To investigate the effect of endovascular embolization of posterior communicating artery (Pcom) aneurysms on concomitant oculomotor nerve palsy (OMNP) and factors affecting the effect of treatment.
Materials and methodsPatients with the Pcom aneurysms concomitant with OMNP were retrospectively enrolled for endovascular treatment of the aneurysms. All patients had the endovascular management. The clinical effect, degree of OMNP, size of the aneurysm, type of treatment, subarachnoid hemorrhage (SAH), and time from onset to treatment were analyzed on the resolution of OMNP.
ResultsNinety-six patients with 99 Pcom aneurysms were enrolled and treated endovascularly, with the success rate of 100%. Immediately after endovascular treatment, 75 aneurysms (75.75%) got complete occlusion, and 24 (24.24%) nearly complete occlusion. Followed up for 3–18 (mean 8.52±0.56) months, complete resolution of the OMNP was achieved in 63 patients (65.63%), partial resolution in 21 (21.88%), and non-recovery in the other 12 (12.50%). The degree of OMNP at onset, SAH, and time from onset to treatment were significantly (P<0.05) correlated with the resolution of OMNP. Univariate analysis revealed that younger age of the patient, degree of OMNP at onset, presence of subarachnoid hemorrhage, and time from disease onset to treatment were significantly (P<0.05) associated with the recovery of OMNP. Multivariate analysis revealed that the younger age, degree of OMNP at onset, and time from disease onset to treatment were significantly (P<0.05) associated with the recovery of OMNP.
ConclusionEndovascular embolization of Pcom aneurysms concomitant with OMNP can effectively improve the OMNP symptoms, especially for patients with moderate and a shorter history of OMNP. Younger age, degree of oculomotor nerve palsy at onset, and time from onset to treatment may significantly affect recovery of oculomotor nerve palsy.
Investigar la eficacia de la embolización intravascular del aneurisma de comunicación posterior (Pcom) en pacientes con parálisis oculomotora (OMNP) y los factores que influyen en la eficacia.
Materiales y métodosSe analizaron retrospectivamente los datos clínicos de la terapia intravascular en pacientes con aneurismas Pcom con OMNP. Todos los pacientes recibieron tratamiento intravascular. Se analizaron los efectos de la eficacia clínica, el grado de OMNP, el tamaño del aneurisma, el método de tratamiento, la hemorragia subaracnoidea y el tiempo desde el inicio hasta el tratamiento en la regresión de OMNP.
ResultadosUn total de 96 pacientes con 99 aneurismas Pcom fueron tratados con éxito. Inmediatamente después del tratamiento intravascular, 75 casos (75,75%) de aneurismas fueron completamente ocluidos y 24 casos (24,24%) casi completamente ocluidos. Durante el seguimiento de 3 a 18 meses (promedio: 8,52±0,56 meses), se logró la resolución completa en 63 casos (65,63%), la resolución parcial en 21 (21,88%) y la no recuperación en los otros 12 (12,50%). El grado de OMNP al inicio, la hemorragia subaracnoidea y el tiempo de inicio a tratamiento se correlacionaron significativamente con la resolución de la OMNP (p<0,05). El análisis univariado mostró que la menor edad del paciente, el grado de OMNP, la presencia de hemorragia subaracnoidea y el tiempo transcurrido desde el inicio de la enfermedad hasta el tratamiento se correlacionaron significativamente con la recuperación de OMNP (p<0,05).
ConclusiónLa embolización intravascular del aneurisma Pcom combinada con OMNP puede mejorar eficazmente los síntomas de OMNP, especialmente en pacientes con OMNP a corto y mediano plazo. La edad temprana, el grado de parálisis del nervio oculomotor al inicio y el tiempo desde el inicio hasta el tratamiento tuvieron un efecto significativo en la recuperación de la parálisis del nervio oculomotor.
Oculomotor nerve palsy (OMNP) may be caused by direct oppression of a lesion or microvascular alterations to affect the inner fibers of the oculomotor nerve.1–3 Compression from aneurysms of the posterior communicating artery (Pcom) is a common external cause of OMNP, and about 20% of patients with Pcom aneurysms suffer from OMNP.4 The aneurysmal pulsatile stimulation was considered the primary cause of the OMNP symptoms like ophthalmoplegia, ipsilateral ptosis, pupillary dysfunction, and diplopia, which may be partially or completely resolved after successful treatment involving surgical clipping and endovascular embolization.1–6 Direct aneurysmal compression of the OMN and irritation of aneurysmal subarachnoid hemorrhage (SAH) may also cause the OMNP symptoms. Endovascular embolization of the Pcom aneurysm eliminates the aneurysmal pulsatility, leading to recovery of the OMNP. However, the factors affecting the effect of endovascular embolization on OMNP caused by Pcom aneurysms are not clear, and this study was consequently performed to investigate these factors affecting recovery of OMNP after endovascular embolization.
Materials and methodsSubjectsThis study was approved by the ethics committee of our hospital, and all patients or their family members had signed the informed consent to participate. Between September 2010 and September 2020, patients with Pcom aneurysms concomitant with OMNP who were treated endovascularly were retrospectively enrolled. The inclusion criteria were patients with Pcom aneurysms confirmed by digital subtraction angiography (DSA) or computed tomography angiography (CTA), treated with endovascular embolization, and without other causes of OMNP like diabetes, syphilis, or oculomotor neuritis. The exclusion criteria were patients with intracranial space occupying lesions including tumors, cysts, and hypertensive cerebral hemorrhage, with concomitant severe cardiac, hepatic, renal and pulmonary dysfunction intolerable to contrast agents.
Endovascular proceduresThe location, size, and morphology of the aneurysm were confirmed by CTA or DSA before the endovascular procedure. Dual antiplatelet therapy was administered in patients with unruptured Pcom aneurysms including Clopidogrel bisulfate (75mg) plus aspirin enteric coated tablets (100mg) 3–4 days before embolization, and clopidogrel bisulfate (300mg) plus aspirin enteric coated tablets (300mg) was administered 2h before embolization for patients with ruptured aneurysms. The dual antiplatelet therapy plus a weight-based intravenous heparin bolus (1000 units) during the procedure was to maintain an activated clotting time of 230–250s during the procedure for prevention of possible thrombosis. Because the microcatheters, guide wires, coils and stents used in the arteries to embolize aneurysms may cause thrombosis, all patients will have to be treated with double antiplatelet therapy to prevent possible cerebral thrombosis and ischemic complications from happening. No anti-inflammatory medication was used in these patients.
The procedure was performed under general anesthesia, and a 6F guiding catheter was navigated into the ipsilateral internal carotid artery after the femoral artery was punctured using the Seldinger technique. Then, a microcatheter was sent into the aneurysm cavity for embolization with the intention of complete occlusion of the aneurysm. Immediately after embolization, head CT scan was performed routinely in the operating theater in all patients to check no hemorrhage intracranially caused by the endovascular treatment. The occlusion status of the aneurysm was divided into three categories: complete occlusion with no contrast agent entering the aneurysm cavity or the neck, near-complete occlusion with the contrast agent entering the neck rather than the aneurysm cavity, and incomplete occlusion with contrast agent entering the aneurysm cavity.
Effect assessmentComplete OMNP was diagnosed if the following parameters were present: vision loss, blepharoptosis, extraocular muscle paralysis, strabismus, mydriasis, decreased or disappeared light reflex. Complete resolution of OMNP was defined as complete disappearance of all these symptoms, whereas incomplete resolution as partial recovery of any initially present symptoms, with no new symptoms present. No effect indicated no resolution or even aggravation of the initial symptoms.
Parameters observedThe degree of onset OMNP, aneurysm size, presence of SAH, and time from symptom onset to treatment were analyzed. The size of aneurysm was categorized as<0.7cm or ≥0.7cm, and the time from onset to treatment was divided into ≤14 days or >14 days.
Statistical analysisThe statistical analysis was performed with the SPSS software (Version 19.0, IBM, Chicago, IL, USA). Continuous measurement data were presented as mean±SD (standard deviation) and tested with the Chi square test. Categorical data were presented with numbers and percentages and tested with the fisher's exact probability method. Univariate and multivariate analyses were performed. The significant P value was set at <0.05.
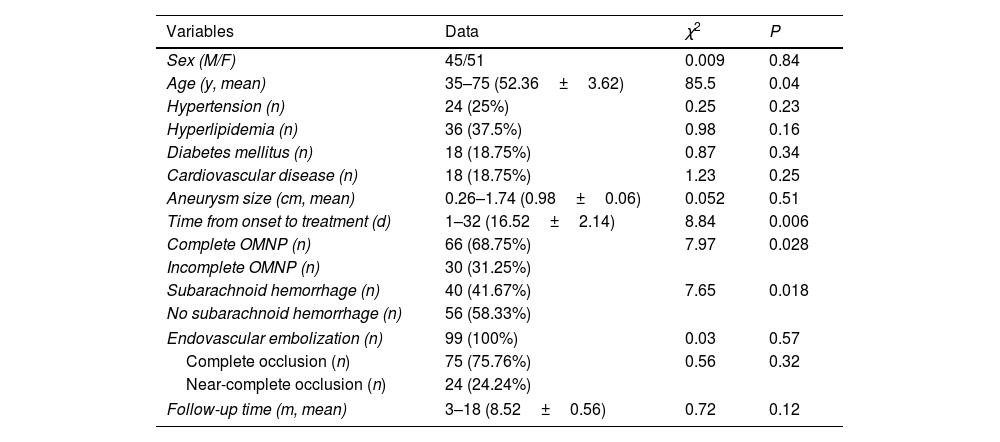
ResultsNinety-six patients who had 99 Pcom aneurysms and concomitant OMNP were enrolled, including 45 male and 51 female patients with an age range of 35–75 (mean 52.36±3.62) (Table 1). The maximal diameter of the aneurysm ranged 0.26–1.74 (mean 0.98±0.06) cm. The time from onset to treatment ranged 1–32 days (mean 16.52±2.14). All patients had varied degrees of ptosis, vision loss, unilateral or bilateral eye movement difficulties, disappearance of pupil light reflex, dizziness and headache. Complete OMNP was present in 66 (68.75%) patients, and incomplete OMNP in the rest 30 patients (31.25%). Subarachnoid hemorrhage was in 40 (41.67%). Among these patients, hypertension was present in 24 (25%) patients, hyperlipidemia in 36 (37.5%), diabetes mellitus in 18 (18.75%), and cardiovascular diseases in 18 (18.75%).
All aneurysms were successfully embolized with the success rate of 100%. Coil embolization alone was performed in 72 aneurysms, balloon-assisted coiling was performed in 10 aneurysms, stent-assisted coiling in 14 aneurysms. No flow-diverters were used in this cohort of patients. Complete occlusion was achieved in 75 aneurysms (75.76%), and near-complete occlusion in 24 (24.24%) aneurysms. Bilateral Pcom aneurysms were present in three patients and were all successfully embolized.
Followed up for 3–18 (mean 8.52±0.56) months, complete recovery of OMNP was achieved in 63 patients (65.63%), partial recovery in 21 (21.88%), and no effect in 12 (12.5%), with the total effective rate of 87.5% (84/96).
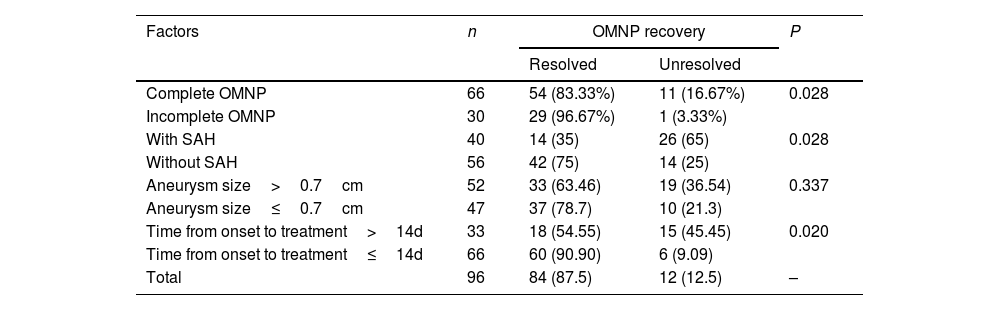
In analyzing factors affecting recovery of OMNP, univariate analysis revealed that younger age of the patient, degree of OMNP at onset, presence of subarachnoid hemorrhage, and time from disease onset to treatment were significantly (P<0.05) associated with the recovery of OMNP (Tables 1 and 2). Non-complete OMNP, no presence of subarachnoid hemorrhage, and short time from onset to endovascular embolization were significantly (P<0.05) associated with good recovery of OMNP. The sex, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular diseases, aneurysm size, treatment type, and occlusion degree at embolization were not significantly (P>0.05) associated with the recovery of OMNP. Multivariate analysis revealed that the younger age, degree of OMNP at onset, and time from disease onset to treatment were significantly (P<0.05) associated with the recovery of OMNP.
Demography and univariate analysis.
| Variables | Data | χ2 | P |
|---|---|---|---|
| Sex (M/F) | 45/51 | 0.009 | 0.84 |
| Age (y, mean) | 35–75 (52.36±3.62) | 85.5 | 0.04 |
| Hypertension (n) | 24 (25%) | 0.25 | 0.23 |
| Hyperlipidemia (n) | 36 (37.5%) | 0.98 | 0.16 |
| Diabetes mellitus (n) | 18 (18.75%) | 0.87 | 0.34 |
| Cardiovascular disease (n) | 18 (18.75%) | 1.23 | 0.25 |
| Aneurysm size (cm, mean) | 0.26–1.74 (0.98±0.06) | 0.052 | 0.51 |
| Time from onset to treatment (d) | 1–32 (16.52±2.14) | 8.84 | 0.006 |
| Complete OMNP (n) | 66 (68.75%) | 7.97 | 0.028 |
| Incomplete OMNP (n) | 30 (31.25%) | ||
| Subarachnoid hemorrhage (n) | 40 (41.67%) | 7.65 | 0.018 |
| No subarachnoid hemorrhage (n) | 56 (58.33%) | ||
| Endovascular embolization (n) | 99 (100%) | 0.03 | 0.57 |
| Complete occlusion (n) | 75 (75.76%) | 0.56 | 0.32 |
| Near-complete occlusion (n) | 24 (24.24%) | ||
| Follow-up time (m, mean) | 3–18 (8.52±0.56) | 0.72 | 0.12 |
Note: OMNP, oculomotor nerve paralysis.
Factors affecting recovery of OMNP [n(%)].
| Factors | n | OMNP recovery | P | |
|---|---|---|---|---|
| Resolved | Unresolved | |||
| Complete OMNP | 66 | 54 (83.33%) | 11 (16.67%) | 0.028 |
| Incomplete OMNP | 30 | 29 (96.67%) | 1 (3.33%) | |
| With SAH | 40 | 14 (35) | 26 (65) | 0.028 |
| Without SAH | 56 | 42 (75) | 14 (25) | |
| Aneurysm size>0.7cm | 52 | 33 (63.46) | 19 (36.54) | 0.337 |
| Aneurysm size≤0.7cm | 47 | 37 (78.7) | 10 (21.3) | |
| Time from onset to treatment>14d | 33 | 18 (54.55) | 15 (45.45) | 0.020 |
| Time from onset to treatment≤14d | 66 | 60 (90.90) | 6 (9.09) | |
| Total | 96 | 84 (87.5) | 12 (12.5) | – |
Note: OMNP, oculomotor nerve paralysis; d, day; SAH, subarachnoid hemorrhage.
In this study investigating the effect of endovascular embolization of Pcom aneurysms on concomitant OMNP and factors affecting the effect of treatment, it was found that endovascular embolization of Pcom aneurysms concomitant with OMNP can effectively improve the OMNP symptoms, especially for patients with moderate and a shorter history of OMNP.
Patients with Pcom aneurysms frequently have OMNP caused by aneurysm compression, arterial blood pulsation stimulation, and chemical irritation resulted from aneurysm rupture and bleeding, leading to ptosis, vision loss, strabismus and other symptoms, which has a great impact on the quality of life of the patients and makes it difficult for patients to live normally.1–4 The most important factor leading to OMNP is the compression of the aneurysm on the oculomotor nerve. The more severe the compression of the aneurysm on the oculomotor nerve and the longer the compression time, the more severe the OMNP symptoms will be, leading to the development of patients from incomplete to complete OMNP and even threatening the life of patients. Some factors can affect the degree of compression of the aneurysm on the oculomotor nerve, such as the direction of aneurysm growth and the size of the aneurysm. If the aneurysm grows outward, backward or downward, it will cause compression on the oculomotor nerve, leading to OMNP. In the early stage of oculomotor nerve compression, the damage of nerve conduction block is reversible, and patients will show symptoms such as ptosis, slight abnormal eye movement, and decreased vision. If it is not treated in a timely manner, the oculomotor nerve injury will become irreversible with extended time of oculomotor nerve compression, resulting in a serious impact on the quality of life.
Embolization of the Pcom aneurysms blocks the continuous impact of pulsatile arterial blood on the aneurysm wall, changes the hemodynamics in the aneurysm cavity, and reduces the risk of aneurysm rupture. At the same time, the size of aneurysms will gradually shrink with self-healing of the aneurysm parent artery, thus reducing the compression and stimulation of aneurysms on the oculomotor nerve. This is conducive to the self-healing of the oculomotor nerve and alleviating the symptoms of PMNP.
Among factors affecting the recovery of OMNP in our study, patients with mild or moderate OMNP, no subarachnoid hemorrhage, and short time from disease onset to embolization can have good recovery of OMNP. Other factors including aneurysm size did not affect the recovery of OMNP. Patients with incomplete OMNP are more likely to have complete recovery of the oculomotor nerve function after endovascular embolization than those with complete OMNP.7–10 Nerve damage at the time of subarachnoid hemorrhage may cause OMNP,11 and after endovascular embolization to protect ruptured aneurysms against further rebleeding, resorption of the subarachnoid blood will eliminate the irritating effect on the oculomotor nerve.12–14 It has been reported that the presence of subarachnoid hemorrhage may be a predictor of OMNP recovery.11,15 If the original injury of the oculomotor nerve was resulted from acute high-pressure blood jet caused by aneurysm rupture, decompression of the nerve following absorption of the subarachnoid hemorrhage may confer some extent of additional benefit.15,16 Studies have demonstrated that if the treatment was performed within 14 days after disease onset, a more satisfactory recovery of the OMNP would be achieved.17,18 It was believed that aneurysmal pulsatility instead of aneurysm size underlay aneurysm-associated OMNP.4,10,13,19,20 Rapid resolution of signs and symptoms of OMNP after endovascular embolization may suggest that other factors rather than aneurysm size play a much more important role in resolving OMNP and oculomotor function.21,22
In the study investigating the effect of endovascular treatment of ruptured Pcom aneurysms on the recovery of OMNP, Zu et al.14 found that patients with initial incomplete OMNP had a trend toward complete recovery even with no significance (P>0.05). In their study, endovascular embolization resulted in complete recovery of OMNP in 61.8% patients but incomplete recovery in 23.5%, with only five patients having no signs of recovery. Signorelli et al.23 had studied the effect of endovascular vs. surgical treatment of ruptured Pcom aneurysms on improving OMNP and found that timely, complete, and durable occlusion of the Pcom aneurysm is the best predictor of OMNP recovery, with improvement of OMNP in 72.7% but persistence or recurrence of OMNP in 27.3%. Liu et al. had also investigated the treatment effect of Pcom aneurysms on the recovery of OMNP using endovascular embolization versus surgical clipping,1 and they found that the postoperative recovery of OMNP was significantly associated with preoperative spontaneous subarachnoid hemorrhage and time from disease onset to treatment, even though the recovery rate and degree of OMNP after surgical treatment was significantly better than those of endovascular embolization in patients with Pcom aneurysms concomitant with OMNP. At the early period of subarachnoid hemorrhage caused by aneurysm rupture, the oculomotor nerve function was probably in the status of reversible conduction block and reversible neuropraxia, and the damage of oculomotor nerve was mild or moderate and could be improved or prevented from further injury by aneurysm compression or pulsation.1 Moreover, early intervention could prevent cerebral vasospasm caused by aneurysmal subarachnoid hemorrhage.
In our study, younger age of the patient was detected to be a significant factor affecting the recovery of OMNP after endovascular embolization of the Pcom aneurysms. The exact mechanism of age on the recovery of OMNP is not clear and no articles have investigated this issue in the current literature. Age is the most important predictor of clinical outcome after peripheral nerve injury, and the age at the time of injury is the most important predictor of outcome of nerve injury and repair.24–26 Children frequently re-obtain near normal function following nerve injury, but adults or aged persons experience slow or absent neuromuscular recovery.24,27 Over 1/2 patients older than the age of 40 do not achieve any functional recovery after nerve repair.26 This may be associated with the stability of the neuromuscular junction (NMJ) after denervation which is central to neuromuscular recovery.24 Stability has the feature of maintenance of the motor endplate and mRNA upregulation of the constituent nicotinic acetylcholinergic receptor (nAChR) subtypes and the muscle regulatory factors (MRFs). Researchers have shown that upregulation of the nAChRs and MRFs may play a very important role in maintaining the NMJ stability after nerve transection and repair. Gamma-nAChR and myogenin do not prevent age-related NMJ fragmentation and loss of endplate area following nerve injury. Impairments of the aged NMJ response to injury may contribute to the poor neuromuscular recovery seen after nerve injury.
Some limitations existed in this study, including a small cohort of patients, Chinese patients enrolled only, single center and retrospective study, no randomization, and no control, which may all affect the generalization of the outcomes of the study. In the future, better studies should be conducted to resolve all these issues for better outcomes.
In short, endovascular embolization of Pcom aneurysms concomitant with OMNP can effectively improve the OMNP symptoms, especially for patients with moderate and a shorter history of OMNP. Factors affecting the recovery of oculomotor nerve palsy mainly include the degree of oculomotor nerve palsy at onset and time from onset to treatment.
Conflict of interestThe authors declare that they have no conflict of interest.