The aim of this study was to compare the effect of five types of PEGlated nanoliposomes (PNLs) on α-synuclein (α-syn) fibrillization, attenuation of microglial activation, and silence of the SNCA gene, which encodes α-syn.
MethodsTo evaluate the inhibition of α-syn fibrillization, we used standard in vitro assay based on Thioflavin T (ThT) fluorescence. Next, to evaluate the attenuation of microglial activation, the concentration of TNF-a and IL-6 was quantified by ELISA assay in BV2 microglia cells treated with 100nM A53T α-syn and PNLs. In order to determine the silencing of the SNCA, real-time PCR and Western blot analysis was used. Finally, the efficacy of PNLs was confirmed in a transgenic mouse model expressing human α-syn.
ResultsThT assay showed both PNL1 and PNL2 significantly inhibited a-syn fibrillization. ELISA test also showed the production of TNF-a and IL-6 was significantly attenuated when microglial cells treated with PNL1 or PNL2. We also found that SNCA gene, at both mRNA and protein levels, was significantly silenced when BV2 microglia cells were treated with PNL1 or PNL2. Importantly, the efficacy of PNL1 and PNL2 was finally confirmed in vivo in a transgenic mouse model.
ConclusionsIn conclusion, the novel multifunctional nanoliposomes tested in our study inhibit α-syn fibrillization, attenuate microglial activation, and silence SNCA gene. Our findings suggest the therapeutic potential of PNL1 and PNL2 for treating synucleinopathies.
El objetivo de este estudio fue comparar el efecto de cinco tipos de nanoliposomas PEGlados (PNL) sobre la fibrilización de la α-sinucleína (α-syn), la atenuación de la activación microglial y el silencio del gen synuclein alpha (SNCA), que codifica α-syn.
MétodosPara evaluar la inhibición de la fibrilización α-syn, utilizamos un ensayo in vitro estándar basado en la fluorescencia de la tioflavina T (ThT). A continuación, para evaluar la atenuación de la activación microglial, se cuantificó la concentración de factor de necrosis tumoral alpha (TNF-a) e interleucina 6 (IL-6)mediante ensayo ELISA en células de microglía BV2 tratadas con 100nM de α-syn de A53T y PNL. Para determinar el silenciamiento del SNCA, se utilizó reacción en cadena de la polimerasa (PCR) en tiempo real y análisis de Western blot. Finalmente, la eficacia de las PNL se confirmó en un modelo de ratón transgénico que expresa α-syn humana.
ResultadosEl ensayo ThT mostró que tanto PNL1 como PNL2 inhibieron significativamente la fibrilización de α-syn. La prueba enzyme-linked immunosorbent assay (ELISA) también mostró que la producción de TNF-a e IL-6 se atenuó significativamente cuando las células microgliales se trataron con PNL1 o PNL2. También encontramos que el gen SNCA, tanto a nivel de ARN mensajero (ARNm) como de proteína, se silenciaba significativamente cuando las células de microglía BV2 se trataban con PNL1 o PNL2. Es importante destacar que la eficacia de PNL1 y PNL2 finalmente se confirmó in vivo en un modelo de ratón transgénico.
ConclusionesLos nuevos nanoliposomas multifuncionales probados en nuestro estudio inhiben la fibrilización α-syn, atenúan la activación microglial y silencian el gen SNCA. Nuestros hallazgos sugieren el potencial terapéutico de PNL1 y PNL2 para el tratamiento de sinucleinopatías.
Synucleinopathies are a group of neurodegenerative diseases characterized by the accumulation of α-synuclein (α-syn) in intra-cytoplasmic inclusions in neurons and/or in glial cells.1 Parkinson's disease (PD), dementia with Lewy bodies, and multiple system atrophy are examples of synucleinopathy.2 The physiological role of α-syn in neurodegenerative disorders is unclear and more studies are needed.3 The aggregation process is complex and involves the formation of various types of oligomers, intermediate supramolecular assemblies, and amyloid fibrils.4 The fibrils are linear rods with 5–10nm in diameter and several micrometers in length. Importantly, oligomeric α-syn intermediates, produced at early stages of α-syn aggregation, appear to be the most toxic species.5
A main important problem of synucleinopathies is neuroinflammation, associated with microglial activation.6 Under normal conditions, microglia remove cell debris and foreign biomolecules.7 But the high concentration of α-syn leads to impair of α-syn clearance pathway and induces a pro-inflammatory microglial phenotype.8 The release of pro-inflammatory mediators, such as tumor necrosis factor (TNF-a), interleukin-6 (IL-6), nitric oxide (NO), reactive oxygen species (ROS), and toxic protein aggregates, are the results of microglial activation.9
We posit that, in order to efficiently treat synucleinopathies, α-syn must be targeted at various stages,10 including (1) reducing α-syn synthesis, (2) increasing α-syn degradation, (3) reducing α-syn aggregation, (4) blocking α-syn propagation, (5) activating immune cells against α-syn, and (6) inhibiting neuroinflammation. It should be noted that, although it is difficult to target all pathways, some of them may be targeted by multifunctional nanoparticles. With new advances in medical nanotechnology over the last years, it can be argued that in the near future, it will be possible to treat synucleinopathies using nanoparticles. There are now some data showing that some nanoparticles, i.e. antioxidant nanoparticles,11 zwitterionic nanoliposomes,12 cerium oxide nanoparticles,13 gold nanoparticles,14 and PEGlated nanoliposome 15 are able to inhibit α-syn fibrillization, reduce α-Syn toxicity, and attenuate microglial activation. These nanoparticles may be a part of future therapeutic alternative.
The aim of this study was to compare five types of multifunctional nanoliposomes for their abilities to inhibit α-syn fibrillization, to attenuate microglial activation, and to silence the SNCA gene.
Materials and methodsSynthesis and characterization of nanoliposomesIn this study, 5 types of nanoliposomes were synthesized and characterized, including:
- 1.
PEGlated nanoliposomes 1 (PNL1) containing sodium percarbonate (SPC) (Merk, Germany), 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) (Merk, Germany), 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine-polyethylene glycol-2000 (DSPE-PEG-2000) (Merk, Germany), carboxy-PEG (Merk, Germany), omega-3 (Merk, Germany), mannitol (Merk, Germany), 7-hydroxyflavone (Merk), and antisense oligonucleotides (Bioneer, South Korea).
- 2.
PEGlated nanoliposomes 2 (PNL2) containing SPC, DOTAP, DSPE-PEG-2000, carboxy-PEG, omega-3, mannitol, and 7-hydroxyflavone.
- 3.
PEGlated nanoliposomes 3 (PNL3) containing SPC, DOTAP, DSPE-PEG-2000, carboxy-PEG, omega-3, and mannitol.
- 4.
PEGlated nanoliposomes 4 (PNL4) containing SPC, DOTAP, DSPE-PEG-2000, carboxy-PEG, and omega-3.
- 5.
PEGlated nanoliposomes 5 (PNL5) containing SPC, DOTAP, DSPE-PEG-2000, and carboxy-PEG.
To synthesize PNLs, reverse-phase evaporation method was used.16 Briefly, SPC, DOTAP, DSPE-PEG-2000, and carboxy-PEG were dissolved in chloroform, and then omega-3, mannitol, 7-hydroxyflavone, and antisense oligonucleotides (5′-CTACATAGAGAACAC-3′) were separately added based on each formulation. The mixture was hardly mixed and homogenized using a probe ultrasound. The pulse was 5s, 2s off, 100% amplitude, and 36joles per pulse. Subsequently, the chloroform was evaporated by a rotary vacuum pump. To hydrate PNLs, PBS was used and their concentrations were adjusted to 1%, 2%, and 4%. Finally, their size distribution was measured by dynamic light scattering (DLS) and their stability was checked until one week at 25°C.
Kinetic of α-syn fibrillizationFirst, 100μL of PNLs at concentration of 1%, 2%, and 4% was separately incubated with 100μL of 100nM recombinant monomeric α-syn (Sigma-Aldrich) in presence of Thioflavin T (ThT) (Acros Organics) for 60min at 37°C, as previously described.17 The α-syn fibrillization was assessed each 20min at the emission wavelength of 490nm and the excitation wavelength of 450nm
Also, after incubation of PNLs with recombinant monomeric α-syn for 60min, 50μL of each mixture was separately placed on a Butvar-coated copper grid, dried, and then negatively stained by uranyl acetate (1.0%, w/v). Here, a transmission electron microscopy (TEM) (Zeiss EM10, Germany) with an excitation voltage of 100kV was applied to observe and compared the size and morphology of α-syn aggregates.
Microglia activationBV2 microglia were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (Atlanta Biologics) and 1% Penicillin-Streptomycin (Merck, Germany). Then, they were plated at 50,000cells/well in a 96-well plate to adhere overnight. Cells were first treated with 100nM A53T α-syn and then with PNLs at a final concentration of 1%, 2%, and 4%. After 24h, supernatants were collected and assayed for TNF-a and IL-6 by ELISA kit (R&D systems) as previously described.11 Briefly, the high binding ELISA plates (Biomat, Italy) were separately coated with anti-TNF-a IgG and anti-IL-6 IgG (R&D systems) at 1μgmL−1. After washing, 50μL of supernatants was added and incubated for 1h at 37°C. Then, plates were washed and 100μL of secondary antibody (anti-TNF-a IgG and anti-IL-6 IgG (R&D systems)) was added. After incubation and washes, 50μL of 3,3′, 5,5′-tetramethylbenzidine was added and then stopped by a stopping solution. Finally, the absorbance of each well was read by a Spectrophotometer at 450nm (BioTek Industries) and the concentration of TNF-a and IL-6 was calculated using standard curve.
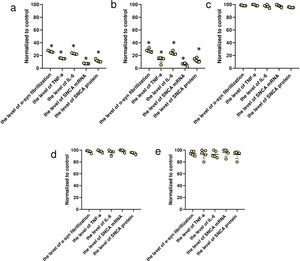
Expression of SNCATo quantify the expression of SNCA at mRNA level, BV2 microglia cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin, plated, and treated with PNLs at a final concentration of 1%, 2%, and 4% for 24h. Then, total RNA was extracted by extraction buffer (QIAGEN) and then cDNA was synthesized by cDNA mastermix (Life Technologies). Finally, quantitative real-time PCR was performed on an ABI real-time PCR system using qPCR Master Mix (Life Technologies). The GAPDH was used as a reference gene and the delta-delta CT formula was applied to evaluate the relative expression. The primers used in this study are listed in Table 1.
The primers used in this study for real-time PCR.
| Forward primer sequence (5′→3′)a | Reverse primer sequence (5′→3′) | Product length (bp) | |
|---|---|---|---|
| SNCA | ATTCGACGACAGTGTGGTGT | GTTTTCTCAGCAGCAGCCAC | 102 |
| GAPDH | GACAGTCAGCCGCATCTTCT | GCGCCCAATACGACCAAATC | 104 |
To quantify the expression of SNCA at the protein level, BV2 microglia cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin, plated, and treated with PNLs at a final concentration of 1%, 2%, and 4% for 24h. Cell lysates were mixed with Laemlli sample buffer, boiled, and separated on polyacrylamide gel (Thermo Fisher Scientific). After protein transfer to polyvinylidene fluoride membranes (Thermo Fisher Scientific), the blots were probed with the primary antibodies (α-syn (BD Transduction Laboratories) and beta-actin (Millipore)), visualized with secondary antibodies, HRP-conjugated anti-mouse IgG (GE Healthcare), and developed using ECL Prime Western Blotting Detection Reagent. The signals of immunoblots were recorded with a Chemidoc Touch Imaging System. Images of blots were cropped from different parts of the same gel for densitometry analysis.
In vivo assessmentFor this part of the study, we used α-syn transgenic mouse model (https://qpsneuro.com/in vivo-services/animal-models/alpha-synuclein-transgenic-mouse-models) to check the efficacy of PNLs. Briefly, transgenic mice were separately treated with 100μL of PNLs 1–5 at a concentration of 4% which taken orally for 5 days. After treatment period, all mice were killed under high dose of anesthesia and then their brains were removed. Then, tissue sections were prepared and fixed. After passing from the serial dilutions of alcohols, the slides were immersed in distilled water and then antigen retrieval was carried out by microwaving in the citric acid buffer. In the next step, 50μL of primary antibody (anti-α-syn IgG (BD Transduction Laboratories)) were added and incubated for 12h. After washing, 50μL of the secondary antibody (FITC-conjugated anti-mouse IgG (GE Healthcare)) were added and incubated for 3h. Finally, the slides were examined by a fluorescent microscopy. The amount of α-syn accumulation was scored and finally normalized to control. To evaluate the neuroinflammation, cerebrospinal fluid (CSF) of mice was used. After treatment of mice, the concentration of TNF-a and IL-6 in CSF was evaluated by ELISA kit (R&D systems) as previously described.11 To evaluate the expression of SNCA, several pieces of brain were cut and hardly crushed by a mechanical pressure. In the next step, the mixture was centrifuged at 5000RPM for 5min and the supernatant is separated for the evaluation of SNCA expression at both mRNA and protein by real-time PCR and Western blot with above mentioned details.
Statistical analysisData are presented as mean±SD with 3 independent repeats (n=3) for in vitro experiments and with 5 independent biological repeats for in vivo experiments. Analysis of different study groups was performed using one-way ANOVA with Tukey's post hoc test with p<0.05.
ResultsCharacterization of PNLsThe size distribution of all synthesized PNLs was approximately between 120 and 130nm and all of them were stable during one week at 25°C.
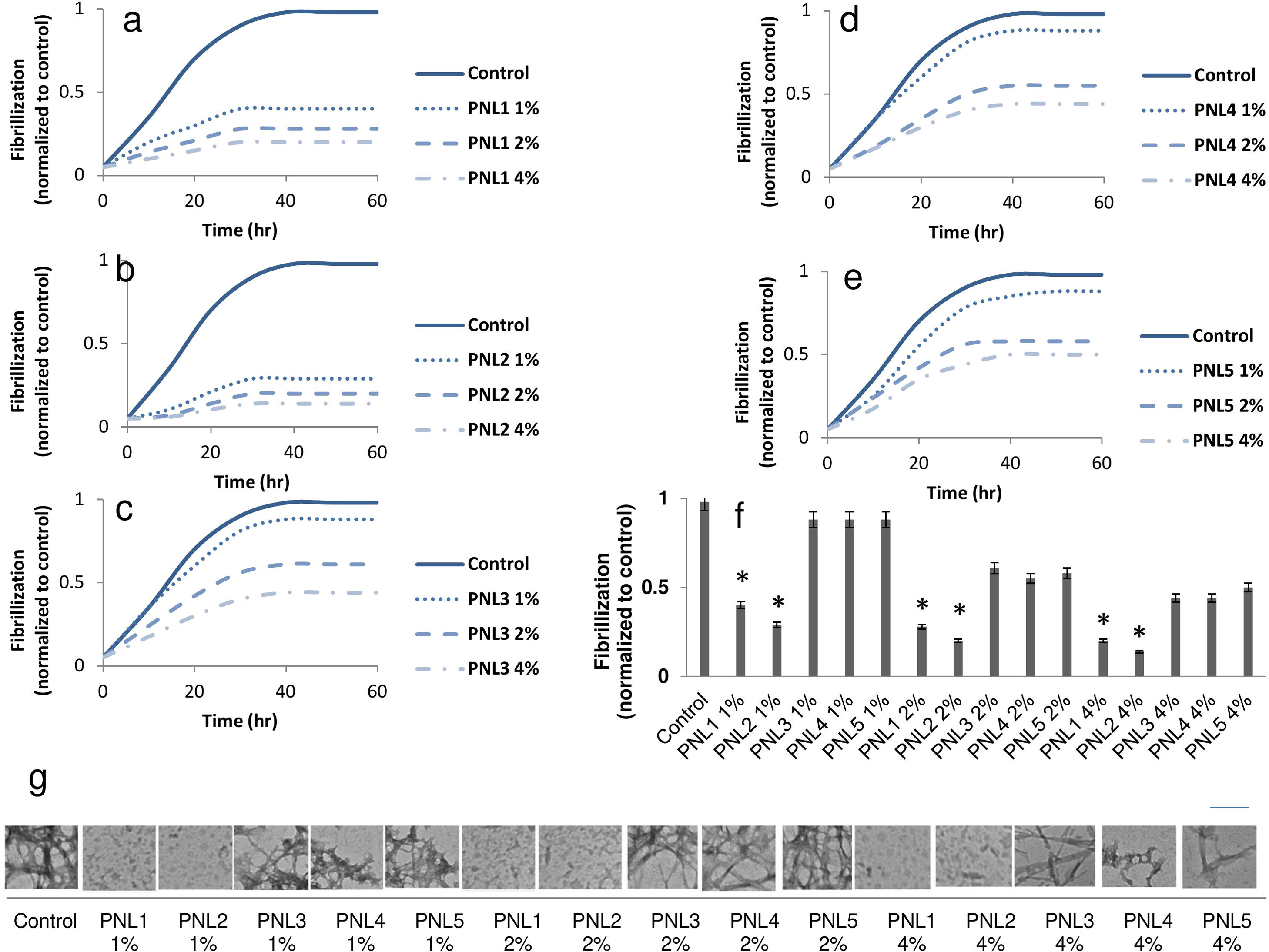
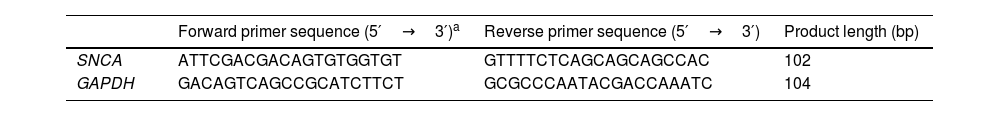
The inhibition of α-syn fibrillizationFirst we explored the effect of PNLs on α-syn fibrillization using a standard ThT kinetic fibrillization assay. We found that all PNLs shortened the α-syn growth phase, suggesting inhibition of α-syn fibrillization (Fig. 1a–e). Importantly, the endpoint ThT assay showed both PNL1 and PNL2 had significantly less fibrillization when compared with other PNLs (Fig. 1f).
The effect of PNL1 (a), PNL2 (b), PNL3 (c), PNL4 (d), and PNL5 (e) on α-syn fibrillization by ThT kinetic fibrillization assay. Endpoint ThT results for different concentrations of PNLs (f). TEM images showed the inhibition of fibrillization by PNL1 and PNL2 (g). As seen, very low fibrils were seen when α-syn treated with PNL1 and PNL2 at a concentration of 1%, 2%, and 4% for 60h. The scale bar is 100nm Data are presented as mean±SD; n=3; *p<0.05 when compared with PNL3, PNL4, and PNL5 by one-way ANOVA. Control was α-syn alone without PNLs.
After incubation of PNLs with recombinant monomeric α-syn, they were negatively stained by uranyl acetate and observed by TEM at 100kV. The inhibition of α-syn fibrillization was also seen when monomeric α-syn incubated with PNL1 or PNL2 at concentration of 1%, 2%, and 4%. In other treated groups, we saw high degrees of fibrillization (Fig. 1g).
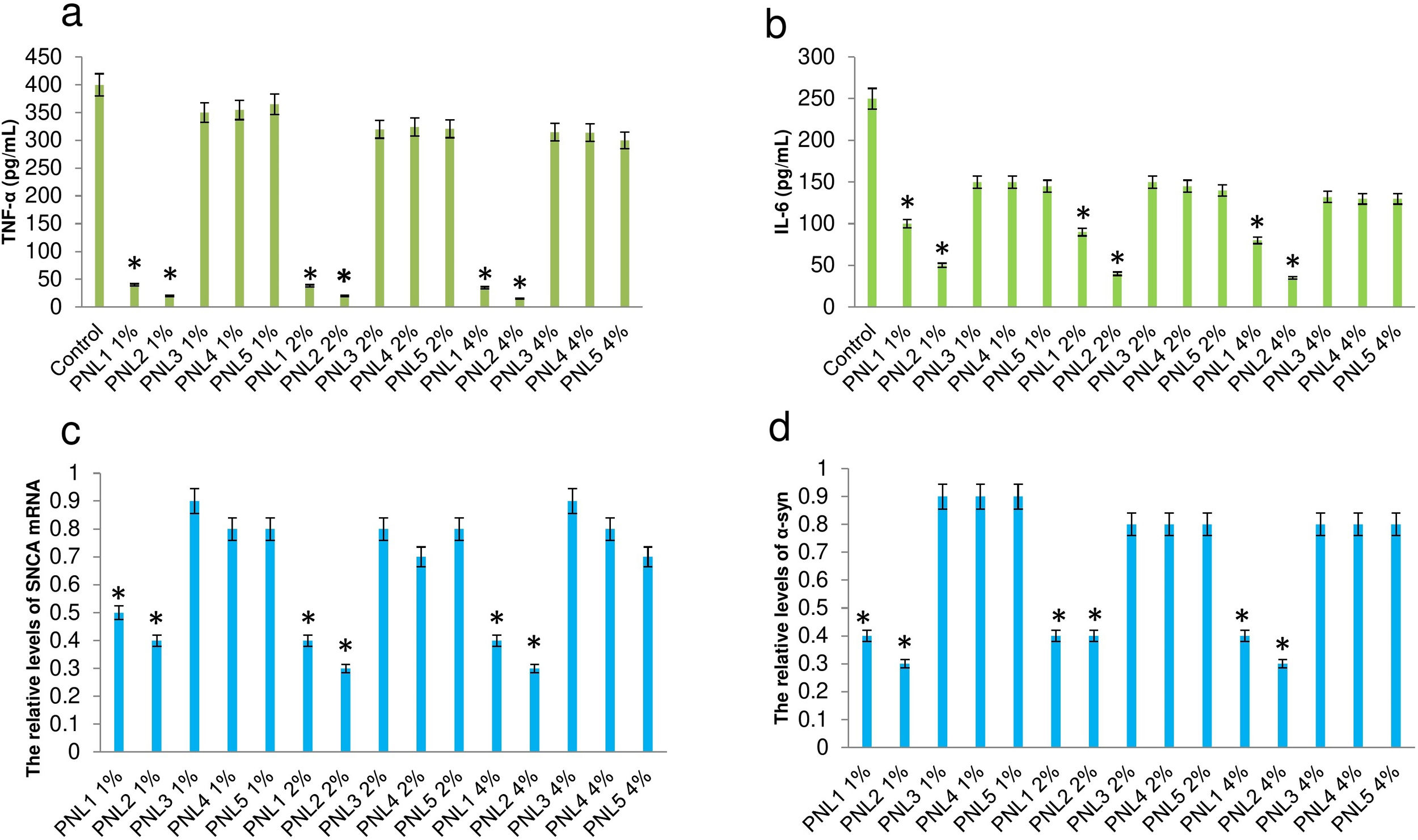
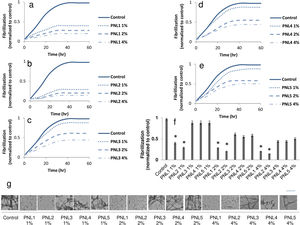
Attenuation of microglial activationActivation of microglia leads to the release of inflammatory agents which particularly are harmful for neurons.18 Here, we investigated the effect of PNLs on the production of TNF-a and IL-6 when microglial cells exposed to 100nM α-syn. It was found that the production of TNF-a (Fig. 2a), and IL-6 (Fig. 2b) was significantly decreased when microglial cells were treated with PNL1 or PNL2 at concentrations of 1%, 2%, and 4%.
Production of TNF-a (a), and IL-6 (b) when microglial cells were first exposed to 100nM α-syn and then treated with PNLs. The expression of SNCA mRNA (c) and SNCA protein (d) when microglial cells were treated with PNLs for 24h. Data are presented as mean±SD; n=3; *p<0.05 when compared with PNL3, PNL4, and PNL5 by one-way ANOVA. Control was microglial cells were exposed to 100nM α-syn and did not treat with PNLs.
The expression of SNCA at the mRNA level was significantly decreased when BV2 microglia cells were treated with PNL1 or PNL2 (Fig. 2c). Also, the expression of SNCA at the protein level was significantly decreased when BV2 microglia cells were treated with PNL1 or PNL2 (Fig. 2d).
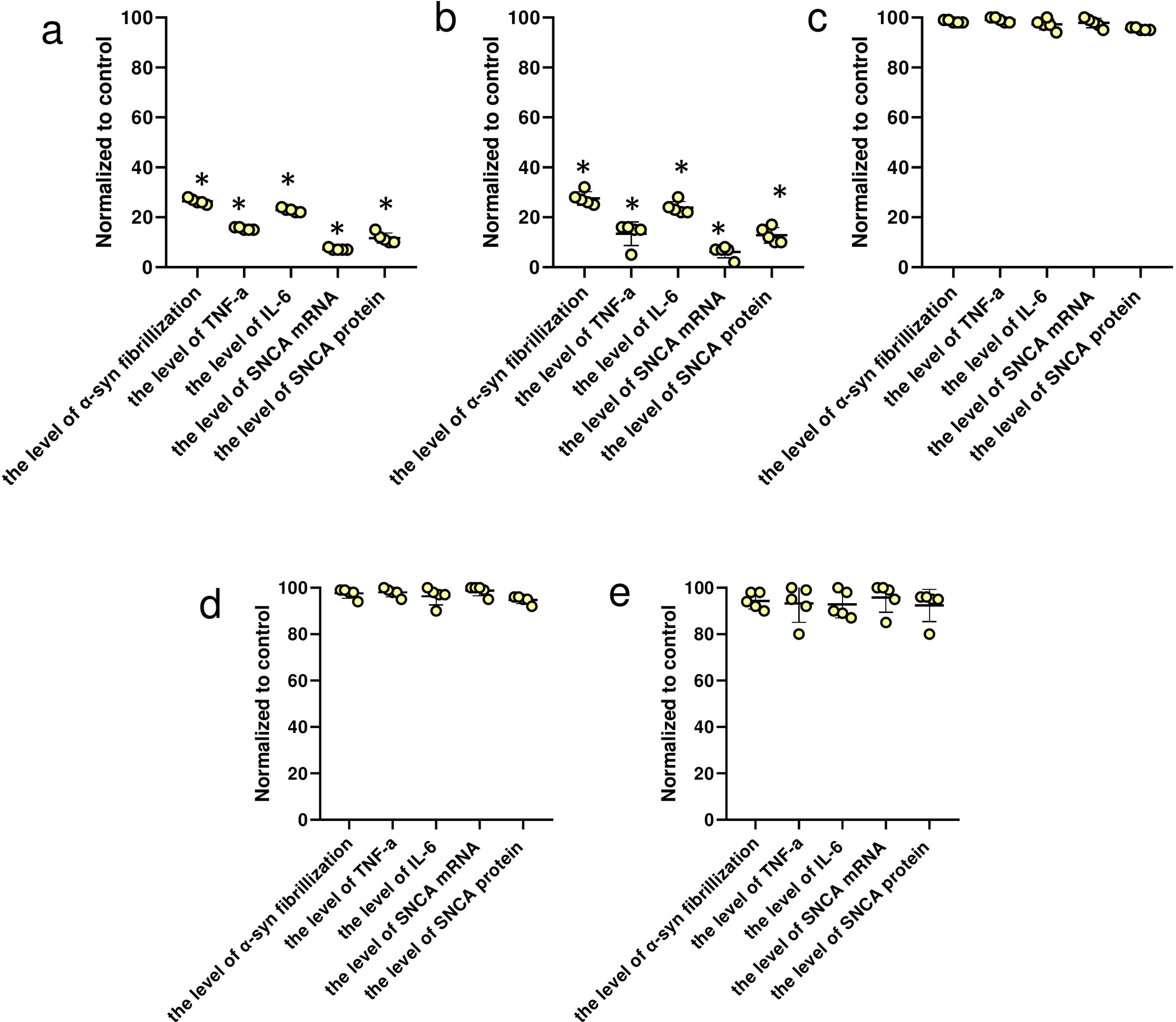
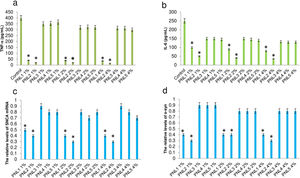
In vivo evaluation of the efficacy of PNLsBased on in vivo experiments, we confirmed the inhibition of α-syn fibrillization, attenuation of microglial activation, and silencing of SNCA in α-syn transgenic mice when treated with PNL1 (Fig. 3a) or PNL2 (Fig. 3b) at concentration of 4%. Importantly, we could not confirm the efficacy of PNL3 (Fig. 3c), PNL4 (Fig. 3d), and PNL5 (Fig. 3e), suggesting these are not good candidates.
Levels of α-syn fibrillization, TNF-a, IL-6, SNCA mRNA, and SNCA protein in α-syn transgenic mouse model when treated with PNL1 (a), PNL2 (b), PNL3 (c), PNL4 (d), and PNL5 (e) for 5 days. Data are presented as mean±SD; n=5; *p<0.05 when compared with PNL3, PNL4, and PNL5 by one-way ANOVA. Control was α-syn transgenic mouse model which was not treated with PNLs.
Finding a chemical compound that can act as a drug for a specific disease is not an easy task and requires many studies and numerous laboratory tests. In the most diseases, several pathways are involved and each pathway must be edited or modified by chemical molecules. In synucleinopathies, several pathways are impaired and their causative agents have not been discovered yet.19 The problem starts with the fact that the expression of SNCA is increased for unknown reasons and then α-syn forms fibrils, which are toxic for nerve cells.20 On the other hand, they cause neuroinflammation at the site of accumulation.21 Based on review literature, various strategies can be considered for the treatment of synucleinopathies.10 First, the overexpression of SNCA must be prevented. The next step is to inhibit aggregation and forming fibril molecules. Another strategy is to decrease neuroinflammation, caused by α-syn molecules and fibrils. With this explanation, it is clear that finding a drug molecule that can affect all of these pathways is very difficult and maybe impossible. Different molecules with different functions can be put inside a nanoliposome and it is possible that different molecules may interact with each other and reduce effects. Contrary, it is also possible that different molecules have a synergistic effect and create a more effective drug.
In this study, we have developed a similar strategy. We synthesized five multifunctional nanoliposomes with different functional molecules. Previous studies had shown that mannitol22 and 7-hydroxyflavone23 have a good ability to inhibit α-syn aggregation. Also, the omega-3 molecule is a powerful anti-inflammatory agent24 and antisense oligonucleotides suppress the expression of SNCA.25 In this study, we found that if all functional molecules are included together, they have a very strong effect and can simultaneously inhibit aggregation of α-syn, silence SNCA gene, and attenuate neuroinflammation. An important point is the difference between PNL1 and PNL2. This study showed that although both PNL1 and PNL2 were significantly effective compared with other PNLs, the efficiency of PNL2 was higher than PNL1. We think that the reason for this phenomenon is that some molecules are incompatible with each other and their accumulation does not cause synergy. It can be said that the addition of antisense oligonucleotides slightly affects the function of other molecules. On the other hand, with a closer look at the results of PNL4 and PNL3, we will find an interesting phenomenon. We found that although these nanoliposomes have omega-3 and/or mannitol, they did not lead to inhibit α-syn aggregation or attenuate neuroinflammation, indicating that the presence of all compounds is necessary for maximum effect. Regarding PNL5, it should be noted that although this nanoliposome did not have any functional molecules, it could slightly inhibit α-syn aggregation, neuroinflammation, and SNCA expression.
Zhao et al. showed that antioxidant nanoparticles inhibit α-syn fibrillization and attenuate microglial activation.11 This study is an example that showed us a nanotherapeutic candidate can target both protein aggregation and neuroinflammation in neurodegenerative diseases. Zwitterionic nanoliposomes were also used to inhibit neurotoxic α-syn aggregation in PD. The effect of neutral (zwitterionic) nanoliposomes, supplemented with cholesterol and decorated with PEG, on α-syn aggregation and neurotoxicity was reported by Aliakbari et al., 12 cerium oxide nanoparticles13 and gold nanoparticles14 also could inhibit α-syn aggregation. Previously, Jebali et al. also showed that PEGlated nanoliposome could attenuate inflammatory response in mice model of multiple sclerosis.15
ConclusionTaken together, this study showed that PNL1 and PNL2 could significantly inhibit α-syn fibrillization, attenuate microglial activation, and silence SNCA gene, when compared with other PNLs. We hypothesize that, in the future, clinical trials using PNL1 and PNL2 should be considered for the treatment of synucleinopathies.
Ethical approvalAll experiments were performed under the guidelines of the National Institute of Health, the provisions of the Declaration of Helsinki, and the National Ethics Committee of Medical Science.
FundingThe main parts of the study were financially supported by Zahedan University of Medical Sciences, Zahedan, Iran (Grant number: 9936 and 9937). Also, some parts were financed by the internal budget of the authors.
Competing interestsThe authors have no conflicts of interest to declare.
We thank the Laboratory staff of Faculty of Advanced Sciences and Technology, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran, and Zahedan University of Medical Sciences, Zahedan, Iran.