyte quality due to the increase in IGF-1 as it has important actions on the proliferation and differentiation of granulosa cells (Casson et al., 2000) and also because of the ability of DHEA to increase the number of cohesins with consequent decrease in oocyte aneuploidy (Barad and Gleicher, 2006; Chu et al., 2017).
There have been several systematic reviews and meta-analyses in which an increase in clinical pregnancy and live birth rate has been reported in patients with POR who received DHEA supplementation prior to IVF/ICSI (Liu et al., 2018; Monteiro et al., 2019; Qin et al., 2017; Schwarze et al., 2018; Xu et al., 2019; Zhang et al., 2016b). However, we have not found any systematic review evaluating the effect of DHEA in patients with normal ovarian reserve (NOR) undergoing assisted reproductive treatment. The aim of this systematic review and meta-analysis was to examine the literature and extract the results of randomized controlled trials (RCTs) that investigated the efficacy of DHEA supplementation in women with normal ovarian reserve undergoing IVF/ICSI compared to a control group.
1In 2011 the European Society of Human Reproduction and Embryology published the Bologna criteria in order to obtain a standardized definition of POR: presence of at least two of the following characteristics (i) patients aged ≥40 years or other risk factor for POR; (II) a previous low response; (III) altered ovarian reserve. (Ferraretti and Gianaroli, 2014).
Material and methodsStudy design and registryThis is a systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating reproductive outcomes after DHEA treatment in patients with NOR undergoing IVF/ICSI. The review was written following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The study protocol was registered in PROSPERO prior to the systematic literature search.
Search strategyThe search for clinical trials was performed through different online databases (Web of Science, the Cochrane Library, Pubmed) to identify studies that evaluated the effect of dehydroepiandrosterone on reproductive outcomes in patients with NOR, published in the last 10 years (March 27, 2011 through March 27, 2021). For this purpose, the search strategy included the combination of the following Medical Subject Heading (MeSH) terms: “Dehydroepiandrosterone” or “DHEA”; and “fertilization in vitro” or “reproductive techniques” or “ovulation induction” or “intracytoplasmic sperm injections”. No language restrictions were established.
Eligibility criteriaSelection criteria were established prior to the literature search. Studies were selected according to the following criteria: (1) controlled, randomized, parallel studies; (2) studies in which DHEA supplementation was used in monotherapy as experimental medication, regardless of dose and duration of treatment; (3) studies in which the control group received placebo or no medication; (4) studies in which patients met at least one criterion of normal ovarian reserve: FSH <10 IU/l, Anti-Müllerian Hormone 2–6.8 ng/ml, inhibin B > 45 pg/ml or antral follicle count (AFC) of 5–15. Trials that included patients of any age or ethnicity were eligible. Studies published only as abstracts in journals were not considered, as their design and quality could not be adequately assessed. Animal studies, studies that were not completed and studies that did not allow data extraction, as well as reviews, comments or letters were also excluded.
Selection of studies and data extractionAccording to the selection criteria mentioned above, two reviewers (MC and MP) selected the studies independently; first through the titles and abstracts and then by checking that they met the inclusion criteria with the full-text review. Subsequently, each reviewer extracted the data of interest from each study. Any disagreements were resolved by discussion among the reviewers. The following data were extracted from the manuscripts: authorship, year of publication, demographics (type of study, number of patients included and definition of normal ovarian reserve), methodological (method of randomization, allocation concealment), procedural (dose and duration of DHEA, type and protocol of ovarian stimulation, number of embryos transferred) and main outcome data (live birth rate and clinical pregnancy rate per embryo transfer and miscarriage rate per clinical pregnancy) and secondary outcome data (mean and standard deviation of oocytes and metaphase II (MII) oocytes retrieved per one IVF cycle).
Quality assessmentTwo reviewers (MP and MC) independently used the Risk of Bias (RoB) tool developed by Cochrane. Risk of bias was assessed using the following parameters: 1) random sequence generation; 2) allocation concealment; 3) blinding of participants and personnel; 4) masking of outcome assessment; 5) incomplete outcome data; 6) selective reporting. The risks of bias graphs were constructed with Review Manager 5.4 software (Cochrane Collaboration, Oxford, UK).
Statistical analysisMeta-analysis was performed using RevMan 5.4 software (Cochrane Collaboration, Oxford, UK). Heterogeneity was measured with Higgins I2 (Higgins et al., n.d.). A value of I2 greater than 50% was considered to indicate significant heterogeneity, in which case a random effects model was applied; otherwise, we applied a fixed effects model. Dichotomous outcomes (clinical pregnancy rate, live birth rate and miscarriage rate) were analyzed by calculating the odds ratio (OR) with 95% confidence intervals (CI) while continuous outcomes (oocytes and MII oocytes retrieved) were analyzed by calculating standard mean differences (MD) with 95% CI. For the analysis of continuous variables, only the results expressed as mean and standard deviation were included. Statistical significance was established at a p value <0.05. The risk of bias between studies was not measured due to the low number of included studies, in accordance with the Cochrane Handbook recommendation (Page et al., 2022).
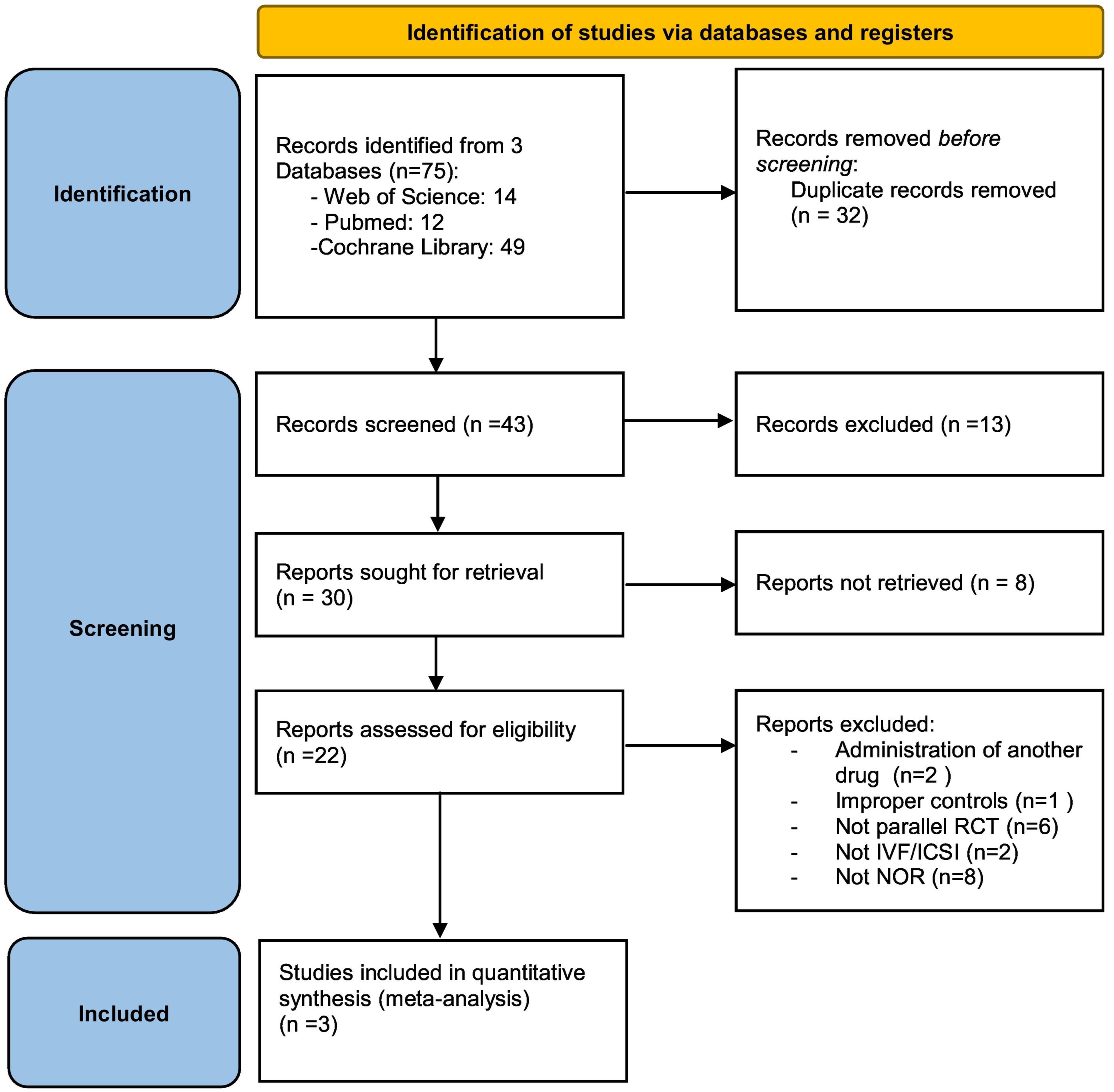
ResultsDescription of included studiesWe identified 75 studies from the 3 databases. After removing 32 duplicate studies and then examining titles and abstracts of the rest, 30 studies remained. After reviewing the full texts of these 30 studies, 3 RCTs were finally included. Fig. 1 shows the detailed data retrieval process by which these 3 RCTs (Mostajeran et al., 2018; Tartagni et al., 2015; Yeung et al., 2016) were eligible for systematic review and meta-analysis.
Of the total of 275 patients included in this systematic review, 134 patients were in the DHEA group and 141 patients were in the control (placebo) group. All included trials targeted patients with normal ovarian reserve whose definition was based primarily on antral follicle count or laboratory parameters. Most of the patients included in the review were between 35 and 40 years old. In all studies, two embryos or more were transferred if possible. However, the stimulation protocol and the administration and duration of DHEA pretreatment were different for each study. The basic characteristics of the included studies are shown in Table 1.
Methodological characteristics of eligible articles.
| Authors and year | Inclusion criteria | Patients (n) | COS protocol | DHEA supplementation | Embryo transfer | Reproductive outcomes | Adverse events | |
|---|---|---|---|---|---|---|---|---|
| DHEA group | Control group | |||||||
| Mostajeran et al. (2018) | Age > 35; BMI 18–25; NOR (defined as FSH < 10 IU/L; AMH 2.0–6.80 ng/ml; inhibin B > 45 pg/m) | 45 | 49 | Long stimulation protocol | 25 mg three times daily 8 weeks before stimulation | Fresh embryo transfer, day 3 embryos (2 embryos per transfer) | 1) Clinical pregnancy rate 2) Miscarriage rate | Not specified |
| Yeung et al. (2016) | Age < 40; subfertility >1 year; NOR (defined as AFC 5–15) | 36 | 36 | Fixed antagonist protocol | 25 mg three times daily 12 weeks before stimulation | Fresh embryo transfer, day 2/5 embryos (2 embryos per transfer) | 1) Oocytes retrieved 2) High quality embryos 3) Clinical pregnancy rate 4) Live birth rate 5)Miscarriage rate | No adverse events |
| Tartagni et al. (2015) | Age 36–40; normal BMI; menstrual cycle length 24–34 days; NOR (defined as FSH < 10 IU/L; AMH 2.0–6.80 ng/ml; inhibin B > 45 pg/ml) | 53 | 56 | Long stimulation protocol | 75 mg/24 h 8 weeks before and during stimulation | Fresh embryo transfer, day 3 embryos (up to 3 embryos per transfer) | 1) Oocytes retrieved 2) Mature oocytes retrieved 3) High quality embryos 4) Clinical pregnancy rate 5) Live birth rate 6) Miscarriage rate | No adverse events |
COS: Controlled ovarian stimulation, BMI:Body mass index, NOR:Normal ovarian reserve, FSH: Follicle stimulating hormone, AMH: Anti-Müllerian Hormone, AFC: Antral follicle count.
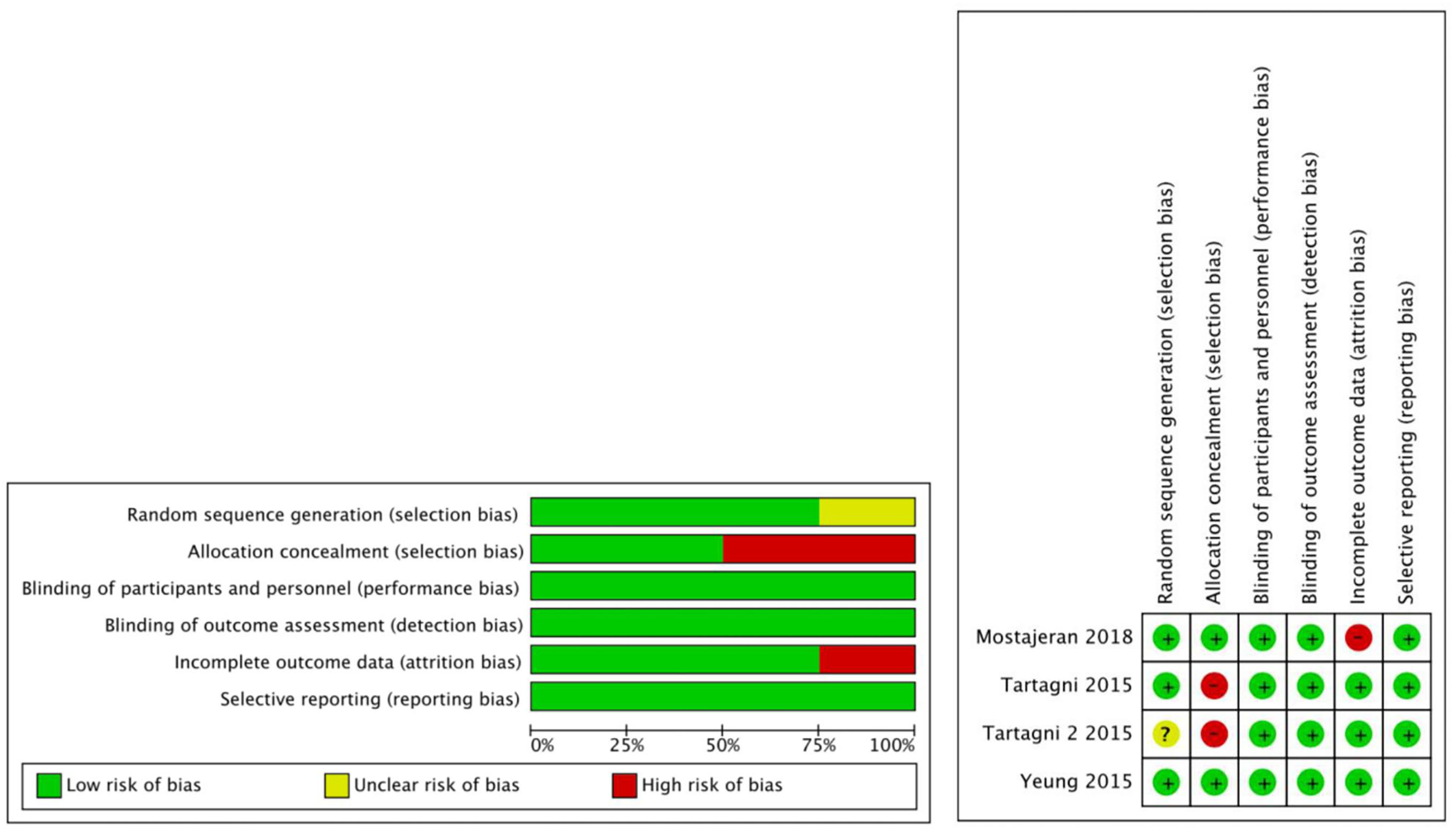
The quality of the included studies was assessed using the RoB tool by evaluating 6 parameters. All included studies were of double-blind, placebo-controlled design with the randomization procedure performed by computer. However, one study (Tartagni et al., 2015) did not specify how allocation was concealed and was therefore considered to be at high risk of selection bias. In addition, one study (Mostajeran et al., 2018) was considered to be at high risk of attrition bias due to the fact that 5 patients assigned to the DHEA group had a spontaneous pregnancy before IVF treatment and were excluded from the study. The details of the RoB are presented in Fig. 2.
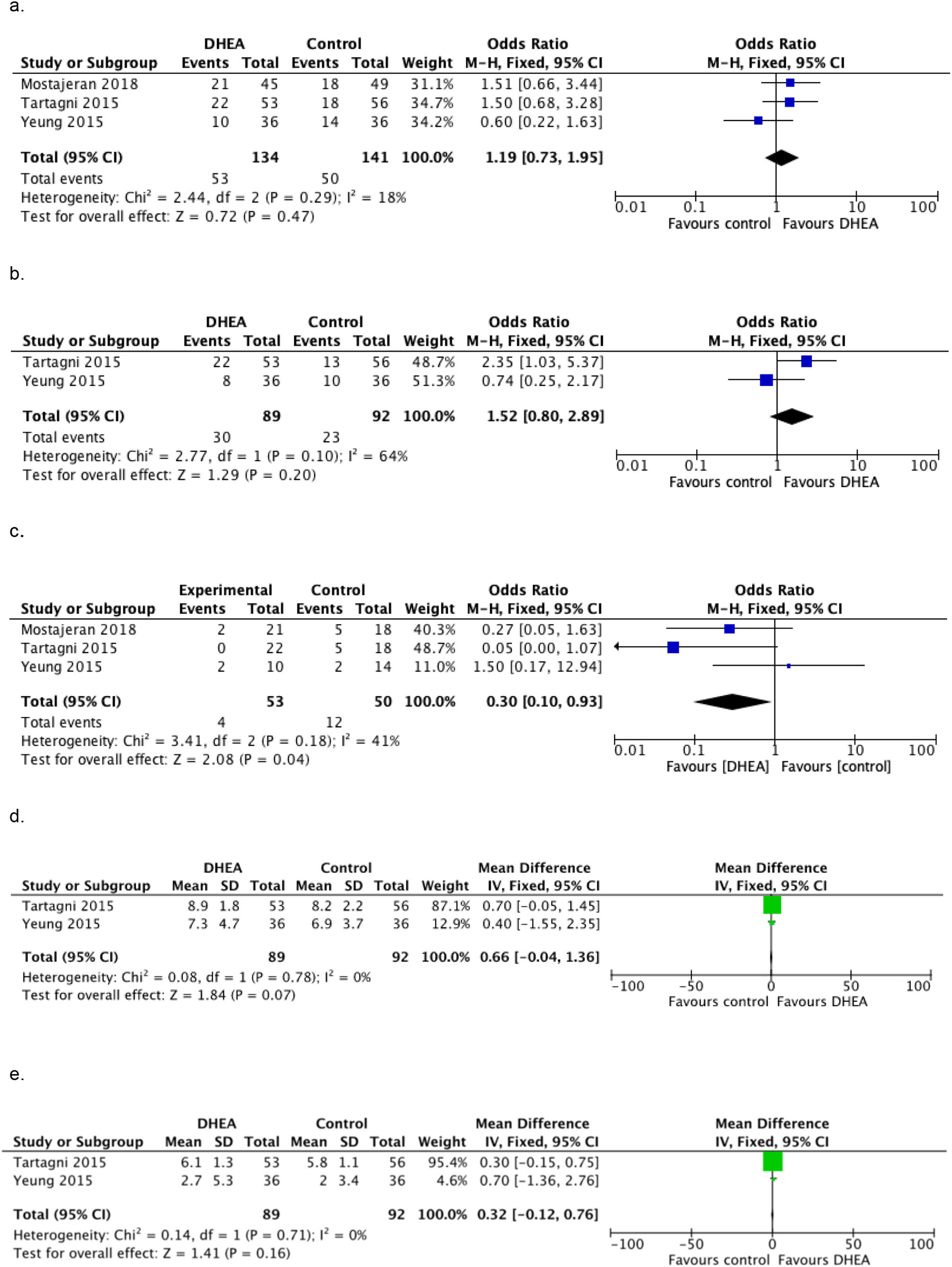
OutcomesPrimary outcomesClinical pregnancy rateThe clinical pregnancy rate of the intervention group was higher than that of the control group in all but one trial (Yeung et al., 2016). Of the total of 134 participants in the intervention group, 53 (39.6%) achieved a clinical pregnancy. In contrast, 50 (35.5%) of 141 women achieved a clinical pregnancy in the control group. The 3 RCTs (Mostajeran et al., 2018; Tartagni et al., 2015; Yeung et al., 2016) were selected for the meta-analysis of clinical pregnancy rate. There were no statistically significant differences between patients who received DHEA supplementation and those who did not in the clinical pregnancy rate [OR = 1.19, 95% CI: 0.73–1.95; p = 0.47], there was no significant heterogeneity between studies according to the fixed effects model [I2 = 18%]. Fig. 3 a.
Live birth rateThe live birth rate was evaluated in 2 RCTs (Tartagni et al., 2015; Yeung et al., 2016); out of 89 patients who received DHEA supplementation, 30 obtained a live newborn (33.7%); while in the control group, 23 out of 92 patients achieved a live newborn (25%). Nevertheless, in the meta-analysis performed, the live birth rate wasn't statistically significant higher in the DHEA supplemented group than in the control group [OR = 1.52, 95% CI: 0.8–2.89; p = 0.2]; significant heterogeneity was found with the fixed effects model [I2 = 64%]. Fig. 3 b
Miscarriage rateMiscarriage rates were published in all included studies and was 7.5% (4/53) in the intervention group and 24% (12/50) in the control group. All 3 RCTs (Mostajeran et al., 2018; Tartagni et al., 2015; Yeung et al., 2016) were included in the meta-analysis. There was a lower rate of miscarriage in DHEA supplemented patients compared to patients in the control group [OR = 0.30, 95% CI: 0.10–0.93; p = 0.04] and moderate but acceptable heterogeneity was found with the fixed effects model [I2 = 41%] Fig. 3 c.
Secondary outcomesOocytes and MII oocytes retrievedThe analysis results of two RCTs (Tartagni et al., 2015; Yeung et al., 2016) couldn't show statistically significant differences in the number of retrieved oocytes or MII oocytes retrieved [MD = 0.66, 95% CI: −0.04–1.36; p = 0.07] [MD = 0.32 CI 95%: −0.12–0.76; p = 0.16]. No significant heterogeneity was found between studies according to the fixed effects model [I2 = 0%] for either of the two outcomes Fig. 3 d-e.
Adverse eventsIn two (Tartagni et al., 2015; Yeung et al., 2016) of the included RCTs it was specified that the included patients did not experience adverse effects, but in Mostajeran et al. study it was not specified (Mostajeran et al., 2018).
DiscussionThis systematic review and meta-analysis summarizes the current scientific evidence on the efficacy of DHEA supplementation before or during controlled ovarian stimulation in patients with normal ovarian reserve undergoing IVF. Three randomized controlled trials were included. The main finding of the review was that the miscarriage rate was significantly decreased in the DHEA-supplemented group compared to the placebo group in patients with normal ovarian reserve who underwent IVF treatment. However, DHEA did not significantly improve clinical pregnancy rate, live birth rate, or number of oocytes retrieved per IVF cycle.
Pretreatment with DHEA to improve fertility has been a topic under constant review in recent years.
Most of the studies have focused on the use of DHEA to improve ovarian response and reproductive outcomes in patients with poor ovarian reserve. In 2013, Narkwichean et al. published a systematic review and meta-analysis in which no statistically significant difference was found in clinical pregnancy rate or miscarriage rate in patients with POR supplemented with DHEA (Narkwichean et al., 2013). Nevertheless, subsequently numerous systematic reviews and meta-analyses were published in which an improvement in the clinical pregnancy rate, live birth rate and in the miscarriage rate were observed in patients with POR treated with DHEA (Liu et al., 2018; Monteiro et al., 2019; Qin et al., 2017; Schwarze et al., 2018; Xu et al., 2019; Zhang et al., 2016a). However, no differences in the number of oocytes retrieved could be demonstrated in these studies; even in the study of Xu et al. a lower number of oocytes retrieved was observed in DHEA-supplemented patients (Xu et al., 2019).
Due to the promising effects on reproductive outcomes of DHEA in patients with POR and the lack of evidence on its effect in patients with normal ovarian reserve, we decided to perform this systematic review and meta-analysis. Unlike the previous systematic reviews discussed above, our review found no statistically significant difference in clinical pregnancy rate or live birth rate.
This meta-analysis included 3 RCT with 275 patients with normal ovarian reserve who underwent IVF. We analyzed the effect of DHEA supplementation on clinical pregnancy rate and live birth rate per fresh embryo transfer, miscarriage rate, and number of oocytes and MII oocytes retrieved per one IVF cycle. We found that patients who received DHEA supplementation as an intervention had a significantly lower miscarriage rate. On the other hand, we did not find statistically significant differences in the live birth rate, the clinical pregnancy rate or in the number of oocytes retrieved. In the included studies, no adverse effects were reported with DHEA treatment.
Because of the delay in childbearing and the oxidative stress to which oocytes are subjected by current lifestyles and exposure to toxins (Petraglia et al., 2013), the number of infertile couples demanding assisted reproduction treatments has been increasing.
Multiple strategies have been studied to increase the quantity and quality of oocytes in infertile patients with the aim of improving reproductive outcomes. Dehydroepiandrosterone supplementation is one of these strategies, although its exact mechanism of action is still unknown. The study by Tsui et al. showed that DHEA supplementation positively affected cumulus cell gene expression, promoted extracellular matrix formation and inhibited apoptosis with a consequent increase in antral follicles (Tsui et al., 2014). On the other hand, many studies have tried to demonstrate how DHEA is capable of improving the embryo and oocyte quality, Chu et al. found that DHEA was able to delay the physiological loss of cohesins in oocytes that has been related to the occurrence of oocyte aneuploidy (Chu et al., 2017); furthermore, Gleicher et al. suggested that DHEA supplementation was able to increase the percentage of euploid embryos obtained in patients with low ovarian reserve with a consequent decrease in the rate of miscarriages (Barad and Gleicher, 2006; Gleicher et al., 2009). In addition, it has also been proposed that DHEA is capable of improving oocyte and embryo quality by increasing mitochondrial activity, decreasing mitochondrial fission and increasing the elimination of dysfunctional mitochondria with mitophagy; this could decrease chromosomal aneuploidies, improve embryonic cleavage, and reduce cytoplasmic fragmentation (Li et al., 2018). All of these findings may be in agreement with our results.
This meta-analysis is the first to our knowledge so far to evaluate the effect of DHEA in patients with normal ovarian reserve. We included only randomized controlled studies in order to minimize biases related to other types of studies. Strict selection criteria and rigorous methodology are the strengths of this study. Nevertheless, our meta-analysis has several limitations. First, there was a study in which the definition of NOR was based on antral follicle count (Yeung et al., 2016), unlike the other studies in which the definition was based on analytical criteria; the definition of normal ovarian reserve should be standardized in order to conduct RCTs with a more homogeneous population. Secondly, the dosage and duration of DHEA treatment was different in each study, as was the ovarian stimulation protocol. Thirdly, the meta-analysis of some results was performed with a low number of patients because we were unable to obtain sufficient data from the original study.
Although no statistically significant differences were observed in the clinical pregnancy rate or the live birth rate, there was a trend towards improvement in both rates with DHEA supplementation; also, as we have commented previously; 5 patients supplemented with DHEA who became pregnant spontaneously were not taken into account for the meta-analysis; these patients could have benefited from DHEA supplementation.
The importance of this study lies in the fact that DHEA seems to be capable of reducing the miscarriage rate in patients with normal ovarian reserve, probably due to the improvement in the quality of the oocytes.
In the future, more randomized controlled trials should be conducted to assess which subgroup of patients might benefit most from DHEA pretreatment. Additionally, further studies are needed to assess whether DHEA is capable of improving cumulative rates of reproductive outcomes with frozen embryos.
ConclusionsThe findings of this meta-analysis are similar to those of other meta-analyses that studied the effect of DHEA in patients with poor ovarian reserve. We found that the use of DHEA before ovarian stimulation in women with NOR was associated with a decrease in the miscarriage rate. In addition, no adverse effects were identified with DHEA supplementation. Nevertheless, more well-designed randomized controlled trials are needed to recommend the use of DHEA in patients with normal ovarian reserve and to study which subpopulations might benefit most from DHEA supplementation.
Conflicts of interestNone of the authors have any conflicts of interest or financial ties to disclose. No funding source had involvement in the publication of this manuscript. This research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.