The aim of this study was to analyse and compare procalcitonin (PCT) and C-reactive protein (CRP) as tools for detecting bacterial meningitis and predicting bacteraemia.
MethodsProspective, observational, and descriptive analytical study of 98 consecutive patients aged ≥15 years and diagnosed with acute meningitis in an emergency department between August 2009 and July 2013.
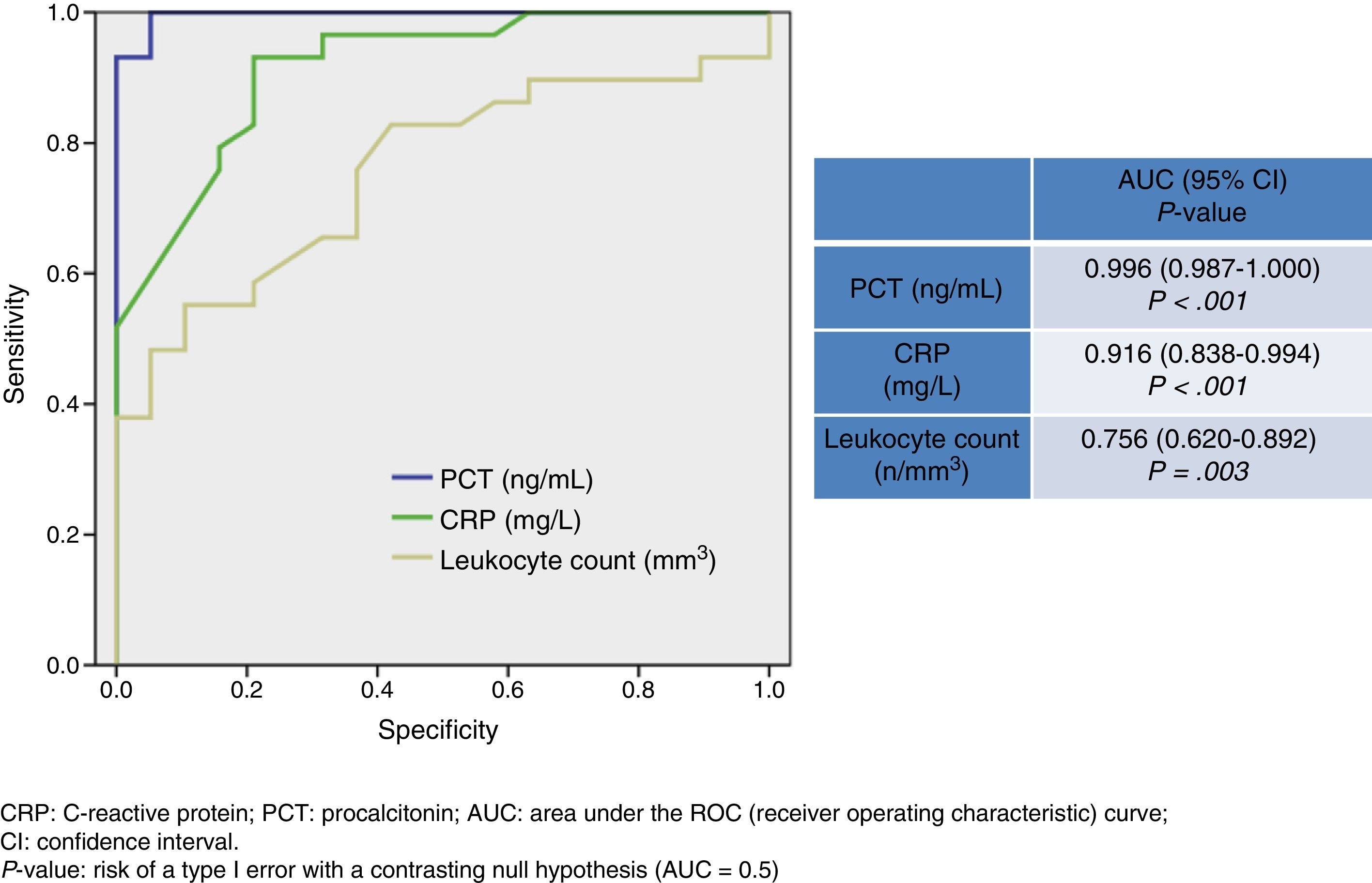
ResultsWe analysed 98 patients with AM (66 males [67%]); mean age was 44±21 years. The diagnosis was bacterial meningitis in 38 patients (20 with bacteraemia); viral meningitis in 33; probable viral meningitis in 15; and presumptively diagnosed partially treated acute meningitis in 12. PCT had the highest area under the ROC curve (AUC) (0.996; 95% CI, 0.987–1; P<.001). With a cutoff of ≥0.74ng/ml, PCT achieved 94.7% sensitivity, 100% specificity, negative predictive value (NPV) of 93.9%, and positive predictive value (PPV) of 100%. The mean levels for PCT were11.47±7.76ng/ml in bacterial meningitis vs 0.10±0.15ng/ml in viral meningitis (P<.001). The AUC for CRP was 0.916 and a cutoff of ≥90mg/L achieved 67.5% sensitivity, 86.3% specificity, PPV of 89.2%, and NPV of 90.4%.
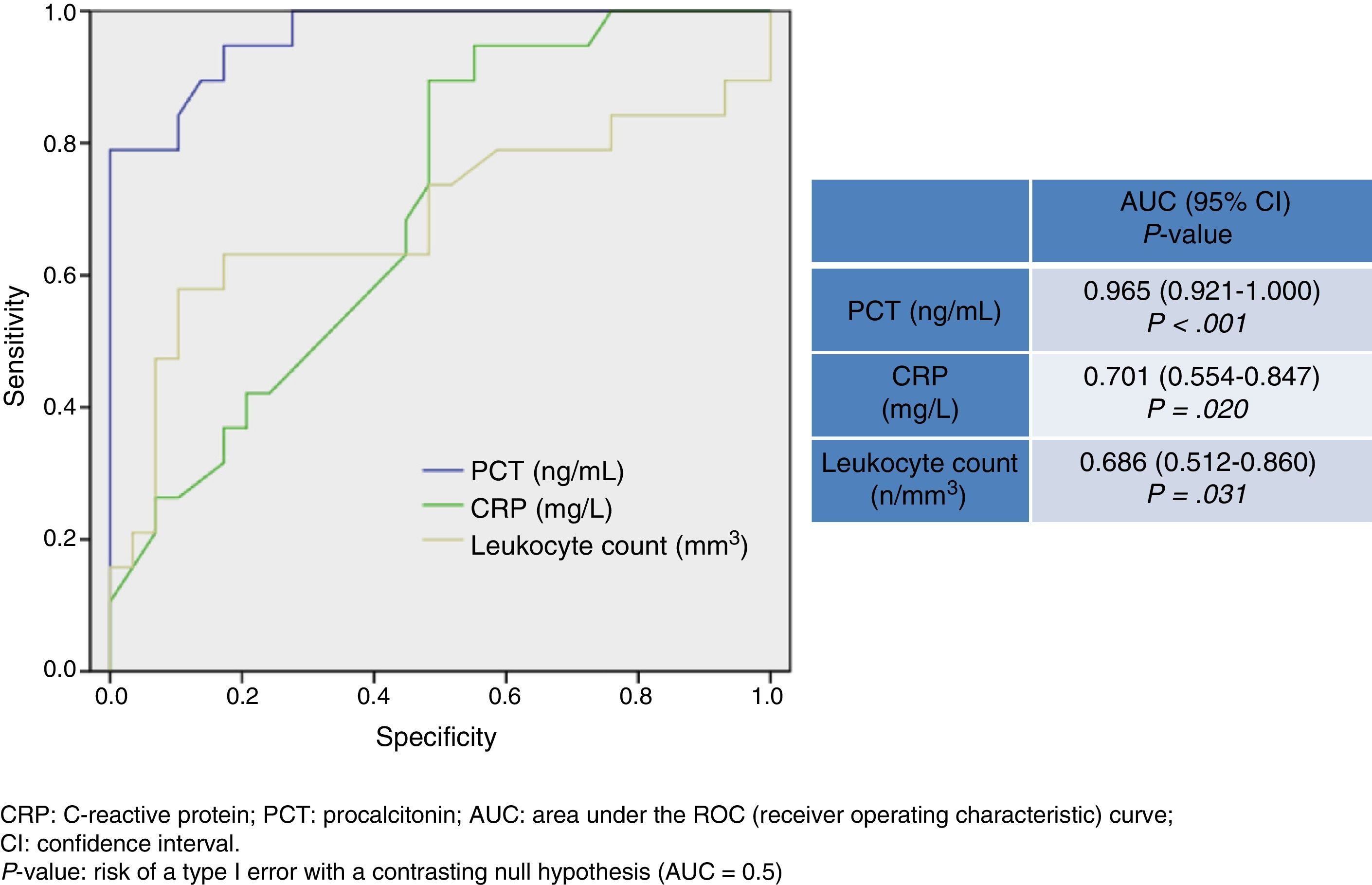
As a predictor of bacteraemia in bacterial meningitis, only PCT delivered a significant difference (14.7±7.1ng/mL vs 4.68±3.54ng/mL, P<.001). A cutoff of ≥1.1ng/mL achieved 94.6% sensitivity, 72.4% specificity, NPV of 95.4%, and PPV of 69.2%; the AUC was 0.965 (95% CI, 0.921–1; P<.001).
ConclusionsPCT has a high diagnostic power for acute meningitis in emergency department patients. PCT outperforms CRP in the detection of bacterial aetiology and is a good predictor of bacteraemia in bacterial meningitis.
El objetivo del estudio fue analizar y comparar la capacidad de la procalcitonina (PCT) y proteína C reactiva (PCR) para detectar meningitis bacteriana (MB) y para predecir la existencia de bacteriemia.
MétodosEstudio observacional, prospectivo, descriptivo y analítico de pacientes adultos (≥15años) diagnosticados de meningitis aguda (MA) en un servicio de urgencias (SU) desde agosto de 2009 hasta julio de 2013.
ResultadosSe incluyeron 98 casos diagnosticados de MA con una edad media de 44±21 años, el 67% varones (66). De ellos 38 fueron MB (20 con bacteriemia), 33 meningitis virales (MV), 15 probable MV y 12 posibles MA decapitadas. La PCT obtiene la mayor área bajo la curva ROC (ABC-ROC), de 0,996 (IC 95%:0,987–1, p<0,001) y con un punto de corte ≥ 0,74ng/ml se consigue una sensibilidad del 94,7%, especificidad del 100%, un VPN de 93,9% y un VPP del 100%. Los valores medios al comparar la PCT en MB y MV fueron 11,47±7,76 vs. 0,10±0,15ng/ml, p<0,001. La PCR consigue un ABC-ROC de 0,916 y con punto de corte ≥ 90mg/L una sensibilidad de 67,5%, especificidad de 86,3%, VPP 89,2% y VPN: 90,4%.
Para la predicción de bacteriemia en las MB solo la PCT consigue diferencias significativas (14,7±7,1 vs. 4,68±3,54ng/ml, p<0,001) y con un PC de 1,1ng/ml una sensibilidad de 94,6%, especificidad 72,4%, VPN 95,4% y VPP 69,2% y un ABC de 0,965 (IC 95%: 0,921–1, p<0,001).
ConclusionesEn los pacientes con MA en SU la PCT consigue un gran rendimiento diagnóstico para sospechar la etiología bacteriana, mayor que la PCR, y para predecir la existencia de bacteriemia en las MB.
Bacterial meningitis (BM) is an inflammatory process involving the leptomeninges. Typical findings in cerebrospinal fluid (CSF) are marked pleocytosis (>500-1000leukocytes/mm3, predominantly polymorphonuclear), elevated protein levels, and low glucose levels.1 BM is not among the 10 most frequent infectious processes seen in adult patients in emergency departments (ED) or among patients requiring hospitalisation and antimicrobial agents.2 Likewise, it is not listed in the 10 most common causes of EDs requesting a consultation with the on-call neurologist.3 However, BM is the infectious process that most frequently meets criteria for sepsis, severe sepsis, and septic shock in EDs, which reflects its severity and clinical relevance.2,4 Furthermore, the associated complications and mortality rates, even in the ED or within 24hours of admission, are high considering the low incidence of BM, although it is not ranked among the 10 most common causes of death in the ED.5 Remaining alert to potential bacterial aetiology of acute meningitis (AM), and confirming this, are therefore essential steps. Nonetheless, the situation still poses a challenge since microbial cultures and tests must be used to determine the bacterial or viral aetiology.6 The aetiology of BM tends to differ by age group. In younger adult patients, Streptococcus pneumoniae (S. pneumoniae) and Neisseria meningitidis (N. meningitidis) B are the most frequently isolated pathogens, but other pathogenic agents frequently found in patients aged 50 and older include Listeria monocytogenes (L. monocytogenes), Haemophilus influenzae (H. influenzae), and gram-negative bacteria.1,6
Non-specificity of clinical manifestations increases with age and is greater in immunodepressed patients, diabetic patients, and others likely to experience severe infections. In these patients, normal signs and symptoms do not provide optimal sensitivity and specificity for distinguishing between potential BM and viral meningitis (VM).1 EDs therefore need accurate and quick-acting tools enabling discrimination between bacterial and viral meningitis.6 Biomarkers of infection and inflammation have been proving themselves useful for more than a decade,7–12 and they can even reduce the chances of inappropriate administration of antimicrobials in EDs, and their subsequent adverse effects.13 In recent years, researchers have published several studies and reviews on the diagnostic utility of these biomarkers, especially for discriminating between infectious agents and other causes of fever, or for identifying sepsis, severe sepsis, or septic shock.7,9,14 However, few of these studies specifically addressed distinguishing between BM and VM in adult patients. Most analysed small samples or used semiquantitative techniques for measuring the biomarkers of infection and inflammation,11,12,14–17 and these techniques are less sensitive than the ones available at present.7
Measuring the C-reactive protein (CRP) released in response to inflammation and bacterial infection in BM is less sensitive and specific than measuring procalcitonin (PCT). Likewise, the kinetic properties of the former are less favourable: CRP levels do not rise until 12 to 24hours after bacterial infection and they remain high for several days after recovery.9 PCT is therefore considered the most appropriate blood biomarker of infection and inflammation to use when discriminating between BM and VM.7–12,14–17
The purpose of our study was to determine the diagnostic ability and utility of PCT for detecting BM, predicting presence of bacteraemia, and establishing a reliable cut-off point for ruling out BM and bacteraemia. These data are helpful for decision-making in the ED (hospital admission, antibiotic use, performing blood cultures or other microbiological tests).4
Patients and methodsDesignWe conducted a prospective, observational, and descriptive analytical study of patients diagnosed with AM in the ED. All patients were monitored for 30 days or until death, regardless of whether they remained in hospital or were discharged.
Study settingThe study was conducted at Complejo Hospitalario de Toledo, a tertiary care hospital with 786 beds, which serves as the reference centre for a population of 437000 inhabitants. During the study period, we attended a mean of 435 emergency visits per day.
Study period and study populationWe included all consecutive adult patients (age≥15 years) diagnosed with AM in the ED between August 2009 and July 2013 who underwent lumbar punctures, blood culture tests, and tests for biomarkers of infection and inflammation (CRP and PCT).
Included patients were categorised as follows: (1) BM, when either the pathogen or its capsular antigens were isolated in CSF (in these cases, we also tested for concomitant bacteraemia); (2) VM, when polymerase chain reaction amplification detected herpesviruses (DNA) and enteroviruses (RNA) in CSF; (3) probable VM, when CSF and blood bacterial cultures were negative; and (4) presumptively diagnosed, partially treated AM, when patients had been treated with antibiotics in the preceding 72hours, and both blood and CSF cultures were negative.
We excluded patients with a different potential primary focus of bacterial infection to avoid false-positive results from biomarkers of infection and inflammation,9 also excluding patients diagnosed with a second episode of AM during the study period or a second episode of tuberculous or autoimmune meningitis during follow-up.
The study complies with the ethical standards of our hospital. All data were coded to preserve confidentiality and were handled only by our research team. Patient follow-up was based on electronic medical histories from the hospital and primary care centres. This study itself did not motivate any treatment decisions and had no clinical implications for selected patients.
Study variablesWe recorded sociodemographic variables (age, sex); comorbidities, including presence of solid or oncohaematological tumours, liver disease, chronic heart disease, kidney disease, cerebrovascular disease, diabetes, HIV infection; and immunosuppression (solid organ transplant recipients, splenectomised patients, patients treated with ≥10mg/day of prednisone or equivalent for more than 30 days, or those treated with immunosuppressants in the past year). The original Charlson and age-adjusted Charlson comorbidity indices were calculated.18 We recorded the following clinical variables: fever (≥38°C), hypothermia (<35°C), confusion or altered level/content of consciousness, headache, nuchal rigidity, and signs of meningeal irritation (Kernig and Brudzinski signs). We also recorded any severe symptoms listed in the diagnostic criteria for sepsis, severe sepsis, and septic shock established by the International Sepsis Definitions Conference held in 2001.19 Additional data included administration of antibiotic treatment in the previous 72hours, time elapsed from symptom onset to ED arrival, hospitalisation time, and mortality rates during hospitalisation and up to 30 days of diagnosis. The microbiological studies performed were blood and CSF cultures, and polymerase chain reaction amplification of DNA (Herpesviridae) and RNA (Enterovirus). The analytical variables included results from complete blood tests, coagulation studies, biochemical studies, and serum CRP and PCT measurements.
Definitions, techniques, and methods used for the samples. Our laboratory's normal reference values were used (CRP 0-8mg/L; PCT<0.5ng/mL). CRP was measured using the quantitative method of enzyme immunoassay with a sensitivity of 1mg/L (VITROS CRP Slide®), whereas PCT was measured with a quantitative electrogenerated chemiluminescence immunoassay with a sensitivity of 0.02ng/mL (Elecsys BRAHMS PCT®).
Results from the CSF analysis were considered normal for leukocyte levels below 10cells/mm3, glucose levels at 60% to 80% of the serum glucose level, and protein levels between 15 and 45mg/dL. Pathological CSF results were classified as bacterial profile (high cell count with a predominance of polymorphonuclear leukocytes, elevated protein levels, and low glucose levels) or lymphocytic profile (predominance of mononuclear cells and normal to low glucose levels).
Statistical analysis. Data describing demographical variables, comorbidities, clinical characteristics, and progression data are presented as means±SD, ranges, medians, or percentages, as appropriate. We calculated 95% confidence limits for means and percentages (exact binomial method, in this case) to estimate population values.
The chi square test or Fisher's exact test, the t test, and the Mann-Whitney U test, as appropriate, were used to compare the distribution of biomarkers and the leukocyte count between subgroups (BM, VM, probable VM, and presumptively diagnosed partially-treated AM); by pathogen; between presence or absence of bacteraemia, sepsis, severe sepsis, or septic shock; and to compare clinical variables and outcome data. Statistical tests were two-tailed and P values <.05 were considered significant. Statistical analysis was performed using IBM-SPSS® Statistics version 19 for Windows.
The effectiveness and capacity of PCT, CRP, and leukocyte count for diagnosing BM and bacteraemia was studied using ROC (receiver operating characteristic) curve analysis. To estimate diagnostic performance, we calculated the 95% confidence interval of the area under the ROC curve (AUC) for each biomarker and compared it to the neutral value (0.5). Standard errors of the AUCs were calculated using non-parametric methods.
We used the Youden index to determine cut-off points for the values of the biomarkers of infection and inflammation with the greatest diagnostic ability and which maximised the difference between true-positive and false-positive rates. Furthermore, we estimated sensitivity, specificity, and positive and negative predictive values (PPV and NPV) for the results, and calculated 95% confidence intervals using the exact binomial method.
ResultsA total of 98 patients diagnosed with AM were included during the study period. Mean age was 44±21 years, and 67% (66 patients) were men. BM was diagnosed in 38 patients (18 with S. pneumoniae, 7 with N. meningitidis, 7 with L. monocytogenes, 4 with H. influenzae, and 2 with Escherichia coli); VM in 33 patients (30 with enteroviruses and 3 with herpes simplex virus); probable VM in 15 patients with negative results from cultures; and presumptively diagnosed partially-treated AM in 12 patients (those with a history of antibiotic treatment and negative results from cultures).
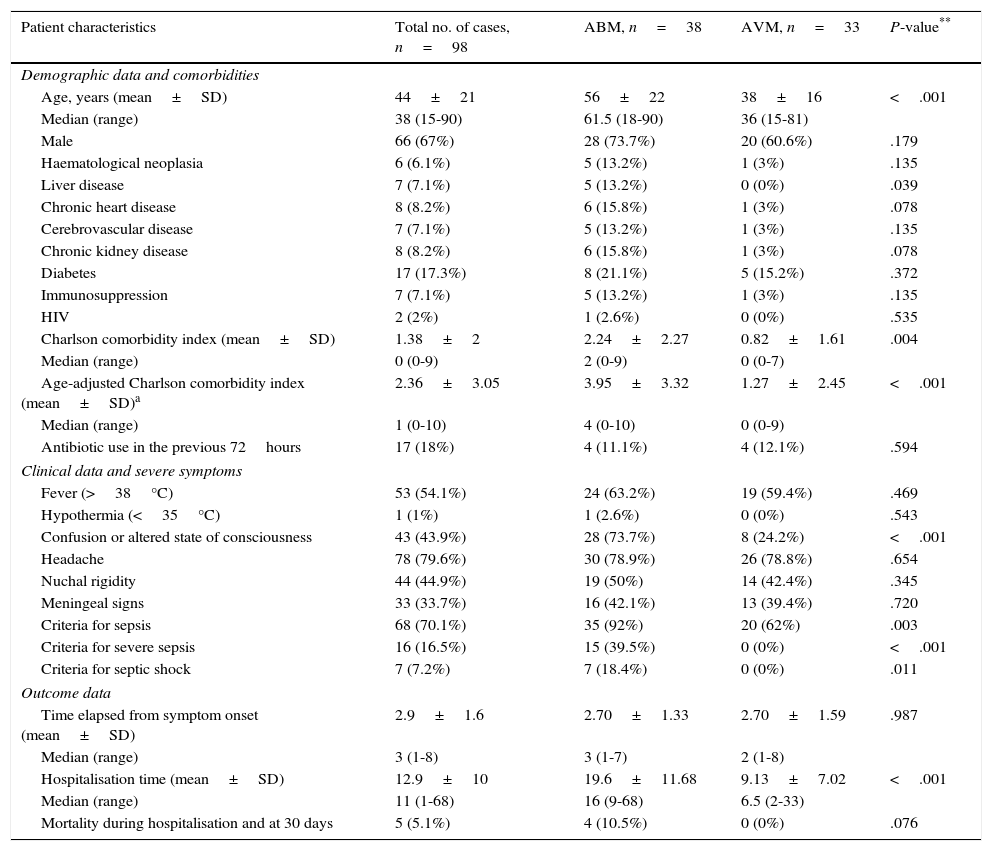
Table 1 displays sociodemographic characteristics, comorbidities, Charlson comorbidity index, clinical data, severe symptoms, and outcome data for all patients, as well as a comparison between the BM and VM subgroups. We found statistically significant differences between these 2 subgroups for the following factors: age (56±22 years vs 38±16 years; P<.001), presence of chronic liver disease (13.2% vs 0%; P=.039), and Charlson comorbidity index (2.2±2.2 vs 0.8±1.6; P=.004), with higher values in the BM subgroup in all cases. Regarding clinical presentation, confusion/altered state of consciousness was more frequent in the BM subgroup (73.7% vs 24.2%; P<.001); no significant differences were found for fever, headache, nuchal rigidity, or signs of meningeal irritation. Likewise, no significant intergroup differences were found regarding previous use of antimicrobials and time elapsed from symptom onset to visit to the ED, but we did find differences in the percentage of patients meeting diagnostic criteria for sepsis (92% vs 62%; P=.003), severe sepsis (39.5% vs 0%; P<.001), and septic shock (7% vs 0%; P=.011). Patients in the BM subgroup showed longer mean hospitalisation times and higher mortality rates during the first 30 days after diagnosis (Table 1).
Sociodemographic and clinical characteristics of patients with acute meningitis seen in the emergency department.
| Patient characteristics | Total no. of cases, n=98 | ABM, n=38 | AVM, n=33 | P-value** |
|---|---|---|---|---|
| Demographic data and comorbidities | ||||
| Age, years (mean±SD) | 44±21 | 56±22 | 38±16 | <.001 |
| Median (range) | 38 (15-90) | 61.5 (18-90) | 36 (15-81) | |
| Male | 66 (67%) | 28 (73.7%) | 20 (60.6%) | .179 |
| Haematological neoplasia | 6 (6.1%) | 5 (13.2%) | 1 (3%) | .135 |
| Liver disease | 7 (7.1%) | 5 (13.2%) | 0 (0%) | .039 |
| Chronic heart disease | 8 (8.2%) | 6 (15.8%) | 1 (3%) | .078 |
| Cerebrovascular disease | 7 (7.1%) | 5 (13.2%) | 1 (3%) | .135 |
| Chronic kidney disease | 8 (8.2%) | 6 (15.8%) | 1 (3%) | .078 |
| Diabetes | 17 (17.3%) | 8 (21.1%) | 5 (15.2%) | .372 |
| Immunosuppression | 7 (7.1%) | 5 (13.2%) | 1 (3%) | .135 |
| HIV | 2 (2%) | 1 (2.6%) | 0 (0%) | .535 |
| Charlson comorbidity index (mean±SD) | 1.38±2 | 2.24±2.27 | 0.82±1.61 | .004 |
| Median (range) | 0 (0-9) | 2 (0-9) | 0 (0-7) | |
| Age-adjusted Charlson comorbidity index (mean±SD)a | 2.36±3.05 | 3.95±3.32 | 1.27±2.45 | <.001 |
| Median (range) | 1 (0-10) | 4 (0-10) | 0 (0-9) | |
| Antibiotic use in the previous 72hours | 17 (18%) | 4 (11.1%) | 4 (12.1%) | .594 |
| Clinical data and severe symptoms | ||||
| Fever (>38°C) | 53 (54.1%) | 24 (63.2%) | 19 (59.4%) | .469 |
| Hypothermia (<35°C) | 1 (1%) | 1 (2.6%) | 0 (0%) | .543 |
| Confusion or altered state of consciousness | 43 (43.9%) | 28 (73.7%) | 8 (24.2%) | <.001 |
| Headache | 78 (79.6%) | 30 (78.9%) | 26 (78.8%) | .654 |
| Nuchal rigidity | 44 (44.9%) | 19 (50%) | 14 (42.4%) | .345 |
| Meningeal signs | 33 (33.7%) | 16 (42.1%) | 13 (39.4%) | .720 |
| Criteria for sepsis | 68 (70.1%) | 35 (92%) | 20 (62%) | .003 |
| Criteria for severe sepsis | 16 (16.5%) | 15 (39.5%) | 0 (0%) | <.001 |
| Criteria for septic shock | 7 (7.2%) | 7 (18.4%) | 0 (0%) | .011 |
| Outcome data | ||||
| Time elapsed from symptom onset (mean±SD) | 2.9±1.6 | 2.70±1.33 | 2.70±1.59 | .987 |
| Median (range) | 3 (1-8) | 3 (1-7) | 2 (1-8) | |
| Hospitalisation time (mean±SD) | 12.9±10 | 19.6±11.68 | 9.13±7.02 | <.001 |
| Median (range) | 11 (1-68) | 16 (9-68) | 6.5 (2-33) | |
| Mortality during hospitalisation and at 30 days | 5 (5.1%) | 4 (10.5%) | 0 (0%) | .076 |
Percentages do not include missing data, when applicable.
SD: standard deviation; ABM: acute bacterial meningitis; AVM: acute viral meningitis; HIV: human immunodeficiency virus.
Table 2 compares mean CRP and PCT levels and leukocyte counts between the BM and VM subgroups; all of these variables, especially mean PCT levels, show significant differences (11.47±7.76ng/mL vs 0.10±0.15ng/mL; P<.001).
Mean values of CRP, PCT, and leukocyte count between BM and VM.
| BM, n=38 | VM, n=33 | P-value | |
|---|---|---|---|
| CRP (mg/L) | 111.89±49.85 | 41.77±29.79 | .045 |
| Procalcitonin (ng/mL) | 11.47±7.76 | 0.10±0.15 | <.001 |
| Leukocyte count (mm3) | 17492±8092 | 11096±4506 | .008 |
BM: bacterial meningitis; VM: viral meningitis; CRP: C-reactive protein.
Viral meningitis: cases confirmed by polymerase chain reaction amplification of RNA for enteroviruses (30 cases) and DNA for herpes simplex virus (3 cases).
Bacterial meningitis: cases confirmed by CSF cultures (S. pneumoniae [18], N. meningitidis [7], L. monocytogenes [7], gram-negative bacteria [6]).
Statistical significance was established at P<.05.
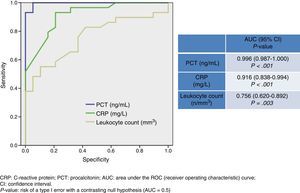
Fig. 1 shows values corresponding to the AUC for PCT level, CRP level, and leukocyte count as predictors of BM. CRP has an AUC of 0.916 (95% CI, 0.838-0.994; P<.001) and therefore its diagnostic utility is significantly greater than that of the leukocyte count. PCT, however, has the highest AUC value of all the biomarkers (0.996; 95% CI, 0.987-1; P<.001). A cut-off point ≥0.74ng/mL was shown to have the greatest diagnostic utility, achieving a sensitivity of 94.7% (95% CI, 82.2%-99.3%), a specificity of 100% (95% CI, 88.7%-100%), an NPV of 93.9% (95% CI, 79.7%-99.2%), and a PPV of 100% (95% CI, 90.2%-100%). For CRP, in turn, a cut-off point ≥90mg/L achieves a sensitivity of 67.5% (95% CI, 50.2%-81.9%), a specificity of 86.3% (95% CI, 65%-97%), a PPV of 89.2% (95% CI, 71.7%-97.7%), and an NPV of 90.4% (95% CI, 69.6%-98.8%).
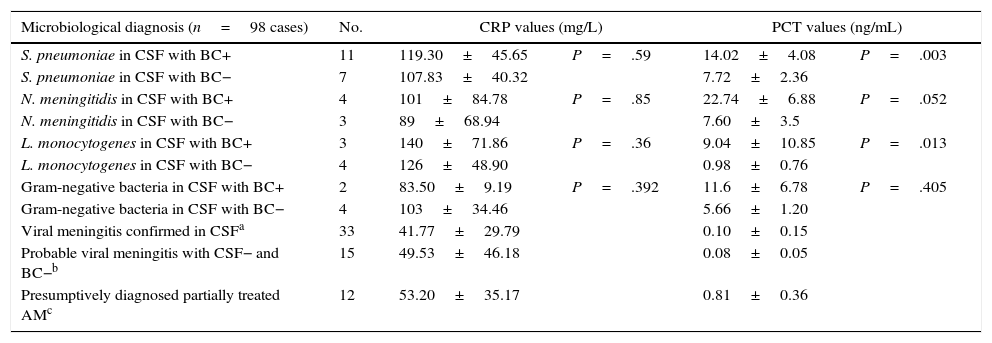
Table 3 shows some of the most relevant results of the comparison of CRP and PCT levels between subgroups with different isolated or suspected pathogens, and with and without confirmed bacteraemia. Table 4 summarises global results of comparisons between all patients with BM and bacteraemia and those in whom presence of these micro-organisms in blood was not confirmed. CRP and PCT levels differed significantly (P<.05 in all cases) between BM and VM subgroups, as shown in Table 2. There were also significant differences (P<.001) in mean PCT levels, but not in CRP, between patients with probable VM (0.08±0.05ng/mL) and patients with BM (11.47±7.76ng/mL). Differences were also significant between patients with probable VM and patients with presumptively diagnosed partially treated AM (0.81±0.36ng/mL; P=.003).
Mean values of C-reactive protein and procalcitonin in acute meningitis broken down by aetiology.
| Microbiological diagnosis (n=98 cases) | No. | CRP values (mg/L) | PCT values (ng/mL) | ||
|---|---|---|---|---|---|
| S. pneumoniae in CSF with BC+ | 11 | 119.30±45.65 | P=.59 | 14.02±4.08 | P=.003 |
| S. pneumoniae in CSF with BC− | 7 | 107.83±40.32 | 7.72±2.36 | ||
| N. meningitidis in CSF with BC+ | 4 | 101±84.78 | P=.85 | 22.74±6.88 | P=.052 |
| N. meningitidis in CSF with BC− | 3 | 89±68.94 | 7.60±3.5 | ||
| L. monocytogenes in CSF with BC+ | 3 | 140±71.86 | P=.36 | 9.04±10.85 | P=.013 |
| L. monocytogenes in CSF with BC− | 4 | 126±48.90 | 0.98±0.76 | ||
| Gram-negative bacteria in CSF with BC+ | 2 | 83.50±9.19 | P=.392 | 11.6±6.78 | P=.405 |
| Gram-negative bacteria in CSF with BC− | 4 | 103±34.46 | 5.66±1.20 | ||
| Viral meningitis confirmed in CSFa | 33 | 41.77±29.79 | 0.10±0.15 | ||
| Probable viral meningitis with CSF− and BC−b | 15 | 49.53±46.18 | 0.08±0.05 | ||
| Presumptively diagnosed partially treated AMc | 12 | 53.20±35.17 | 0.81±0.36 | ||
BC+, positive blood culture; BC–, negative blood culture; CSF, cerebrospinal fluid; CSF+, positive bacterial culture in cerebrospinal fluid; CSF–, negative bacterial culture in cerebrospinal fluid; CRP, C-reactive protein; PCT, procalcitonin.
Gram-negative bacteria: 2 cases of E. coli and 4 of H. influenzae.
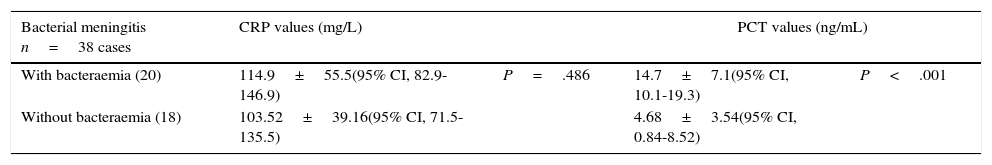
Mean values of C-reactive protein and procalcitonin in acute bacterial meningitis with or without associated bacteraemia.
| Bacterial meningitis n=38 cases | CRP values (mg/L) | PCT values (ng/mL) | ||
|---|---|---|---|---|
| With bacteraemia (20) | 114.9±55.5(95% CI, 82.9-146.9) | P=.486 | 14.7±7.1(95% CI, 10.1-19.3) | P<.001 |
| Without bacteraemia (18) | 103.52±39.16(95% CI, 71.5-135.5) | 4.68±3.54(95% CI, 0.84-8.52) | ||
CRP: C-reactive protein; PCT: procalcitonin.
Statistical significance is established at P<.05.
Regarding diagnosis of bacteraemia associated with BM, no significant differences in CRP levels were found either among the BM patient total (Table 4), or between individual cases representing different pathogen subgroups: S. pneumoniae, N. meningitidis, L. monocytogenes, or gram-negative bacteria (Table 3). For PCT, however, significant differences (P<.001) were found between patients with BM and bacteraemia (20) and patients with BM and no bacteraemia (18), with a higher mean PCT level in the former group (14.7±7.1 vs 4.68±3.54ng/mL). The same is true for patients with S. pneumoniae (P=.003) or L. monocytogenes (P=.013), but it was not observed in the very small subgroups with N. meningitidis (P=.052) and gram-negative bacteria (P=.405) (Table 3).
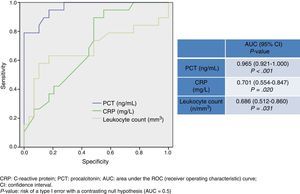
Fig. 2 shows AUC values for PCT level, CRP level, and leukocyte count for determining presence of bacteraemia in BM. PCT presents a high diagnostic utility for predicting bacteraemia in patients with BM. Its predictive ability is greater than that of CRP and leukocyte count, and its AUC is 0.965 (95% CI, 0.921–1; P<.001). A cut-off point of 1.1ng/mL has been found to achieve the greatest diagnostic utility, yielding a sensitivity of 94.7% (95% CI, 73.9%-99.8%), a specificity of 72.4% (95% CI, 52.7%-87.8%), an NPV of 95.4% (95% CI, 77.1%-99.8%), and a PPV of 69.2% (95% CI, 48.2%-85.6%).
DiscussionIn line with previous studies,11,12,15–17 our results confirm that PCT level has a high predictive ability for BM in emergency department patients diagnosed with probable AM. Furthermore, and depending on the cut-off point chosen, it displays a high predictive ability for bacteraemia in BM cases confirmed by isolating the pathogen in CSF cultures.7,11 In both situations, the diagnostic utility of PCT is significantly higher than that of CRP. On the other hand, diagnostic utility of CRP is known to decrease in older patients, but this is not true for PCT.9 This must be considered when evaluating and interpreting levels of the different biomarkers of infection and inflammation, among other reasons because patients with BM (56±22 years) are older than patients with VM (38±16 years), as in our sample. Clinical manifestations become more non-specific with age, in immunodepressed patients, diabetic patients, and other patients likely to experience severe infections. In such cases, normal signs and symptoms do not provide optimal sensitivity and specificity for discriminating between potential cases of BM and VM.1 In our sample, neither fever, nor headache, signs of meningeal irritation, or nuchal rigidity were suggestive of a bacterial aetiology; only confusion or altered level/content of consciousness was more frequent in BM than in VM (73.7% vs 24.2%), although this sign is also seen in patients with viral meningoencephalitis. This being the case, ED doctors need quick and reliable tests for diagnosing BM (it only takes 20-30minutes to obtain blood test results).11 These tests may help with triage decisions which will have a crucial impact on the patients’ prognosis and outcomes from the time they enter the hospital,20 since they will result in activating ‘code sepsis’ or ‘code meningitis’. These protocols include extraction of cultures and samples for microbiology studies, early administration of the appropriate antibiotics, hospitalisation in the most suitable unit, etc.
Regarding the ability to discriminate between BM and VM, our results coincide with those reported in previous studies11,12,15–17 in which PCT was shown to have greater diagnostic utility than CRP and leukocyte count. There is controversy, however, regarding the optimal cut-off point, which varies greatly from study to study (mainly due to differences in sample size and the technique used for measuring PCT). It ranges from 0.2 to 5ng/mL (for sensitivity and specificity values above 90%).11,12,15–17,21–24 According to some researchers, PCT values ranging between 0.2 and 0.5ng/mL are optimal for diagnosing BM,12,16,21,22 with a specificity and an NPV of 90%. Other researchers, such as Ray et al.,22 achieved a sensitivity of 87%, a specificity of 100%, a PPV of 100%, and an NPV of 99% with a cut-off point of 2.13ng/mL. According to one of the most relevant studies, conducted by Viallon et al.12 and including 254 patients with AM (35 with BM and 181 with VM), cut-off points ≥0.28ng/mL for PCT provided the greatest diagnostic utility, achieving a sensitivity of 95%, a specificity of 100%, a PPV of 100%, and an NPV of 97%, with an AUC of 0.99 (95% CI, 0.99-1). These results coincide with our own except for the cut-off point: in our study, the same specificity and PPV were achieved with a higher cut-off point (PCT≥0.74ng/mL). In light of these results and in order to achieve the maximum diagnostic certainty, ED doctors must regard initial PCT levels >0.25ng/mL as diagnostic of BM. The necessary microbiology tests should then be performed6 and appropriate antibiotic treatment should be started immediately.9
A novel finding from our study is that there are significant differences in PCT levels between patients with probable VM (without a diagnosis) and both confirmed BM (P<.001) and presumptively diagnosed partially treated AM (P=.003): 0.08±0.05, 11.47±7.76, and 0.81±0.36, respectively. When diagnosis is unclear, PCT may provide helpful clues for determining the aetiology of AM cases.
As shown by Viallon et al.,12 combining serum PCT levels and CSF lactate levels increases the diagnostic utility beyond that of either factor alone.
Although a few studies16,17,25 have analysed and compared the diagnostic abilities of CSF and serum PCT, they have a low statistical power. In any case, CSF PCT levels in these studies have demonstrated a lower predictive value than serum PCT levels. CSF PCT alone is therefore not recommended for diagnosing BM, but it can be useful when used in combination with CSF lactate results.12
Future studies should follow up on our findings by analysing the ability of serum PCT combined with CSF lactate levels for differentiating BM and presumptively diagnosed partially treated AM from confirmed or suspected VM cases.
As previously described in patients with severe bacterial infections9,10 and other infectious processes which have been more thoroughly investigated,26 presence of bacteraemia led to higher PCT levels in patients with BM in our sample (14.7±7.1 vs 4.68±3.54, P<.001). CRP levels show no significant differences, either among the total sample of patients with BM or between individual cases with different isolated pathogens. PCT shows no significant differences for cases with N. meningitidis (P=.052) and gram-negative bacteria (P=.405), but this may be due to the small sample sizes in both subgroups. In any case, global differences in PCT levels between BM patients with and without bacteraemia are obvious. The cut-off point for predicting bacteraemia varies greatly from study to study,9,10 ranging from 0.4 to 5ng/mL. In our study, a cut-off point of 1.1ng/mL achieved a sensitivity of 94.7%, a specificity of 72.4%, an NPV of 95.4%, and a PPV of 69.2% with an AUC of 0.965. These results are similar (cut-off point of 0.95-1ng/mL) to those reported by other studies on infectious aetiologies9,26 and by the Tudela et al. study,10 which proposes a predictive model designed for EDs which includes a PCT measurement and uses a cut-off point of 0.4ng/mL. For these reasons, bacteraemia and BM should be suspected in patients whose ED tests yield PCT levels >1ng/mL, as suggested in a recent review article.9
Our study has several limitations, including its single-centre study design and its small sample size (we cannot rule out presence of type II errors). This sample size was insufficient for some comparisons and may have affected cut-off points and performance. Our analysis did not include complications (encephalitis, seizures, etc.), although this data might have provided more information. Including CSF lactate levels in our analysis might also have increased its diagnostic ability, which could have led us to design a more reliable predictive model for BM. Another limitation is the lack of a healthy control group, which would have strengthened the study's internal validity. Despite these limitations, we feel that our study reflects the clinical reality of how our ED handles suspected and confirmed meningitis, and it shows the major role PCT levels may play in diagnosing BM and predicting bacteraemia.
In conclusion, as we await results from future studies with greater statistical power and larger sample sizes, PCT has been proved unquestionably useful in EDs based on its high diagnostic power for distinguishing between BM and VM and for determining presence of bacteraemia. In fact, it has been included in validated diagnostic protocols for other infectious processes such as severe pneumonia,27 and in sepsis and septic shock.28 In the near future, PCT measurement is very likely to be added to diagnostic protocols for BM in EDs as an additional tool for emergency doctors and neurologists.
FundingThis study has not been presented at any scientific event, nor has it received funding from any public or private institutions.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Morales Casado MI, Moreno Alonso F, Juárez Belaunde AL, Heredero Gálvez E, Talavera Encinas O, Julián-Jiménez A. Capacidad de la procalcitonina para predecir meningitis bacterianas en el servicio de urgencias. Neurología. 2016;31:9–17.