To determine the analgesic effect of whole body cryotherapy (WBC) in patients with trapezius myofascial pain syndrome.
MethodsTwenty patients from an outpatient rehabilitation clinic were recruited. Patients were required to have clinical data for their diagnosis of myofascial pain syndrome in the trapezius. Twenty WBC sessions (−160°C) were prescribed at four sessions per week for five weeks. A visual analog scale for pain (VAS) and algometry at trigger points (TPs) were applied before and after each session to measure the Pressure Pain Threshold (PPT). Six blood samples per patient were obtained during the study to measure the serum concentration of pro-inflammatory and anti-inflammatory cytokines.
ResultsA significant decrease in pain immediately after WBC was found from session 1 (p<0.001) onwards. Furthermore, a significant difference was observed in VAS at baseline compared to the value before each cryotherapy session starting from session 6 (p<0.001). Significant differences were found in algometry at each session (p<0.001) and when comparing the initial and pre-session values starting from session 6 (p<0.001). No significant differences were found in the concentrations of inflammatory or anti-inflammatory factors throughout the study (p>0.05).
ConclusionsWBC is useful as an analgesic treatment for myofascial pain syndrome in trapezius.’
Musculoskeletal processes are the most common cause of both acute and chronic pain, as well as temporary or permanent disability.1 It is estimated that the global prevalence of these diseases ranges from 13.5 to 47%. In Mexico, the prevalence of musculoskeletal pain is approximately 20% and is consistently more prevalent in women and urban areas.2 It is estimated that one-third of patients with musculoskeletal pain meet the diagnostic criteria for myofascial pain syndrome.3,4 Myofascial pain syndrome affects up to 85% of the population at some point in their lives.5
Myofascial pain syndrome (MPS) refers to soft tissue pain resulting from irritation of local points within the skeletal muscle and myotendinous junctions. These local points are known as trigger points.6 These trigger points (TPs) have been described as hyperirritable sites located in a taut, muscular band, which are felt as small painful nodules that produce nociceptive pain caused by compression.3
It has been postulated that TPs are the result of acute muscle injury due to overuse or repetitive activity. When TPs persist for more than three weeks, the diagnosis of MPS is made.7
Physical examination and the clinical data thus found are of vital importance for the diagnosis of MPS. When MPS is suspected, the presence of a well-defined trigger point within a tight muscle band that causes pain when firm pressure is applied for 5s and does not follow established nerve patterns can be suggestive of the disease. This pain should be reproducible with each compression of approximately 3kg/cm2 per second or less.6 During the physical examination, there is sometimes an obvious response of muscle twitches and a perception of weakness by the patient.8 According to the symptomatology, regardless of the clinical findings, TPs can be divided into active and latent.6 It is important to consider fibromyalgia, bursitis, tendinosis, fasciitis, and disorders of joint hypermobility as differential diagnoses of MPS.6,9,10
MPS is a pathology that, while not fatal, represents a significant reduction in quality of life and an important cause of absence from work, generating an enormous cost for its treatment in health systems.11
Despite its high incidence, there is no specific treatment for the disease. Several conservative treatments have been described, among them analgesic and anti-inflammatory drugs, and in recent years, acupuncture, dry needle therapy, and botulinum toxin application.3,4,12,13 Physical rehabilitation has been shown to have significant value in the management of this disease and, due to its analgesic effect in various musculoskeletal diseases, cryotherapy is one of the methods frequently used.14
The effects of cryotherapy consist of the provocation of several physiological effects: vasoconstriction, decreased edema, and decreased spasticity. Its direct analgesic effect is to reduce nociceptive conduction velocity in peripheral nerves.14–17
There are many different methods for applying cryotherapy. One of these methods is whole body cryotherapy, which, in addition to having a local effect, provides a systemic effect by modifying the levels of pro- and anti-inflammatory markers.18
Whole-body cryotherapy (WBC) or total body cryotherapy consists of exposing the body to cold air at temperatures below −110°C in a chamber that controls the temperature. This type of therapy has been applied in several pathologies to relieve pain and inflammation.19
The anti-inflammatory effect observed in subjects after treatment with whole-body cryotherapy has been an increase in anti-inflammatory cytokines (IL-10) and a decrease in proinflammatory cytokines (IL-2, IL-8) and prostaglandin E2.20
Recent studies suggest that WBC may be effective in inflammatory processes in patients with some rheumatic diseases such as rheumatoid arthritis, ankylosing spondylitis, osteoarthrosis, and fibromyalgia.15 Despite this recent introduction of WBC to the field of treatment of rheumatological diseases, there is no evidence of its use in MPS7,13,21 and therefore it is important to continue searching for new therapeutic methods that could be more successful than those already known.
The aim of this study was to demonstrate the analgesic effect of total body cryotherapy in patients with myofascial pain syndrome of the trapezius muscle.
Material and methodsPatient selectionPatients of the Sports Medicine and Rehabilitation Department of a third level hospital in northeastern México with a clinical diagnosis of myofascial syndrome were recruited. These patients were chosen based on their willingness to participate in the study regardless of whether or not they had undergone another treatment. The trial was conducted between February and July of 2016. A sample of 20 subjects was calculated using a hypothesis test formula and a difference of two means, or with the proportion of a reference value considering a zα value of 1.96 with a level of significance of 95% for a queue, and a zβ value of 1.28 with a power of 90%, with a difference from the initial visual analog scale (VAS) of at least 3 points.
Patients of both genders aged 18–60 years, with a clinical diagnosis of myofascial pain syndrome and with a pain equivalent to 3 or more points on the VAS and who provided signed informed consent were included. During this trial, other analgesic measures such as NSAIDs, physical therapy, and/or other drugs were suspended. Pregnant women and individuals with heart diseases, respiratory diseases, cancer, sensitivity alterations, claustrophobia, poorly controlled hypertension, mental disorders and skin wounds, as well as those intolerant to cold or who had experienced adverse reactions to cold were excluded. Patients who did not comply with the total number of sessions (n=1) were eliminated.
Study designThis study was a longitudinal, prospective, experimental, and non-blinded, that was approved by the Ethics Committee of a third-level hospital in northeastern México. Informed consent was obtained from patients after a verbal explanation of the procedure, clarification of questions, and the signing of the document.
MeasurementsEach participant was examined by the only resident physician in the specialty of sports medicine and rehabilitation to assess the presence of a myofascial trigger point in the trapezius muscle. The following diagnostic criteria were used: the presence of a palpable band, a sensitive point, and the recognition of pain referred to the neck or head.
An analog pressure algometer, the Wagner Pain Test™ Model FPK 10 (Wagner Instruments, Greenwich, CT) was used for measuring the pressure pain threshold (PPT), which is defined as the minimum amount of pressure required to produce a sense of discomfort or pain at the point being measured. A pain evaluation was performed using a VAS before and after each session.
Six blood samples per patient were obtained during the study to measure the serum concentration of pro-inflammatory and anti-inflammatory cytokines. These were drawn at the beginning of the protocol and after sessions 4, 8, 12, 16 and 20. IL-1β, IL-6, IL-8, IL-10, PCR, CPK and cortisol were evaluated in each session.
A plate reader (Multi-Mode Synergy 2; Biotec, USA) was used for the CPK and cortisol determinations, and a flow cytometer (BD AccuriTM C6; BD Biosciences, USA) was used for cytokine analysis.
Whole body cryotherapy sessionsWe used the CRYO-B Chillout Elite booth. We chose a total 20 session treatment distributed by 4 sessions per week as recommended by the Cryo-B manufacturers and other studies.19
ProcessAfter locating the trigger point, the algometer was placed on it perpendicular to the muscular plane of the trapezium. Gradually, the pressure on the trigger point increased at a rate of 1kg/cm2/s. The value indicated on the algometer was recorded at the time the subject reported pain. An evaluation of vital signs (heart rate and blood pressure) was performed before and after each cryotherapy session.
Patients underwent 20 full-body cryotherapy sessions at an average temperature of −160°C for 3min in a liquid nitrogen-cooled CRYO-B booth. The sessions were distributed in 4 sessions per week over five weeks. All subjects were instructed in the following safety measures: the use of gloves, dry socks and shoes inside the cryotherapy booth; arm and leg movement during the session; and avoiding holding his/her breath with the intention of reducing the risk of adverse effects.
Data analysisWe used SPSS version 20 (SPSS, Inc., Armon, NY) to perform hypothesis testing and the difference of two means. Descriptive statistics were obtained and a hypothesis test formula was used with the difference of two means as a reference value for the evaluation of VAS and PPT. For the analysis of laboratory results, we used the Kolmogorov Smirnov test to determine the distribution of the data, the Student's t-test for tests related to parametric distribution (IL-1β, IL-10, TNF, CPK, and Cortisol) and the Wilcoxon rank-sum test for nonparametric variables (IL-6 and IL-8).
ResultsTwenty subjects were included in the study, of which 15 were women, and 5 were men. The mean age of the patients was 40.86±11.04 years, with an age range of 23–56 years.
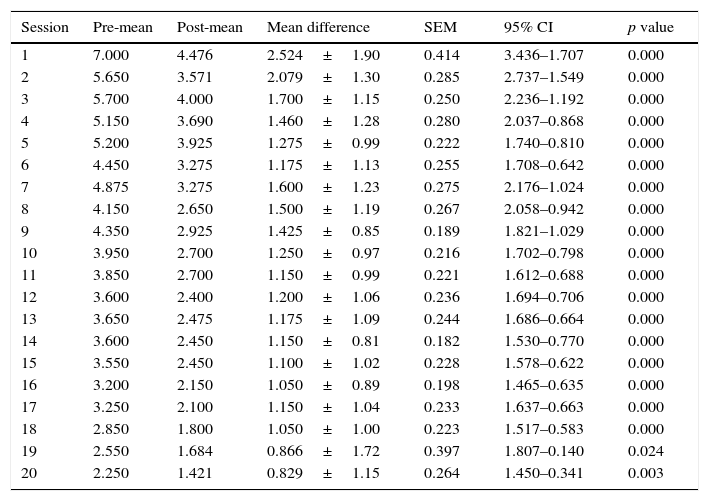
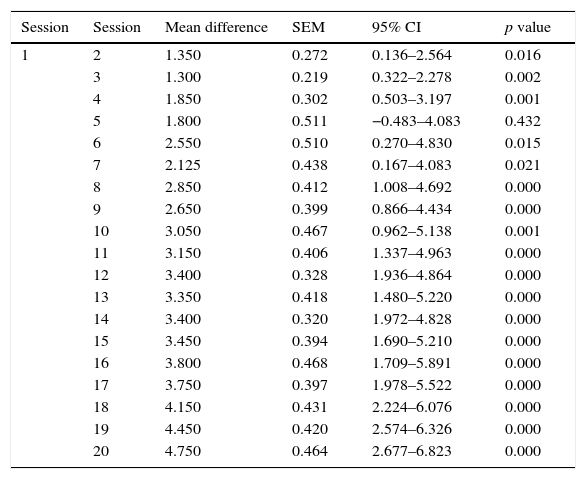
The mean VAS of pain at the start of the study was 7.00±1.37 points and was 2.25±2.09 points at the end of the study. A statistically significant reduction in pain immediately after application of full-body cryotherapy was found from session 1 (p<0.001). (Table 1) Also, a significant difference was observed in the value of VAS at baseline compared with the previous value at each cryotherapy session starting from the 6th session (p<0.001) (Table 2).
Difference in VAS score per session.
| Session | Pre-mean | Post-mean | Mean difference | SEM | 95% CI | p value |
|---|---|---|---|---|---|---|
| 1 | 7.000 | 4.476 | 2.524±1.90 | 0.414 | 3.436–1.707 | 0.000 |
| 2 | 5.650 | 3.571 | 2.079±1.30 | 0.285 | 2.737–1.549 | 0.000 |
| 3 | 5.700 | 4.000 | 1.700±1.15 | 0.250 | 2.236–1.192 | 0.000 |
| 4 | 5.150 | 3.690 | 1.460±1.28 | 0.280 | 2.037–0.868 | 0.000 |
| 5 | 5.200 | 3.925 | 1.275±0.99 | 0.222 | 1.740–0.810 | 0.000 |
| 6 | 4.450 | 3.275 | 1.175±1.13 | 0.255 | 1.708–0.642 | 0.000 |
| 7 | 4.875 | 3.275 | 1.600±1.23 | 0.275 | 2.176–1.024 | 0.000 |
| 8 | 4.150 | 2.650 | 1.500±1.19 | 0.267 | 2.058–0.942 | 0.000 |
| 9 | 4.350 | 2.925 | 1.425±0.85 | 0.189 | 1.821–1.029 | 0.000 |
| 10 | 3.950 | 2.700 | 1.250±0.97 | 0.216 | 1.702–0.798 | 0.000 |
| 11 | 3.850 | 2.700 | 1.150±0.99 | 0.221 | 1.612–0.688 | 0.000 |
| 12 | 3.600 | 2.400 | 1.200±1.06 | 0.236 | 1.694–0.706 | 0.000 |
| 13 | 3.650 | 2.475 | 1.175±1.09 | 0.244 | 1.686–0.664 | 0.000 |
| 14 | 3.600 | 2.450 | 1.150±0.81 | 0.182 | 1.530–0.770 | 0.000 |
| 15 | 3.550 | 2.450 | 1.100±1.02 | 0.228 | 1.578–0.622 | 0.000 |
| 16 | 3.200 | 2.150 | 1.050±0.89 | 0.198 | 1.465–0.635 | 0.000 |
| 17 | 3.250 | 2.100 | 1.150±1.04 | 0.233 | 1.637–0.663 | 0.000 |
| 18 | 2.850 | 1.800 | 1.050±1.00 | 0.223 | 1.517–0.583 | 0.000 |
| 19 | 2.550 | 1.684 | 0.866±1.72 | 0.397 | 1.807–0.140 | 0.024 |
| 20 | 2.250 | 1.421 | 0.829±1.15 | 0.264 | 1.450–0.341 | 0.003 |
SEM: standard error of the mean, 95% CI: 95% confidence interval.
Comparison of the difference in VAS pre-WBC from the first session to the subsequent ones.
| Session | Session | Mean difference | SEM | 95% CI | p value |
|---|---|---|---|---|---|
| 1 | 2 | 1.350 | 0.272 | 0.136–2.564 | 0.016 |
| 3 | 1.300 | 0.219 | 0.322–2.278 | 0.002 | |
| 4 | 1.850 | 0.302 | 0.503–3.197 | 0.001 | |
| 5 | 1.800 | 0.511 | −0.483–4.083 | 0.432 | |
| 6 | 2.550 | 0.510 | 0.270–4.830 | 0.015 | |
| 7 | 2.125 | 0.438 | 0.167–4.083 | 0.021 | |
| 8 | 2.850 | 0.412 | 1.008–4.692 | 0.000 | |
| 9 | 2.650 | 0.399 | 0.866–4.434 | 0.000 | |
| 10 | 3.050 | 0.467 | 0.962–5.138 | 0.001 | |
| 11 | 3.150 | 0.406 | 1.337–4.963 | 0.000 | |
| 12 | 3.400 | 0.328 | 1.936–4.864 | 0.000 | |
| 13 | 3.350 | 0.418 | 1.480–5.220 | 0.000 | |
| 14 | 3.400 | 0.320 | 1.972–4.828 | 0.000 | |
| 15 | 3.450 | 0.394 | 1.690–5.210 | 0.000 | |
| 16 | 3.800 | 0.468 | 1.709–5.891 | 0.000 | |
| 17 | 3.750 | 0.397 | 1.978–5.522 | 0.000 | |
| 18 | 4.150 | 0.431 | 2.224–6.076 | 0.000 | |
| 19 | 4.450 | 0.420 | 2.574–6.326 | 0.000 | |
| 20 | 4.750 | 0.464 | 2.677–6.823 | 0.000 |
SEM: standard error of the mean, 95% CI: 95% confidence interval.
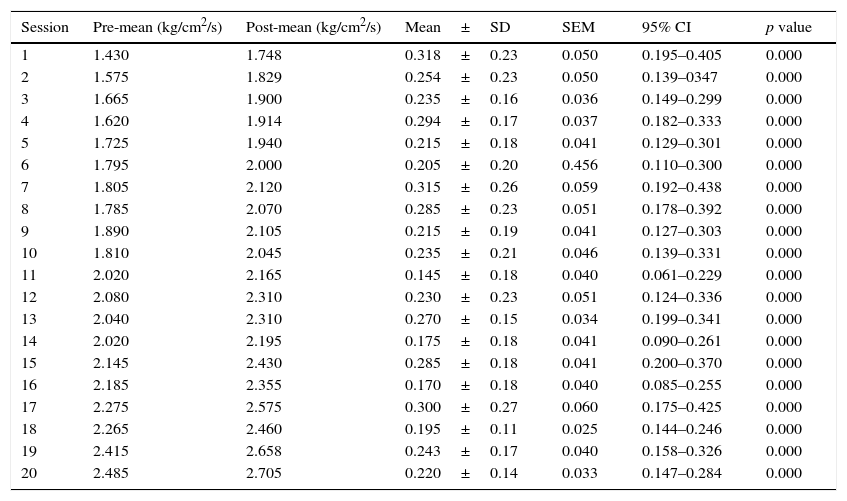
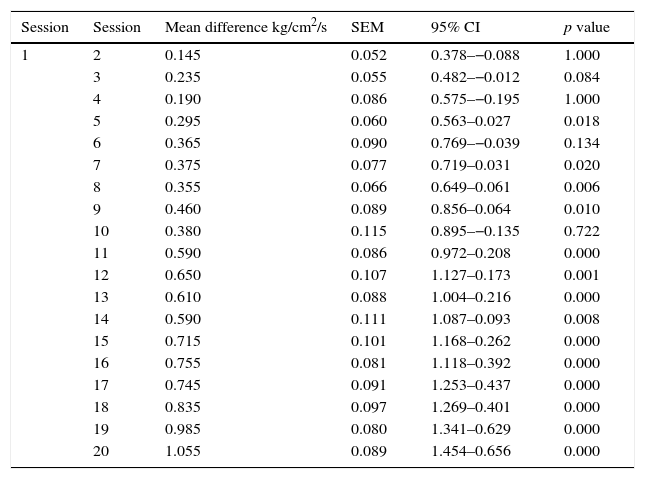
As for the PPT measured by the pressure algometer, a significant difference was also found. The mean algometry value at the beginning of the study was 1.44±0.35kg/cm2/s of pressure, and at the end it was 2.49±0.34kg/cm2/s of pressure, with significant differences in algometry values at each full-body cryotherapy session (p=<0.001) (Table 3), as well as in comparisons made between the initial and pre-session values starting from session 6 (p<0.001) (Table 4).
Difference in pressure pain threshold.
| Session | Pre-mean (kg/cm2/s) | Post-mean (kg/cm2/s) | Mean±SD | SEM | 95% CI | p value |
|---|---|---|---|---|---|---|
| 1 | 1.430 | 1.748 | 0.318±0.23 | 0.050 | 0.195–0.405 | 0.000 |
| 2 | 1.575 | 1.829 | 0.254±0.23 | 0.050 | 0.139–0347 | 0.000 |
| 3 | 1.665 | 1.900 | 0.235±0.16 | 0.036 | 0.149–0.299 | 0.000 |
| 4 | 1.620 | 1.914 | 0.294±0.17 | 0.037 | 0.182–0.333 | 0.000 |
| 5 | 1.725 | 1.940 | 0.215±0.18 | 0.041 | 0.129–0.301 | 0.000 |
| 6 | 1.795 | 2.000 | 0.205±0.20 | 0.456 | 0.110–0.300 | 0.000 |
| 7 | 1.805 | 2.120 | 0.315±0.26 | 0.059 | 0.192–0.438 | 0.000 |
| 8 | 1.785 | 2.070 | 0.285±0.23 | 0.051 | 0.178–0.392 | 0.000 |
| 9 | 1.890 | 2.105 | 0.215±0.19 | 0.041 | 0.127–0.303 | 0.000 |
| 10 | 1.810 | 2.045 | 0.235±0.21 | 0.046 | 0.139–0.331 | 0.000 |
| 11 | 2.020 | 2.165 | 0.145±0.18 | 0.040 | 0.061–0.229 | 0.000 |
| 12 | 2.080 | 2.310 | 0.230±0.23 | 0.051 | 0.124–0.336 | 0.000 |
| 13 | 2.040 | 2.310 | 0.270±0.15 | 0.034 | 0.199–0.341 | 0.000 |
| 14 | 2.020 | 2.195 | 0.175±0.18 | 0.041 | 0.090–0.261 | 0.000 |
| 15 | 2.145 | 2.430 | 0.285±0.18 | 0.041 | 0.200–0.370 | 0.000 |
| 16 | 2.185 | 2.355 | 0.170±0.18 | 0.040 | 0.085–0.255 | 0.000 |
| 17 | 2.275 | 2.575 | 0.300±0.27 | 0.060 | 0.175–0.425 | 0.000 |
| 18 | 2.265 | 2.460 | 0.195±0.11 | 0.025 | 0.144–0.246 | 0.000 |
| 19 | 2.415 | 2.658 | 0.243±0.17 | 0.040 | 0.158–0.326 | 0.000 |
| 20 | 2.485 | 2.705 | 0.220±0.14 | 0.033 | 0.147–0.284 | 0.000 |
SEM: standard error of the mean; mean±SD: mean±standard deviation; 95% CI: 95% confidence interval; bilateral p value is shown.
Comparison of the difference of Pressure Pain Threshold measured pre-WBC comparing the first session to subsequent ones.
| Session | Session | Mean difference kg/cm2/s | SEM | 95% CI | p value |
|---|---|---|---|---|---|
| 1 | 2 | 0.145 | 0.052 | 0.378–−0.088 | 1.000 |
| 3 | 0.235 | 0.055 | 0.482–−0.012 | 0.084 | |
| 4 | 0.190 | 0.086 | 0.575–−0.195 | 1.000 | |
| 5 | 0.295 | 0.060 | 0.563–0.027 | 0.018 | |
| 6 | 0.365 | 0.090 | 0.769–−0.039 | 0.134 | |
| 7 | 0.375 | 0.077 | 0.719–0.031 | 0.020 | |
| 8 | 0.355 | 0.066 | 0.649–0.061 | 0.006 | |
| 9 | 0.460 | 0.089 | 0.856–0.064 | 0.010 | |
| 10 | 0.380 | 0.115 | 0.895–−0.135 | 0.722 | |
| 11 | 0.590 | 0.086 | 0.972–0.208 | 0.000 | |
| 12 | 0.650 | 0.107 | 1.127–0.173 | 0.001 | |
| 13 | 0.610 | 0.088 | 1.004–0.216 | 0.000 | |
| 14 | 0.590 | 0.111 | 1.087–0.093 | 0.008 | |
| 15 | 0.715 | 0.101 | 1.168–0.262 | 0.000 | |
| 16 | 0.755 | 0.081 | 1.118–0.392 | 0.000 | |
| 17 | 0.745 | 0.091 | 1.253–0.437 | 0.000 | |
| 18 | 0.835 | 0.097 | 1.269–0.401 | 0.000 | |
| 19 | 0.985 | 0.080 | 1.341–0.629 | 0.000 | |
| 20 | 1.055 | 0.089 | 1.454–0.656 | 0.000 |
SEM: standard error of the mean, 95% CI: 95% confidence interval.
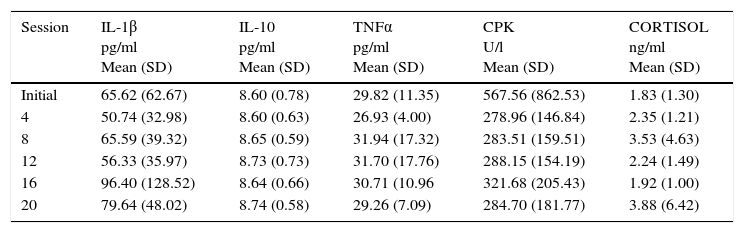
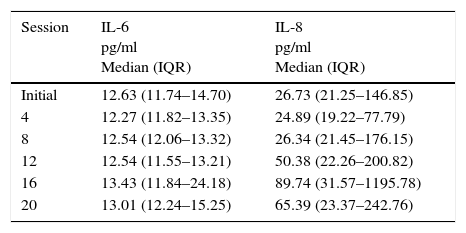
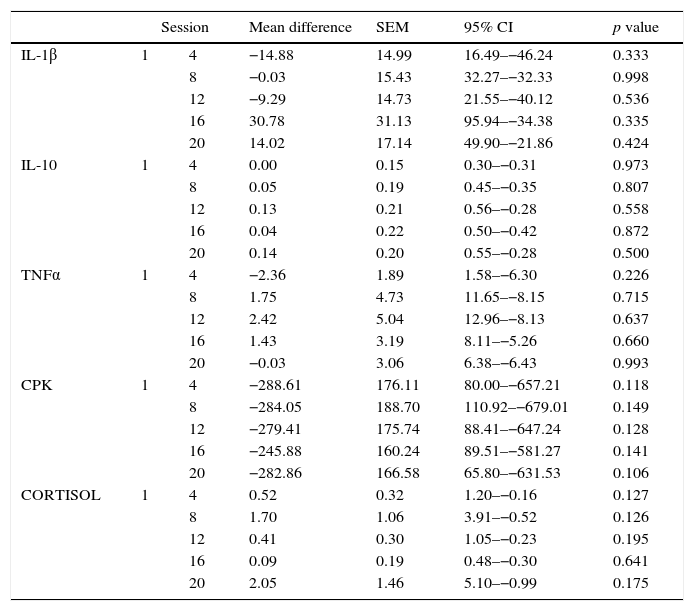
Initial and follow-up results at the 4th, 8th, 12th, 16th, and 20th sessions of the measured substances (Cortisol, ILs) are shown in Tables 5 and 6. Tables 7 and 8 show the changes in the follow-up sessions compared to the initial measurement.
Mean levels of serum cytokine concentration throughout the study.
| Session | IL-1β pg/ml Mean (SD) | IL-10 pg/ml Mean (SD) | TNFα pg/ml Mean (SD) | CPK U/l Mean (SD) | CORTISOL ng/ml Mean (SD) |
|---|---|---|---|---|---|
| Initial | 65.62 (62.67) | 8.60 (0.78) | 29.82 (11.35) | 567.56 (862.53) | 1.83 (1.30) |
| 4 | 50.74 (32.98) | 8.60 (0.63) | 26.93 (4.00) | 278.96 (146.84) | 2.35 (1.21) |
| 8 | 65.59 (39.32) | 8.65 (0.59) | 31.94 (17.32) | 283.51 (159.51) | 3.53 (4.63) |
| 12 | 56.33 (35.97) | 8.73 (0.73) | 31.70 (17.76) | 288.15 (154.19) | 2.24 (1.49) |
| 16 | 96.40 (128.52) | 8.64 (0.66) | 30.71 (10.96 | 321.68 (205.43) | 1.92 (1.00) |
| 20 | 79.64 (48.02) | 8.74 (0.58) | 29.26 (7.09) | 284.70 (181.77) | 3.88 (6.42) |
IL: interleukin; TNF: tumor necrosis factor; CPK: creatine phosphokinase; SD: standard deviation.
Range of distribution of serum cytokines concentration throughout the study.
| Session | IL-6 pg/ml Median (IQR) | IL-8 pg/ml Median (IQR) |
|---|---|---|
| Initial | 12.63 (11.74–14.70) | 26.73 (21.25–146.85) |
| 4 | 12.27 (11.82–13.35) | 24.89 (19.22–77.79) |
| 8 | 12.54 (12.06–13.32) | 26.34 (21.45–176.15) |
| 12 | 12.54 (11.55–13.21) | 50.38 (22.26–200.82) |
| 16 | 13.43 (11.84–24.18) | 89.74 (31.57–1195.78) |
| 20 | 13.01 (12.24–15.25) | 65.39 (23.37–242.76) |
IL: interleukin; IQR: interquartile range 25%–75%.
Comparison of the difference between initial and subsequent serum cytokine concentration per session throughout the study.
| Session | Mean difference | SEM | 95% CI | p value | ||
|---|---|---|---|---|---|---|
| IL-1β | 1 | 4 | −14.88 | 14.99 | 16.49–−46.24 | 0.333 |
| 8 | −0.03 | 15.43 | 32.27–−32.33 | 0.998 | ||
| 12 | −9.29 | 14.73 | 21.55–−40.12 | 0.536 | ||
| 16 | 30.78 | 31.13 | 95.94–−34.38 | 0.335 | ||
| 20 | 14.02 | 17.14 | 49.90–−21.86 | 0.424 | ||
| IL-10 | 1 | 4 | 0.00 | 0.15 | 0.30–−0.31 | 0.973 |
| 8 | 0.05 | 0.19 | 0.45–−0.35 | 0.807 | ||
| 12 | 0.13 | 0.21 | 0.56–−0.28 | 0.558 | ||
| 16 | 0.04 | 0.22 | 0.50–−0.42 | 0.872 | ||
| 20 | 0.14 | 0.20 | 0.55–−0.28 | 0.500 | ||
| TNFα | 1 | 4 | −2.36 | 1.89 | 1.58–−6.30 | 0.226 |
| 8 | 1.75 | 4.73 | 11.65–−8.15 | 0.715 | ||
| 12 | 2.42 | 5.04 | 12.96–−8.13 | 0.637 | ||
| 16 | 1.43 | 3.19 | 8.11–−5.26 | 0.660 | ||
| 20 | −0.03 | 3.06 | 6.38–−6.43 | 0.993 | ||
| CPK | 1 | 4 | −288.61 | 176.11 | 80.00–−657.21 | 0.118 |
| 8 | −284.05 | 188.70 | 110.92–−679.01 | 0.149 | ||
| 12 | −279.41 | 175.74 | 88.41–−647.24 | 0.128 | ||
| 16 | −245.88 | 160.24 | 89.51–−581.27 | 0.141 | ||
| 20 | −282.86 | 166.58 | 65.80–−631.53 | 0.106 | ||
| CORTISOL | 1 | 4 | 0.52 | 0.32 | 1.20–−0.16 | 0.127 |
| 8 | 1.70 | 1.06 | 3.91–−0.52 | 0.126 | ||
| 12 | 0.41 | 0.30 | 1.05–−0.23 | 0.195 | ||
| 16 | 0.09 | 0.19 | 0.48–−0.30 | 0.641 | ||
| 20 | 2.05 | 1.46 | 5.10–−0.99 | 0.175 | ||
SEM: standard error of the mean, 95% CI: 95% confidence interval.
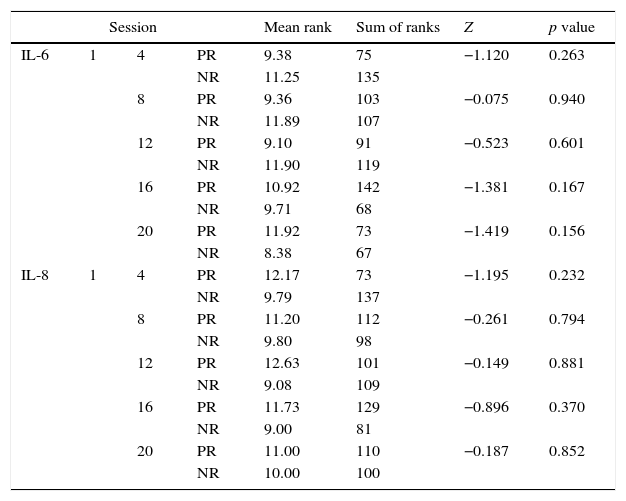
Comparison of the distribution values between the initial and subsequent sessions blood test parameters throughout the study.
| Session | Mean rank | Sum of ranks | Z | p value | |||
|---|---|---|---|---|---|---|---|
| IL-6 | 1 | 4 | PR | 9.38 | 75 | −1.120 | 0.263 |
| NR | 11.25 | 135 | |||||
| 8 | PR | 9.36 | 103 | −0.075 | 0.940 | ||
| NR | 11.89 | 107 | |||||
| 12 | PR | 9.10 | 91 | −0.523 | 0.601 | ||
| NR | 11.90 | 119 | |||||
| 16 | PR | 10.92 | 142 | −1.381 | 0.167 | ||
| NR | 9.71 | 68 | |||||
| 20 | PR | 11.92 | 73 | −1.419 | 0.156 | ||
| NR | 8.38 | 67 | |||||
| IL-8 | 1 | 4 | PR | 12.17 | 73 | −1.195 | 0.232 |
| NR | 9.79 | 137 | |||||
| 8 | PR | 11.20 | 112 | −0.261 | 0.794 | ||
| NR | 9.80 | 98 | |||||
| 12 | PR | 12.63 | 101 | −0.149 | 0.881 | ||
| NR | 9.08 | 109 | |||||
| 16 | PR | 11.73 | 129 | −0.896 | 0.370 | ||
| NR | 9.00 | 81 | |||||
| 20 | PR | 11.00 | 110 | −0.187 | 0.852 | ||
| NR | 10.00 | 100 | |||||
PR: positive ranks, NR: negative ranks.
There is a tendency toward the relation of exposure of WBC and elevation of some of the quantified substances (IL-10, Cortisol), as well as the decrease of others, such as CPK; However, this association was not statistically significant (p>0.05).
No adverse effects occurred during the study.
DiscussionAs previously mentioned, there is no specific treatment for myofascial pain syndrome. This is probably due to limited evidence of the efficacy of procedures used to treat it. This forces researchers to continue the search for the perfect treatment. Although there are several studies on the treatment of myofascial syndrome with pharmacological therapy, physical therapy, botulinum toxin application and dry needle therapy, there are no studies evaluating the efficacy of whole body cryotherapy to treat this condition. Algafly and George, and Bleakley et al. described the effects of cryotherapy on nerve conduction, pain threshold, and pain tolerance, finding that such effects were maintained during short-term exposure to cold through full-body cryotherapy.14,19
In this study, the application of whole body cryotherapy produced a decrease in perceived pain, measured with VAS. The analgesic effect was observed in each of the 20 sessions of cryotherapy, becoming more evident starting from the sixth session, where a cumulative analgesic effect is perceived. PTP measurements using algometry supports these results. Banfi et al. described the effects of the WBC on the immune and inflammatory system, inducing a decrease in IL-8 and other inflammatory factors and an increase in anti-inflammatory substances like IL-10.20
In this study, we observed a significant variability in the measured values of inflammatory and anti-inflammatory factors during the 20 sessions, representing an obstacle for the analysis of the anti-inflammatory effect of exposure to WBC. A trend toward increased IL-10 was found, which is consistent with what has been described in the literature. However, such changes were not statistically significant. There were no significant changes in IL1β and IL-6 during this study, which also coincides with the above described. In contrast, IL-8 showed a tendency to increase during the study, which does not coincide with the literature, although this change was also not statistically significant.
It is known that CPK is a muscle enzyme whose concentration in blood rises in various circumstances related to muscle injuries such as those that occur in rhabdomyolysis or physical training. Banfi et al. also described the decrease of this enzyme after exposure to WBC. In our study, we observed that CPK tended to decrease in blood. However, these data were not significant.20 Regarding the effect on cortisol, our study also did not show significant changes during the 20 sessions.
The authors attribute this absence of homogeneity and the amplitude of the results due to the lack of rigorous control in the blood sampling schedule, which may have been affected by the circadian cycle of these biomarkers. Also, we did not consider other factors that may have contributed to the physiological variability of these substances’ concentration, such as the practice and intensity of physical activity, the stage of the menstrual cycle, or the presence of concomitant diseases. Considering that the pain perceived by the patient and objectively measured pressure pain had a significant decrease over the course of the WBC sessions, and was cumulative even after the sixth session, it might be suggested to consider total body cryotherapy for myofascial syndrome of the trapezium as a treatment, requiring more than 6 sessions of WBC.
It remains to be determined whether myofascial syndrome anywhere else in the body could be improved with this therapy, if the analgesic effect lasts after sessions end and if it is superior to other treatments. Therefore, it is recommended to continue this line of research to validate this effect with a case-control study and long-term follow-up.
This study has some limitations. A control group was not included, making it difficult to accurately determine the positive effect of patients upon the application of full-body cryotherapy, as well as the role of the placebo effect. Similarly, the study sample consisted of a homogeneous group of patients with trapezius myofascial pain syndrome; therefore, it would be important to evaluate the effect of total body cryotherapy on myofascial syndrome in other areas of the body. Although we asked patients to suspend any analgesic measures (drugs and physical therapy), we did not supervise this variable outside the clinic, but we did encourage honesty from the patient. Finally, no follow-up data was collected, so it is not possible to determine the duration of the analgesic effect.
ConclusionThe findings in this study show statistically significant data on the analgesic effects of whole body cryotherapy in patients with myofascial syndrome, as evidenced by the decrease in perceived pain (VAS) and measured pain (PPT). Despite its limitations, this study suggests that WBC is an effective treatment for the reduction of pain in myofascial syndrome of the trapezius. It is important to extend the research of its use in other areas of the body, its comparison with a control group, and the duration of its therapeutic effect, to be considered a feasible therapy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingFinancing was carried out through in-house resources of the Department of Sports Medicine and Physical Rehabilitation of the “Dr. Jose Eleuterio Gonzalez” University Hospital of the Universidad Autonoma de Nuevo Leon.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Sergio Lozano-Rodríguez, M.D. for his help in the translation and editing of this manuscript, and Nery Alvarez, M.D. for his knowledge and help in statistical analysis.