Astrocytes have been considered mere supporting cells in the CNS. However, we now know that astrocytes are actively involved in many of the functions of the CNS and may play an important role in neurodegenerative diseases.
DevelopmentThis article reviews the roles astrocytes play in CNS development and plasticity; control of synaptic transmission; regulation of blood flow, energy, and metabolism; formation of the blood-brain barrier; regulation of the circadian rhythms, lipid metabolism and secretion of lipoproteins; and in neurogenesis. Astrocyte markers and the functions of astrogliosis are also described.
ConclusionAstrocytes play an active role in the CNS. A good knowledge of astrocytes is essential to understanding the mechanisms of neurodegenerative diseases.
Los astrocitos han sido considerados como células de sostén en el SNC. Sin embargo, hoy día se sabe que participan de forma activa en muchas de las funciones del SNC y que pueden tener un papel destacado en las enfermedades neurodegenerativas.
DesarrolloSe revisan las funciones del astrocito en el desarrollo y plasticidad del SNC, en el control sináptico, regulación del flujo sanguíneo, energía y metabolismo, en la barrera hematoencefálica, regulación de los ritmos circadianos, metabolismo lipídico y secreción de lipoproteínas y en la neurogénesis. Asimismo, se revisan sus marcadores y el papel de la astrogliosis.
ConclusiónLos astrocitos tienen un papel activo en el SNC. Su conocimiento parece esencial para comprender los mecanismos de las enfermedades neurodegenerativas.
Glial cells make up the largest part of the cells in the nervous system. Also known as glia (from the Greek word for glue), these cells have been evolutionarily conserved. The proportion of these cells in each different nervous system seems to correlate with the size of the animal: for example, we find 25% in fruit flies, 65% in mice, 90% in humans, and 97% in elephants.1 Based on their shape, function, and location, glial cells are classified as follows: (1) microglia, the only glial cells of immune origin, which reach the brain through the blood during early development; (2) astrocytes; and (3) Schwann cells and oligodendrocytes, which form myelin layers around axons in the peripheral and central nervous systems, respectively. Some authors describe an additional special type of glial cells, glia-NG2, which receive synaptic input directly from neurons.2 Astrocytes, accounting for 25% of the total brain volume, are the most abundant glial cells.3 Whereas the respective functions of the microglia and oligodendrocytes are well-known (local defence and myelination), the astrocytes play a more enigmatic role. When they were first described by Ramón y Cajal, and in later works by Río-Hortega, they were considered mere supporting cells, but their function has been reconsidered in recent years. As scientists’ understanding of these structures grew, they were found to be necessary elements in the maintenance of the microenvironment permitting proper function. A wide variety of specific functions has been attributed to these cells in the last 20 years. Molecular studies of these cells show that they play a key role in transmitting information in the nervous system. This 2-part review aims to analyse the function of astrocytes in mechanisms potentially at work in the most common neurodegenerative diseases.
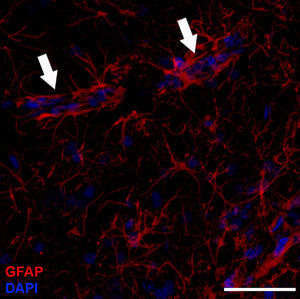
Astrocyte morphology and organisationBased on their shape, antigen phenotype, and location, astrocytes are classified into 2 major groups: protoplasmic and fibrous. Protoplasmic astrocytes are found in the grey matter and their processes contact both synapses –about 100 000 per astrocyte4– and blood vessels (Fig. 1). They have a rounded shape with several main branches that terminate in very ramified, uniformly distributed processes. Fibrous astrocytes are located in the white matter and they contact the nodes of Ranvier and the blood vessels. They are less ramified than protoplasmic astrocytes, and their processes are longer and more fibre-like. Although astrocytes occupy discrete domains and their processes do not overlap in adult brains, electron microscope analysis reveals that both subtypes establish gap junctions with the processes of neighbouring astrocytes. While this classification system is widely used, astrocytes form a very heterogeneous population containing many different subtypes. Furthermore, astrocytes even differ within the same region of the brain. This is not surprising when we consider that they must carry out their functions in specific regions of the nervous system.1 For example, we find such specialised astrocytes as the retinal Müller cells and the Bergmann glia in the cerebellum.5 The astrocytic cells in the subventricular zone (SVZ) belong to a subtype of astrocyte that is able to proliferate in the adult brain. Beginning in the postnatal period, astrocytes are arranged in the nervous system in an orderly manner with scarcely any overlaps, in parallel with vascular and neuronal territories.6 In grey matter, only the distal ends of protoplasmic astrocytes intertwine to provide the substrate for forming gap junctions.7–9 A similar arrangement may exist for fibrous astrocytes in the white matter, but this has not yet been demonstrated.10
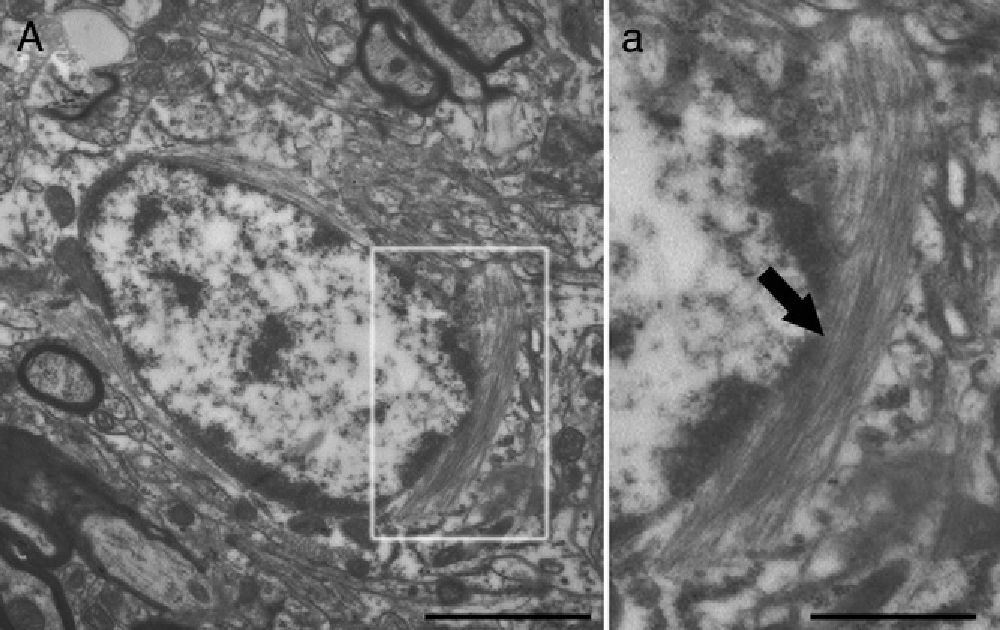
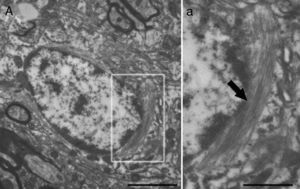
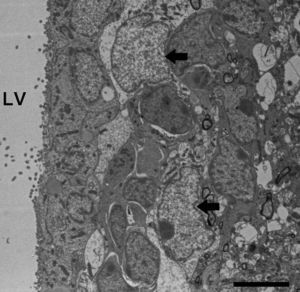
Molecular description, glial fibrillary acidic protein, and other astrocyte markersThe structure of the astrocyte cytoskeleton is supported by the network of intermediate filaments (Fig. 2), and the fundamental component of these filaments is glial fibrillary acidic protein (GFAP). In addition to their structural properties, they may play other roles having to do with the transduction of biomechanical and molecular signals.11 GFAP is elevated in processes causing brain damage and degeneration of the central nervous system (CNS), and its expression increases with age. This is the classic marker used in the immunohistochemical identification of astrocytes. It was first isolated from multiple sclerosis plaques made up of demyelinated axons and fibrous astrocytes.12,13
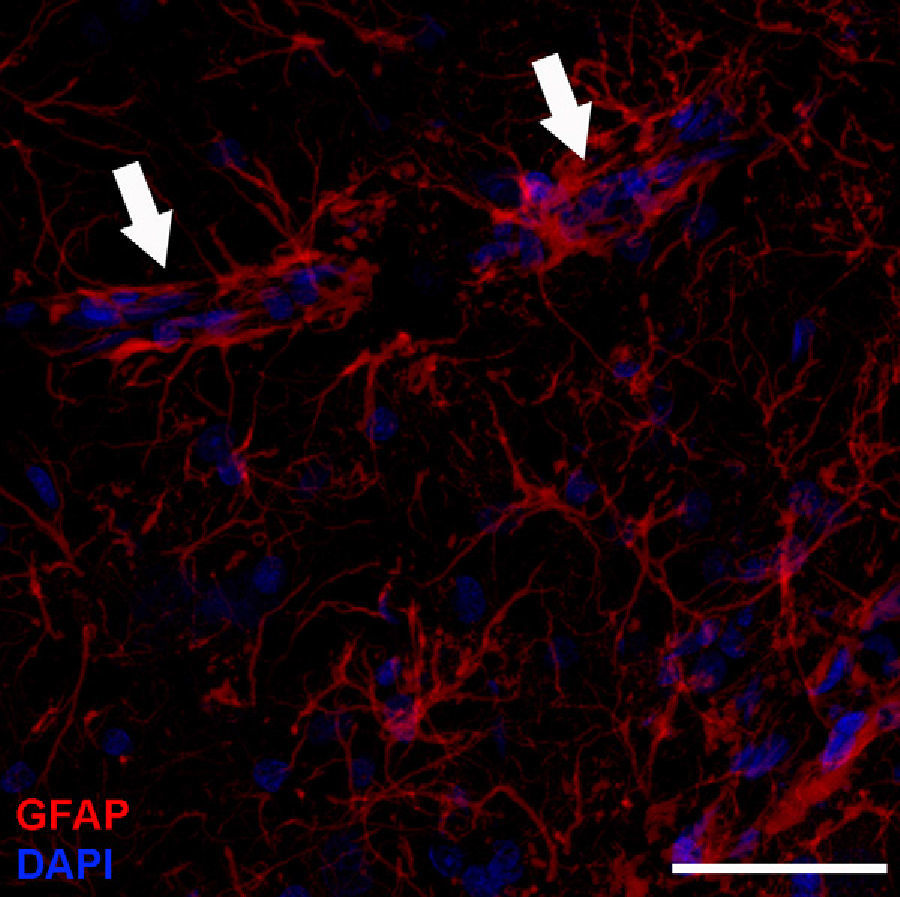
Electron microscope image showing an astrocyte (A) and the following key characteristics: cytoplasm is clearer and some ribosomes are apparent. The nucleus of these cells shows densely packed chromatin. These cells contain abundant intermediate filaments (a, arrow) whose material includes a specific protein, GFAP (box a). Bar in A=2μm; a=0.5μm.
GFAP has 8 isoforms generated by alternative splicing. Each type is expressed by a specific astrocyte subgroup and it confers different structural properties to the network of intermediate filaments. The most abundant isoform, GFAPα, was the first one to be identified.14 Scientists subsequently discovered the isoforms β (the only one to be found only in rats, not in humans),15 γ,16 ¿,17,18 κ,19 and Δ135, Δ164, and Δexon6.20
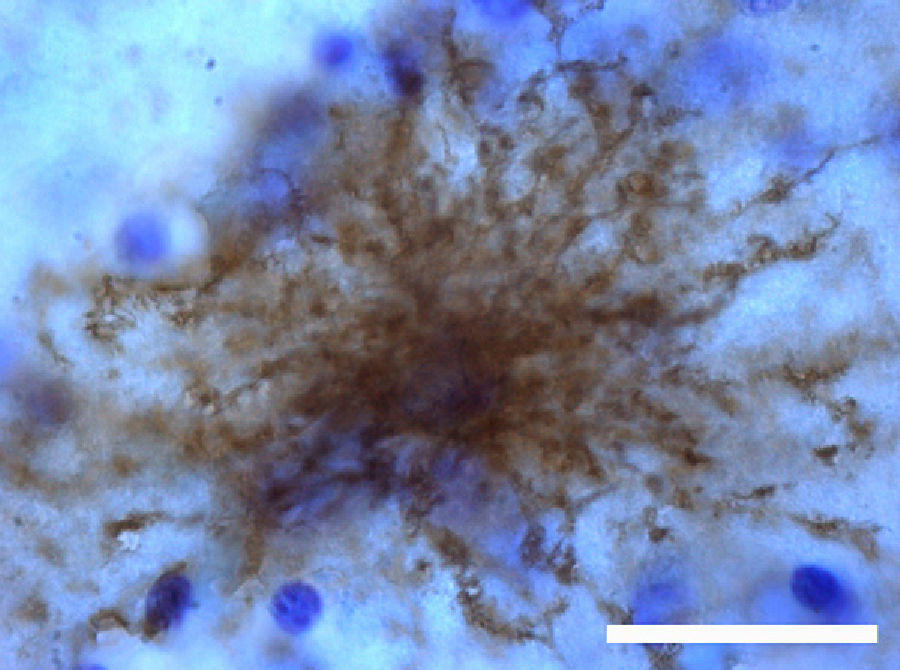
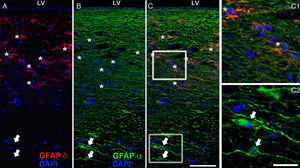
The most relevant human isoform is probably GFAPδ. A specific subpopulation of astrocytes situated in the SVZ and the subpial zone of the ventricles expresses GFAPδ17 (Fig. 3). The subventricular location of these astrocytes suggests that they are in fact neural stem cells (NSC) in the brains of adult humans.21–23 During early embryonic development (gestation weeks 13-15), GFAPδ is expressed in the radial glia of the ventricular zone, in parallel with GFAPα. Beginning in week 17, GFAPδ is also expressed in the SVZ, and this expression continues until the foetus is born.24 GFAP is also expressed in the human hippocampus, SVZ25 (Fig. 3), and areas of the spinal cord with abundant astrocytes, including its central canal.26 Furthermore, GFAPδ expression has been observed in different types of gliosis. Strangely, GFAPδ and vimentin coexist in normal tissue and in gliosis, but not in gliomas.27
GFAP-α (in green) and GFAP-δ (in red) are isoforms of the GFAP protein. The α isoform, expressed in all astrocytes, is the classic marker used for identifying those cells with immunohistochemical techniques (B, c2). The δ isoform is only expressed in those astrocyte populations that are closely linked to neurogenic niches such as the SVZ (A, c1). The image shows confocal microscope images of the 2 isoforms in the human SVZ. C and c1 display the colocation of GFAP-α and GFAP-δ (asterisks), whereas c2 shows only GFAP-α expression (arrows). Bars A-C: 75μm; c1 and c2: 25μm.
While immunohistochemical staining is the classic method used to identify astrocytes, this marker also shows certain limitations.10 (1) GFAP is expressed by most of the reactive astrocytes that respond to CNS lesions, but the protein is not always detectable by an immunohistochemical study in astrocytes from healthy tissue or those distant from the lesion site. Furthermore, GFAP expression is variable and regulated by a large number of intracellular and intercellular signalling molecules.28 (2) GFAP is not present throughout the cytoplasm, but rather can only be found in the main branches. This being the case, immunohistochemical studies of GFAP are limited when it comes to estimating astrocyte size and degree of ramification. (3) Lastly, GFAP expression is not exclusive to protoplasmic and fibrous astrocytes. Within the CNS, GFAP is also expressed by retinal Müller glial cells, Bergmann glia in the cerebellum, tanycytes at the base of the third ventricle, pituicytes in the posterior pituitary, and others; it is also expressed by the NSCs in embryonic and adult brains.29 Other cells outside the CNS that express GFAP include non-myelinating Schwann cells of the peripheral nervous system and a population of enteric glia that extend through the neural plexuses of the enteric nervous system. Enteric glia surround the cell bodies and axons of the enteric nervous system and contact with blood vessels and epithelial cells. Furthermore, some studies seem to indicate that their functions are analogous to those of astrocytes in the CNS.30,31 GFAP is also expressed by mesenchymal stellate cells in many organs, including the liver, kidneys, pancreas, lungs, and testicles.32
Due to these limitations, other markers are used for astrocyte identification, including glutamine synthetase33,34 and S100β35. Likewise, transcriptome analysis enables the identification of the molecular markers that are typical of astrocytes.36,37 Such markers include the Aldh1L1 gene, which shows a more ample expression pattern in astrocytes than GFAP, and the phagocytic pathways Draper/Megf10 and Merk/integrin alpha(v)beta5, a fact which suggests that astrocytes may be true phagocytes.5 The Draper/Megf10 pathway has already been identified in Drosophila astrocytes, where it mediates axon pruning during metamorphosis,38 and also in the Schwann cells that mediate axon removal in the neuromuscular junction during development.39 These findings suggest that astrocytes may mediate synapse removal during mammalian development. Other genes specific to the transcriptome include ApoE, ApoJ, MFGE8, and cystatin C. While the function of these genes is not yet well-understood, it seems that the first 3 contribute to the secretion of lipoprotein particles by astrocytes, and they probably behave like opsonins that facilitate phagocytosis.5
Astrocyte physiologyAstrocytes do not generate action potentials, but they are excitable cells and communication elements. They can be activated by internal or external signals and they send specific messages to adjacent cells in a process known as gliotransmission.6 Astrocytes present temporary increases in intracellular calcium concentration [Ca2+]i. These calcium peaks are responsible for astrocyte-to-astrocyte and astrocyte-to-neuron communication. They occur (1) as intrinsic oscillations resulting from release of calcium stored in the cell (spontaneous excitation), or (2) peaks induced by transmitters released by neurons. In the latter case, neurons release substances such as ATP or glutamate that activate receptors coupled to G proteins which generate an increase in inositol triphosphate (IP3). In turn, IP3 mediates release of calcium by the endoplasmic reticulum.40 Surprisingly, Schummers et al.41 found that these calcium waves do not propagate to other astrocytes in vivo. This suggests that astrocytes respond as individual cells and have unique response patterns, as is also true of neurons. As a result of an increase in [Ca2+]i, astrocytes release gliotransmitters into the extracellular space, thereby inducing receptor-mediated currents in neurons. These gliotransmitters also reach neighbouring astrocytes. The presence of this calcium-mediated signalling suggests that astrocytes play an active role in synaptic transmission, as we will discuss below.
FunctionsDevelopment of the nervous system and synaptic plasticityAstrocytes play a fundamental role in nervous system development. Growing axons are directed towards their targets by astrocyte-derived guide molecules, such as tenascin C and proteoglycans.42 Researchers have also suggested that astrocytes may play a role in synaptic pruning by means of the Draper/Megf10 and Merk/integrin alpha(v)beta5 phagocytic pathways5 and the release of signals that induce expression of the C1q protein, which activates the classical complement pathway.43 Furthermore, changes in gap junctions between astrocytes due to loss of connexins 43 and 30 are a cause of demyelination.44
Astrocytes are also active participants in synaptogenesis, not only during development, but also following lesion to the CNS. In a study of retinal ganglion cells, Pfrieger and Barres observed that, in the absence of glia, these cells presented little synaptic activity, whereas synaptic activity with astrocytes was 100 times higher. Curiously, when co-cultured with other types of cells, such as oligodendrocytes, synaptic activity of retinal ganglion cells did not increase.45 This increase in synaptic activity mediated by astrocytes is due precisely to the increase in the number of synapses, which is 7 times higher in retinal ganglion cells co-cultured with astrocytes than in cells cultured without astrocytes.46
This increase in the number of synapses is mediated by matricellular proteins named thrombospondins.47,48 Thrombospondins are a family of 5 homologous proteins inducing synaptogenesis, and astrocytes express at least 4 of them during development and following damage to brain tissue. Thrombospondins are able to induce the formation of ultrastructurally normal synapses on both the presynaptic (synapsin clustering) and postsynaptic levels (PSD-95).5 Nevertheless, these synapses are silent and require astrocytes to secrete another protein, as yet unidentified, which induces the postsynaptic response to glutamate (AMPA).48 Furthermore, the cholesterol that forms complexes with ApoE-containing lipoproteins also increases presynaptic function, according to Mauch et al.49 Secretion of thrombospondins by immature astrocytes is mediated by ATP and other neurotransmitters,50 which suggests that neuronal activity can in turn regulate the synaptogenic capacity of astrocytes. Paradoxically, the thrombospondin gene is one of the few to be expressed considerably more in humans than in other primates, suggesting that it contributes to the tremendous cerebral plasticity that characterises the human species.51 Due to the above, and to the role of thrombospondin in synaptic pruning, it has also been suggested that astrocytes may participate in creating new circuits and repairing them after lesions.
Control of synaptic functionEvidence shows that astrocytes participate directly in synaptic transmission by releasing synaptically active molecules called ‘gliotransmitters’. These molecules are released by astrocytes in response to neuronal synaptic activity that excites astrocytes, thereby generating waves of [Ca2+]i and neuronal excitability. Increasing evidence indicates that astrocytes have an impact on synaptic activity. For example, Kang et al. showed that astrocytes in hippocampus slices mediate the potentiation of inhibitory synaptic transmission.52 Fellin et al. provided the earliest evidence that astrocytes induce neuronal synchrony mediated by glutamate53; Shigetomi et al. showed that 2 forms of astrocyte calcium excitability have different effects on NMDA receptors in CA1 pyramidal neurons.54
One of the best-studied gliotransmitters is glutamate. Research has shown glutamate release by the subpopulation of NG2-positive astrocytes, which are oligodendrocyte precursor cells,55 but the pathway in question remains controversial. One theory is that astrocytes release glutamate using vesicular compartments, but other authors remain sceptical. Barres lists 2 reasons why vesicular release would be unlikely.5 (1) Astrocytes, unlike neurons, contain high levels of the enzyme glutamine synthetase, the catalyst for glutamate to glutamine degradation, whereas glutamate levels in astrocytes are relatively low and hard to detect by immunohistochemistry techniques. (2) In vivo, astrocytes express none of the components that intervene in vesicular release of neurotransmitters in neurons. Neither astrocytes nor SNAP25 have been shown to contain the synaptic vesicle proteins synaptotagmin I and synaptophysin, nor do they contain synaptic vesicle protein 2 (SV2).56–58
Although astrocytes are not theoretically capable of vesicular release, they do have microvesicles similar to synaptic vesicles, known as synaptic-like microvesicles (SLMV).59 Some studies have shown that these cells secrete gliotransmitters by way of lysosomal exocytosis.60–62 Secretory lysosomes are especially abundant in immune cells and the glia. Secretory lysosomes in astrocytes release ATP and blocking this ATP release will inhibit propagation of calcium waves to nearby astrocytes.5 Furthermore, in vivo studies show that astrocytes regulate synaptic transmission and plasticity by releasing ATP.63
Another substance released by astrocytes and that acts as a gliotransmitter is D-serine, which together with glutamate is a coagonist of the NMDA receptor.64,65 Although serine racemase is also expressed by neurons, only astrocytes are able to synthesise serine. Therefore, D-serine levels in the synapse depend on the quantity of serine produced by astrocytes.7 Another enzyme expressed fundamentally by astrocytes is pyruvate carboxylase, which provides the 4-carbon skeleton necessary for de novo synthesis of glutamate and neuronal GABA.66 This suggests that the rate at which astrocytes release this precursor determines the rate at which neurons will fire.
Furthermore, astrocytes release growth factors and cytokines that exert more potent and prolonged effects on the synapse. For example, TNFα induces insertion of AMPA receptors in the presynaptic membrane,67 but it remains unclear whether the factor is generated by the microglia or by astrocytes. Other substances secreted by astrocytes and which may contribute to synaptic function include polyunsaturated fatty acids and steroids such as oestradiol, progesterone, and other neuroactive intermediaries and metabolites with a particular affinity for GABA receptors.68
All this evidence points to the ‘tripartite synapse’ theory according to which all astrocytes are directly and interactively involved in synaptic activity as well as being necessary for proper processing of information in cerebral circuits.69–71
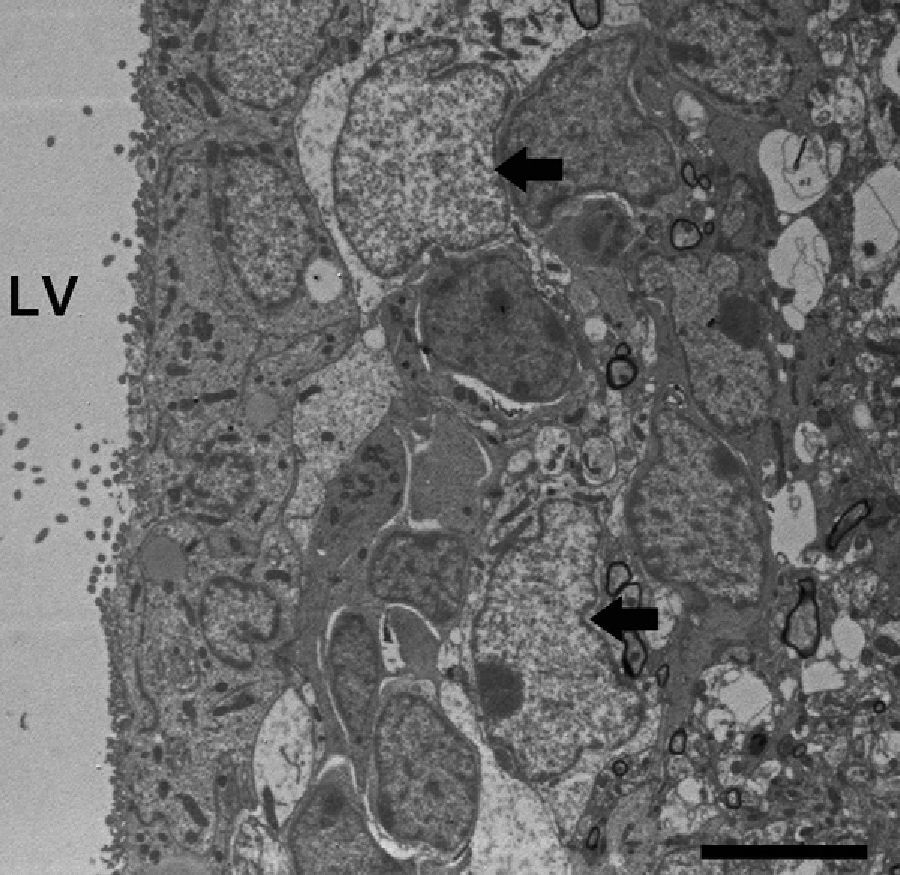
In addition to releasing gliotransmitters, astrocytes participate in correct synaptic function by maintaining synaptic homeostasis of the interstitial fluid. Astrocytes surround the synapse and maintain appropriate levels of pH, ions, neurotransmitters, and fluid.72 For example, astrocyte projections have a high aquaporin 4 content (Fig. 4) for transporting water, as well as transporters for K+ uptake. Astrocyte membranes also include Na+/H+ transporters, different types of bicarbonate transporters, monocarboxylic acid transporters, and vacuolar proton ATPase,73 all of which are involved in pH regulation.
Blood flow regulationAstrocytes also regulate the blood flow that reaches the nervous system, and changes in cerebral microcirculation are linked to neuronal activity.74 In fact, calcium waves in astrocytes are correlated with increases in microcirculation. Evidence suggests that neuron signals induce calcium waves in astrocytes which then release mediators such as prostaglandin E, nitric oxide, or arachidonic acid, substances with vasodilator or vasoconstrictor effects.75–78
Astrocytes perform this function since they have 2 domains: one vascular (Fig. 1) and the other, neuronal. This intersection of neurons, astrocytes, and blood vessels is called the neurovascular junction (Fig. 1). By means of these junctions, astrocytes adjust vascular flow to the synaptic activity, as has been shown in recent studies of the visual cortex. Changes in microcirculation mediated by astrocytes in response to visual stimuli have been detected using fMRI.41,79 Homeostasis of the neurovascular junction is fundamental for cognitive function, and imbalances may be linked to cognitive changes, including Alzheimer disease.80
Energy and metabolism in the central nervous systemAstrocytes contribute to proper metabolic function in the CNS. Since their projections reach blood vessels, astrocytes take up glucose from circulation and provide energy metabolites to neurons.10 In fact, astrocytes contain the main reserve of glycogen granules in the CNS, and these granules are more abundant where synaptic density is highest.81 In addition, evidence shows that levels of glycogen in astrocytes are modulated by glutamate,82 and that glucose metabolites are transmitted to nearby astrocytes by gap junctions in a process that is also modulated by that chemical.83
Blood-brain barrierThe blood-brain barrier (BBB) consists of endothelial cells that form tight junctions enveloped by basal lamina, perivascular pericytes, and astrocyte endfeet. The function of astrocytes in the BBB is not well understood, but evidence suggests that they lend barrier-like properties to endothelial cells by releasing such factors as TGFβ, GDNF, bFGF, and angiopoietin 1,84 as well as exerting an effect on BBB polarity.85
Regulation of circadian rhythmsIn Drosophila and in mammals, glial cells exhibit circadian morphological and biochemical changes.86 For example, the suprachiasmatic nucleus (SCN), which works like an internal pacemaker, displays rhythmic changes in GFAP levels and astrocyte morphology in hamsters.87 Astrocytes communicate with neurons via adenosine, and they are involved in sleep homeostasis and the cognitive effects that arise from sleep deprivation. In fact, inhibiting gliotransmission prevents the cognitive impairment associated with sleep deprivation.88 No animal models in mammals have been able to show that astrocytes directly regulate the function of pacemaker neurons of the CNS. However, studies in cultured astrocytes indicate that the presence of vasoactive intestinal peptide is essential to the opposite function: neuronal-glial communication in circadian rhythms.89
Lipid metabolism and lipoprotein secretionIn the human body, the brain is the organ with the highest cholesterol content. Cholesterol levels are meticulously regulated in neurons and glial cells, and changes in the metabolism of lipids, especially cholesterol, are closely linked to the development of neurodegenerative diseases including Alzheimer disease or Niemann-Pick disease type C.90–92 Lipoproteins and cholesterol in the CNS do not come from peripheral blood; rather, they are synthesised by glial cells, fundamentally astrocytes. ApoE is the main type of apo in the CNS and lipoproteins with glial ApoE provide neurons with cholesterol and other molecules by means of LDL family receptors.93 In addition to taking up lipoproteins, these receptors also function as signal transducers for lipoprotein ligand-binding. In this way, lipoproteins with ApoE stimulate axonal growth in the CNS94 and cholesterol bound to lipoproteins with ApoE participates in synaptogenesis. Researchers have also observed that ApoE mediates the neuroprotective effect of oestrogens in global ischaemia in a mouse model.95 In addition, ApoE has anti-inflammatory properties96 and protects against apoptosis.97,98 Lipids produced by glial cells, especially by astrocytes, mediate essential functions and any changes may affect CNS homeostasis. In fact, altered biosynthesis of cerebral cholesterol and reduced secretion of ApoE-containing lipoproteins have been described in Huntington disease in humans as well as in animal models.99,100 The relationship between Alzheimer disease and ApoE has also been thoroughly studied given that inheritance of ApoE ¿4 allele is a risk factor for presenting the disease.101,102 The allele is also linked to less effective elimination of Aβ.103
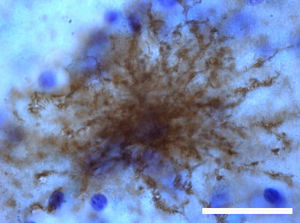
Adult neurogenesisOne of the recently discovered functions of astrocytes is their neurogenic ability in adult brains. Mammal brains contain NSCs not only during the developmental stage, but also in adulthood; these cells are located in the SVZ in the wall of the lateral ventricles. These cells generate new neurons104–106 that use the rostral migratory stream (RMS) to migrate to the olfactory bulb (OB), where they differentiate into granular and periglomerular interneurons.107,108 Stem cells of the SVZ, also known as B cells, express GFAP and display astrocyte morphology and ultrastructure21 (Figs. 2 and 5).
Neuronal precursor cells migrate to the OB in chains surrounded by astrocyte projections, using the network of blood vessels that delimits the RMS as scaffolding. It was recently shown that astrocytes arrange for the generation and structural reorganisation of this vascular scaffolding through expression of the vascular endothelial growth factor.109
Regarding the human brain, astrocytes in the SVZ behave like NSCs in vitro, but their functional purpose in vivo has yet to be resolved. Initial reports described stem cells in the SVZ,23 but no evidence of migratory chains has been found in adult humans.22 A later study discovered evidence of RMS, and therefore of migration, in the adult human brain.110 More recently, Sanai et al. delivered evidence that postnatal neurogenesis and migration are present in humans until 18 months of age before decreasing in older children. These phenomena are minimal in adults.111 Surprisingly, during this short window of time, cells migrate to the prefrontal cortex as well as to the OB.
Reactive astrogliosis and glial scar formationThe term ‘astrogliosis’ refers to a series of changes in astrocytes occurring on the molecular, cellular, and functional levels in response to damage and different types of CNS diseases. Changes affecting reactive astrocytes vary depending on the severity of the lesion and they are regulated by intercellular and intracellular molecules. Astrocyte activity may be modified by a gain or a loss of functions, processes which may affect neighbouring cells.28 According to this definition, reactive astrogliosis is not a ‘binary state’, but rather a continuum of progressive changes. As such, we can distinguish 3 levels of severity.10 (1) Mild to moderate reactive astrogliosis. This level is characterised by increased GFAP expression and hypertrophy of both the cell body and the astrocyte projections. This occurs within the astrocyte's own domain with no overlap with neighbouring astrocytes. Little to no proliferation is present. This degree of reactive astrogliosis is reversible, and it occurs in cases of mild, non-penetrating trauma, diffuse activation of the innate immune system, and areas distant from the focal lesion site. (2) Severe diffuse reactive astrogliosis. In cases of severe focal lesions, infections, or areas with chronic neurodegeneration, overexpression of GFAP and hypertrophy of the cell body and projections are more pronounced. Furthermore, astrocyte overlap and increased astrocyte proliferation occur. These changes may lead to lasting tissue reorganisation. (3) Severe reactive astrogliosis with compact glial scar formation. In this case, a glial scar forms in addition to the previously listed changes. This scar inhibits axonal regeneration and cell migration,112 but it also protects against the arrival of inflammatory cells and infectious agents.113–115 This state is brought about by severe penetrating and/or continuous CNS lesions, invasive infections and abscesses, chronic neurogeneration, and even systemic infections. Glial scar formation includes tissue reorganisation and lasting structural changes that will remain after the causal factor has vanished.
Although only inhibition of axonal regeneration was considered during a long period of time, it is true that reactive astrocytes also perform beneficial functions. For example, they protect CNS cells by capturing potentially excitotoxic glutamate,116,117 releasing glutathione to counteract oxidative stress,118–120 degrading the β-amyloid peptide,121 and facilitating BBB reparation.113 Furthermore, as mentioned above, they limit diffusion of inflammatory cells and infectious agents.113–115
Experimental models for investigating astrocyte functionTransgenic and knock-out mice have provided valuable information about the effects of changes in astrocytes. Deletion of GFAP did not produce any specific diseases in mice with no lesions, although it did elicit certain abnormalities in response to lesions.122 Expression of human GFAP in mice causes astrocytes to form Rosenthal fibres, structures that are characteristic of Alexander disease.123 Lack of GFAP and vimentin resulted in a more marked disease form in an entorhinal cortex lesion model, although researchers observed better regenerative capacity and the number of synapses was restored to the previous level between days 4 and 14.124 In an animal model in which astrocyte ablation prevented the formation of glial scar, researchers observed leucocyte infiltration, neurodegeneration, and neurite outgrowth after a deep mechanical injury to the forebrain.113 Another study also found errors in BBB repair, tissue degeneration, leukocyte infiltration, severe demyelination, neuronal and oligodendrocyte death, and marked motor deficits after spinal cord lesion.114 Alteration of the BBB with a resulting increase in inflammation and infection was also reported in an experimental model of autoimmune encephalitis.115
Another important area of study is the role played by astrocytes in glutamate excitotoxicity. Researchers have described 3 glutamate transporters in rats: astroglial transporters GLAST and GLT-1 (also called EAAT2), and neuronal transporter EAAC1. The loss of glial transporters GLAST and GLT-1 in a rat model elicits elevation of extracellular levels of glutamate, increase in excitotoxicity, and progressing paralysis. The loss of neuronal transporter EAAC1 did not raise the extracellular glutamate concentration, but it did cause specific behavioural changes and epilepsy. These phenomena probably arose because changes in intrasynaptic glutamate affected depolarisation and neurotransmitter release.116 Homozygotic mice lacking GLT-1 displayed epilepsy and increased damage following brain lesion.125 This increased susceptibility to brain damage was also apparent in an ischaemia model in the hippocampal CA1; here, rats lacking GLT-1 showed higher levels of glutamate.126 A study of the GLAST transporter found that GLAST(–/–) mice presented more severe seizures after administration of pentylenetetrazole than GLAST(+/+).127 One polymorphism of glial transporter EAAT2, described in humans, is specifically related to the increase in plasma glutamate levels and higher frequency of neurological deterioration after a cerebrovascular event.128
Altered communication between astrocytes at the gap junctions also has negative effects. Mice with the connexin 43 null mutation demonstrated a larger infarct volume after occlusion of the middle cerebral artery than wild-type mice.129 Deletion of astrocyte connexins 43 and 30 in another mouse model gave rise to a demyelinating phenotype and vacuolisation in hippocampal CA1,44 but this did not change susceptibility to, or severity of, acute experimental autoimmune encephalomyelitis in mice.130
Astrocyte involvement in central nervous system diseaseAstrocytes, especially reactive astrocytes, fulfil essential functions for proper CNS function. Abundant evidence shows that changes in astrocyte function may contribute to or even cause disease in the CNS, especially neurodegenerative diseases. On the one hand, loss of astrocyte function may have negative effects; on the other, excessive astrocyte reactivity may function like inflammation to harm the CNS, as we will explore in a future article.
Conflict of interestThe authors have no conflict of interest to declare.
We would like to thank Prof. José Manuel García Verdugo for his assistance in capturing the electron microscope images, and also the Confocal Microscopy Unit at the Centre for Cytometry and Microscopy, Universidad Complutense de Madrid.
Please cite this article as: Guillamón-Vivancos T, Gómez-Pinedo U, Matías-Guiu J. Astrocitos en las enfermedades neurodegenerativas (I): función y caracterización molecular. Neurología. 2015;30:119–29.