Glioblastoma is the most common primary brain tumour. Despite advances in treatment, its prognosis remains dismal, with a mean survival time of about 14 months. Many articles have addressed direct costs, those associated with the diagnosis and treatment of the disease. Indirect costs, those associated with loss of productivity due to the disease, have seldom been described.
Material and methodWe conducted a retrospective study in patients diagnosed with glioblastoma at Hospital Universitario Donostia between January 1, 2010 and December 31, 2013. We collected demographics, data regarding the treatment received, and survival times. We calculated the indirect costs with the human capital approach, adjusting the mean salaries of comparable individuals by sex and age and obtaining mortality data for the general population from the Spanish National Statistics Institute. Past salaries were updated to 2015 euros according to the annual inflation rate and we applied a discount of 3.5% compounded yearly to future salaries.
ResultsWe reviewed the records of 99 patients: 46 women (mean age 63.53) and 53 men (mean age 59.94); 29 patients underwent a biopsy and the remaining 70 underwent excisional surgery. Mean survival was 18.092 months for the whole series. The total indirect cost for the series was €11080762 (2015). Mean indirect cost per patient was €111926 (2015).
DiscussionAlthough glioblastoma is a relatively uncommon type of tumour, accounting for only 4% of all cancers, its poor prognosis and potential sequelae generate disproportionately large morbidity and mortality rates which translate to high indirect costs. Clinicians should be aware of the societal impact of glioblastoma and indirect costs should be taken into account when cost effectiveness studies are performed to better illustrate the overall consequences of this disease.
El glioblastoma es el tumor cerebral más frecuente. A pesar de los avances en su tratamiento, el pronóstico sigue siendo pobre, con una supervivencia media en torno a los 14 meses. Los costes directos, aquellos asociados al diagnóstico y el tratamiento de la enfermedad, han sido descritos ampliamente. Los costes indirectos, aquellos derivados de la pérdida de productividad debido a la enfermedad, han sido descritos en escasas ocasiones.
Material y métodoRealizamos un estudio retrospectivo, incluyendo a los pacientes diagnosticados entre el 1 de enero del 2010 y el 31 de diciembre del 2013 de glioblastoma en el Hospital Universitario Donostia. Recogimos datos demográficos, relativos al tratamiento ofertado y la supervivencia. Calculamos los costes indirectos a través del método del capital humano, obteniendo datos de sujetos comparables según sexo y edad, y de mortalidad de la población general a través del Instituto Nacional de Estadística. Los salarios pasados fueron actualizados a euros de 2015 según la tasa de inflación interanual y los salarios futuros fueron descontados en un 3,5% anual en forma de interés compuesto.
ResultadosRevisamos a 99 pacientes, 46 mujeres (edad media 63,53 años) y 53 hombres (edad media 59,94 años). En 29 pacientes se realizó una biopsia y en los 70 restantes se realizó una cirugía resectiva. La supervivencia global media fue de 18,092 meses. Los costes indirectos totales fueron de 11.080.762 € (2015). El coste indirecto medio por paciente fue de 111.926 € (2015).
DiscusiónA pesar de que el glioblastoma es un tipo relativamente poco frecuente de tumor, que supone el 4% de todos los tipos de cáncer, su mal pronóstico y sus posibles secuelas generan una mortalidad y morbilidad desproporcionadamente altas. Esto se traduce en unos costes indirectos muy elevados. El clínico debe ser consciente del impacto del glioblastoma en la sociedad y los costes indirectos deben ser tenidos en cuenta en los estudios de coste-efectividad para conocer las consecuencias globales de esta enfermedad.
Glioblastoma (GB) is the most common and most aggressive primary tumour of the central nervous system.1,2 Its incidence ranges between 0.59 and 3.69 cases per 100000 person-years. GB accounts for approximately 1.4% of all cancer diagnoses.1,3,4 The first-line treatment for GB involves maximum surgical resection of the lesion without affecting eloquent areas, followed by chemoradiotherapy with temozolomide plus 6 sequential cycles of chemotherapy with temozolomide, according to the Stupp protocol.2,4–6
The impact of neurological diseases on healthcare costs depends on their prevalence and incidence, the number and complexity of techniques needed for diagnosis and treatment, and the degree of disability and dependence they cause.7,8 In the case of GB, the permanent brain lesion it causes and its resistance to most treatments lead to extremely high morbidity and mortality, with a 5-year survival rate of 0.05% to 4.7%.1,3
This study aims to provide information on the economic impact of GB on society and on the healthcare system, with a view to helping clinicians to rationalise healthcare resources.7 Studies conducted in our setting addressing the health-related costs of GB usually focus on direct costs associated with surgery, radiotherapy, and chemotherapy, whether with temozolomide or with carmustine implants.6,9 However, little attention has been paid to indirect costs, associated with loss of productivity; these should be considered when calculating the total economic impact of the disease.10
Material and methodsThis study (project UND-CGM-2015-01) was approved by our hospital's ethics committee. All participants gave written, informed consent to the collection and disclosure of personal and clinical data for scientific purposes.
We conducted a systematic, retrospective study of all patients diagnosed with GB at our centre between 1 January 2010 and 31 December 2013 using the anatomical pathology department's database. Diagnosis was based on the WHO criteria published in 2007.11 Treatment strategies were designed by a multidisciplinary team including neurosurgeons, medical oncologists, radiation oncologists, neurologists, radiologists, pharmacists, and pathologists.12
We reviewed patients’ clinical histories and gathered epidemiological data on treatment, date of diagnosis, and date of last contact with the patient or date of death, as applicable. Data were entered into a database using Microsoft Access 2013 (Microsoft Corporation, WA, USA). Statistical analysis was performed using Microsoft Excel 2013 (Microsoft Corporation, WA, US) and SPSS v.21 (IBM Corporation, NY, USA).
All costs are expressed in 2015 euros. Costs were calculated using the average annual wage by sex, age, and year (data from Spain's National Statistics Institute13). Pre-2015 costs were transformed into 2015 euros by accounting for the annual rate of inflation. To estimate future costs in 2015 euros, we used the 2015 average wage and applied a discount rate of 3.5%, compounded annually, as prescribed for this type of study by the National Institute for Health and Care Excellence (NICE)14 in 2013. Furthermore, future costs were adjusted for the risk of death in the general population by sex and age; these data were also gathered from Spain's National Statistics Institute.13
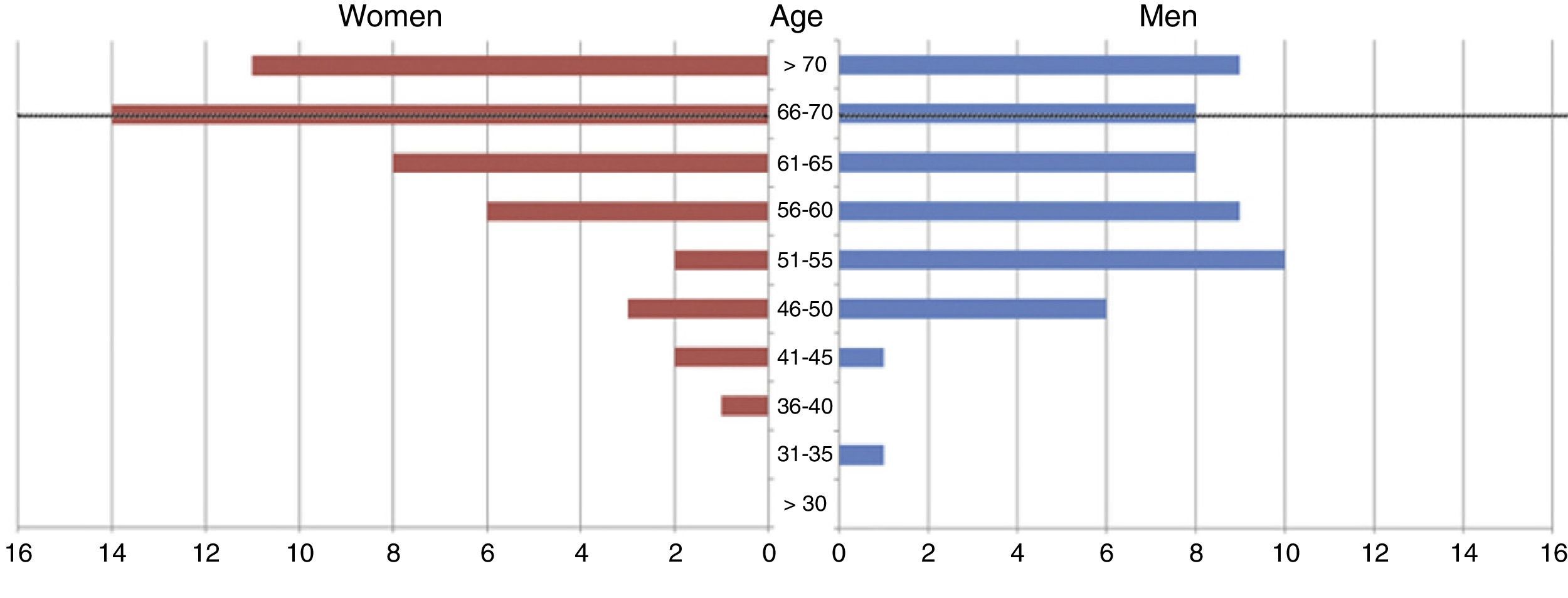
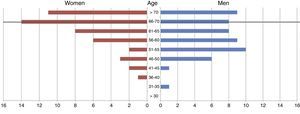
ResultsWe gathered data from 99 consecutive patients. Mean age in our sample was 61.61±9.89 years (range, 31-80 years). Of all patients, 46.46% were women (n=46) and 53.53% were men (n=53) (Fig. 1). Women had a slightly higher mean age than men (63.53±9.69 vs 59.94±9.84 years).
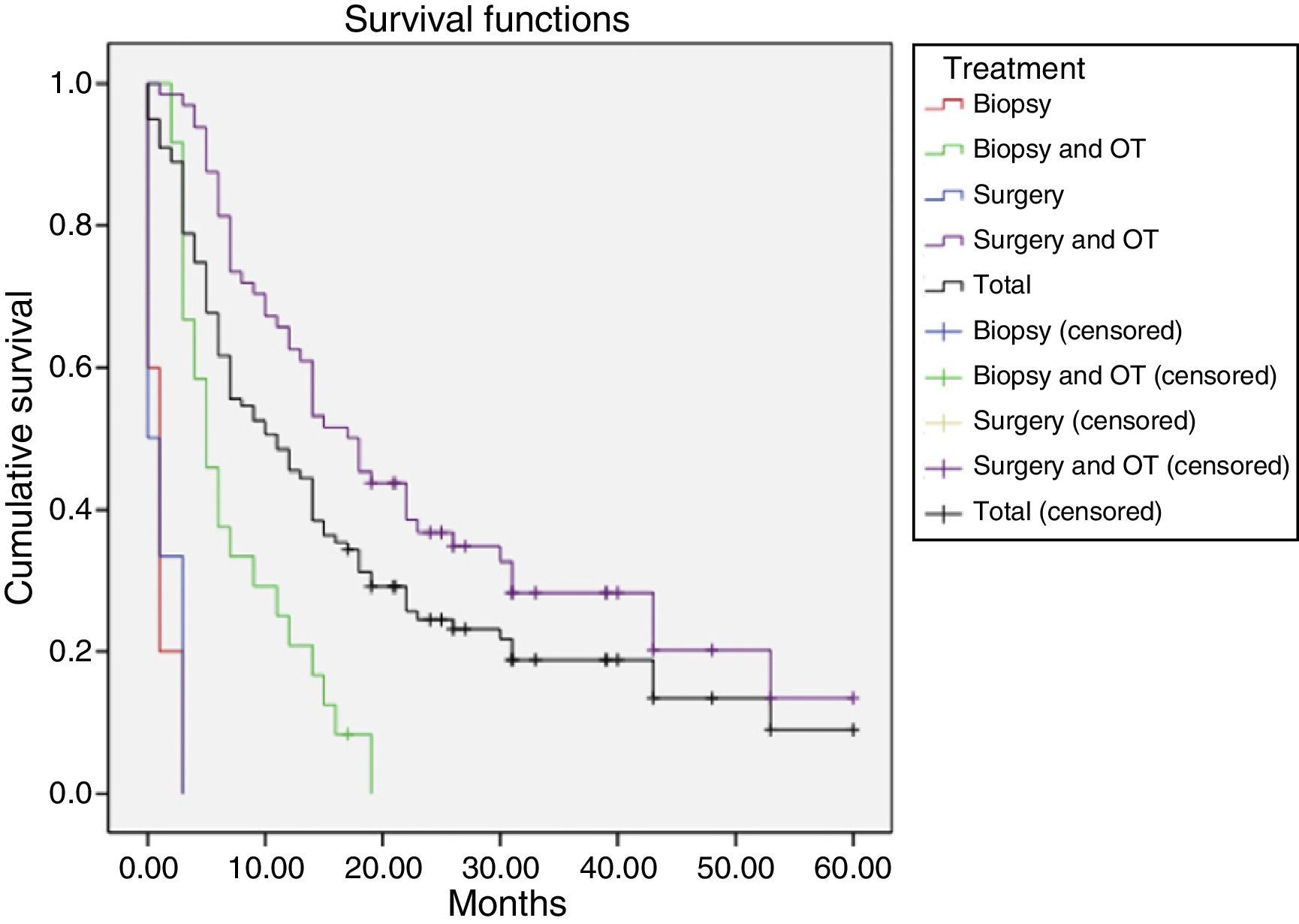
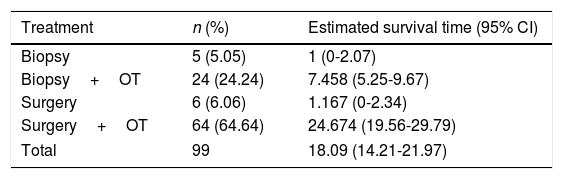
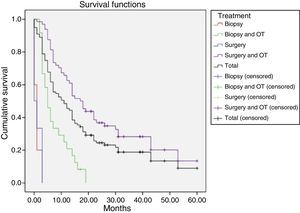
The 99 participants underwent a total of 111 surgical interventions. Eight patients (8.08%) died as a consequence of surgery. Eleven patients (11.11%) received no complementary treatment, either due to postoperative complications, the patient's refusal, poor functional status (low Karnofsky score), or tumour progression immediately after surgery. A total of 86 patients (86.86%) were deceased at the time the study was completed. Patients were followed up for a median of 39 months (mean 39.72±18.3; range, 18.2-63). The median survival time was 10.9 months and the mean survival time was 18.1 months (95% CI, 14.2-21.97) (Table 1, Fig. 2). We found significant differences in survival times between treatments (Mantel–Cox test, P<.001): patients undergoing surgical resection of the lesion and receiving some type of oncological treatment survived for a mean of 24.64 months.
Mean survival time (in months) for each treatment, using the Kaplan–Meier method.
| Treatment | n (%) | Estimated survival time (95% CI) |
|---|---|---|
| Biopsy | 5 (5.05) | 1 (0-2.07) |
| Biopsy+OT | 24 (24.24) | 7.458 (5.25-9.67) |
| Surgery | 6 (6.06) | 1.167 (0-2.34) |
| Surgery+OT | 64 (64.64) | 24.674 (19.56-29.79) |
| Total | 99 | 18.09 (14.21-21.97) |
OT: oncological treatment.
At the time of death, 58.58% of patients (n=58) were no older than 67, that is, they were of working age (Fig. 1). The total indirect cost of our series was €11080762.84. The mean cost per patient was €111926.90±148958.47.
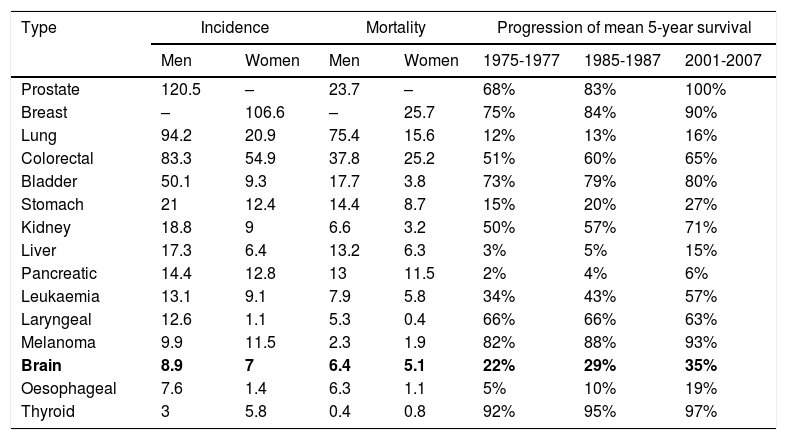
DiscussionThis study analyses the indirect costs of GB in a series of patients diagnosed with the condition at our centre between 2010 and 2013. According to 2012 WHO data, brain tumours have an incidence of 7.9 cases per 100000 person-years in Spain.15 This incidence rate is far lower than those of other types of tumours, such as breast cancer (the most frequent type of tumour in women), prostate cancer (the most frequent in men), or lung or colorectal cancer (the second and third most frequent in both sexes) (Table 2).15,16 The types of cancer which represent the largest contribution to cancer-related mortality are breast and colorectal cancer in women, and lung and colorectal cancer in men (Table 2).15,16 The 5-year survival rate of patients with brain tumours is among the lowest for all cancer types (35%) (Table 2).15,16 GB is the most frequent primary brain tumour; however, its incidence is relatively low compared to other types of cancer, ranging between 0.59 and 3.69 cases per 100000 person-years. Despite advances in the surgical and oncological treatment of GB, prognosis is still poor, with a 5-year survival rate of between 0.05% and 4.7%.1,3,4
Incidence, mortality, and progression of mean survival of the main types of cancer, by location.
| Type | Incidence | Mortality | Progression of mean 5-year survival | ||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | 1975-1977 | 1985-1987 | 2001-2007 | |
| Prostate | 120.5 | – | 23.7 | – | 68% | 83% | 100% |
| Breast | – | 106.6 | – | 25.7 | 75% | 84% | 90% |
| Lung | 94.2 | 20.9 | 75.4 | 15.6 | 12% | 13% | 16% |
| Colorectal | 83.3 | 54.9 | 37.8 | 25.2 | 51% | 60% | 65% |
| Bladder | 50.1 | 9.3 | 17.7 | 3.8 | 73% | 79% | 80% |
| Stomach | 21 | 12.4 | 14.4 | 8.7 | 15% | 20% | 27% |
| Kidney | 18.8 | 9 | 6.6 | 3.2 | 50% | 57% | 71% |
| Liver | 17.3 | 6.4 | 13.2 | 6.3 | 3% | 5% | 15% |
| Pancreatic | 14.4 | 12.8 | 13 | 11.5 | 2% | 4% | 6% |
| Leukaemia | 13.1 | 9.1 | 7.9 | 5.8 | 34% | 43% | 57% |
| Laryngeal | 12.6 | 1.1 | 5.3 | 0.4 | 66% | 66% | 63% |
| Melanoma | 9.9 | 11.5 | 2.3 | 1.9 | 82% | 88% | 93% |
| Brain | 8.9 | 7 | 6.4 | 5.1 | 22% | 29% | 35% |
| Oesophageal | 7.6 | 1.4 | 6.3 | 1.1 | 5% | 10% | 19% |
| Thyroid | 3 | 5.8 | 0.4 | 0.8 | 92% | 95% | 97% |
Epidemiological data from our series are in line with those reported by other authors in similar settings.17,18 In our setting, Graus et al.2 studied 834 patients with GB from 19 Spanish centres; 61.3% were men. Their sample had a mean age of 62 years and a median survival time of 11.8 months (95% CI, 10.7-12.8). In 2015, Brodbelt et al.17 analysed 10743 patients with GB in England between 2007 and 2011 and reported a higher incidence among men (60.04%), with peak incidence between the ages of 65 and 75. This population-based study found a median survival time of 6.1 months. Population-based studies typically report shorter survival times than hospital-based studies since they include all patients from a specific population, whereas hospital series only include patients receiving specialised care. Yabroff et al.18 analysed 1202 patients with GB in the USA, reporting a median survival time of 10 months (95% CI, 9–11) and a 3-year survival rate of 12.6%. All studies coincide with our own in reporting significantly longer survival times in patients undergoing surgery and subsequent chemotherapy and radiation therapy. In addition to treatment, multiple factors affect survival in patients with GB, particularly age at the time of diagnosis and functional status (typically expressed as a Karnofsky Performance Status Scale score). However, a detailed analysis of our patients’ prognoses is beyond the scope of this study.
The cost of neurological diseases in Spain is estimated at 10.4% of the total healthcare expenditure,19 and is expected to reach 14.4% by 2030.20 A systematic review by Parés-Badell et al.21 estimated the total cost of neurological diseases in Spain in 2010 at €83749 million. The total costs of a disease can be divided into direct, indirect, intangible, and informal costs. Direct costs are linked to healthcare activities and reflect the costs of healthcare resources used in prevention, diagnosis, and treatment of the disease. These are the costs most frequently addressed in the literature and are calculated based on the costs of hospital admission, treatment, and follow-up consultations. There is therefore less susceptibility to differing criteria in the calculation of these costs. Direct costs also include those associated with patient transport, social services, and home adaptations due to disability.7,22–24 Indirect costs are opportunity costs and represent the productivity lost due to the disease. They include the costs associated with death, early retirement, inability to attend work, and reduced productivity due to disability.7,10,22,24 Informal costs are those associated with unpaid care provided by the patient's relatives, and are sometimes included in the calculation of direct costs. As they are difficult to determine and are frequently not addressed in the literature, they have also been referred to as the “invisible healthcare system”.7,10,25 Intangible costs are those associated with the patient's loss of quality of life, and are sometimes included in the calculation of indirect costs. This study focuses on indirect costs, that is the loss of productivity associated with the disease.
Since the publication of the 2005 Stupp protocol, first-line treatment for GB has comprised maximum surgical resection of the lesion without affecting eloquent areas, followed by chemoradiotherapy with temozolomide (75mg/m2/day while the patient is receiving radiation up to 60Gy) plus 6 sequential cycles of chemotherapy with temozolomide (150-200mg/m2/day for 5 days every 28 days).5 These complex diagnostic and therapeutic processes entail high direct costs.9,26 In 2004, Chang et al.27 studied the direct cost of treating 7 types of cancer in patients treated between 1998 and 2000 in the USA. In their study of 653 patients with brain tumours, the direct healthcare costs of these patients were $46452±12492 higher per patient than those of a control population. The costs of radiotherapy and surgery contributed the most to direct costs, although it should be noted that the study was conducted before temozolomide was introduced. Silverstein et al.’s28 1996 study of patients with GB reports a total direct cost per patient of $91368 and also points to radiotherapy as contributing the most to direct costs. In both studies, direct costs plateau after a year of treatment due to patients’ short survival time. In our setting, a previous study by our research group9 estimated the cost of surgery for brain tumours at a mean of €13540.56 per patient.
Calculating the indirect costs of a disease is not straightforward due to the need to predict long-term future costs.29 A number of approaches have been developed to estimate indirect costs, the most common being the calculation of loss of income. The calculation uses the human-capital method, which estimates a patient's potential costs or loss of productivity until the theoretical age of retirement,22 taking the salary of comparable individuals from the same population as reference. This type of analysis includes those patients doing unpaid work, such as homemakers and voluntary workers.10 Despite this calculation method being the most commonly used, it has been criticised, with some authors arguing that it tends to overestimate indirect costs.22,23 It has been argued that in a market with an unlimited workforce (due to the presence of unemployed workers), the work not performed by a patient due to the disease will be performed by another individual, who may previously have been unemployed or working elsewhere. In this case, we use the friction-cost approach, which calculates productivity loss due to the reduced experience of the new worker, and training costs.10,22 Neither of these methods aim to quantify the value of human life, but rather the economic impact of a disease on society.30 Our study took the mean salary of comparable individuals (in terms of age and sex) as a reference; the salary was adjusted for the probability of death in the general population, and we applied a 3.5% annual discount rate, as recommended in the NICE guide.14 This model is therefore variable, as it depends on the discount rate applied (3%-5%, depending on the study14), salary forecasts, and differences in salaries between regions or countries.
In 1996, Blomqvist et al.31 estimated the indirect costs of brain tumours at $101058 per patient (78218 in 1996 dollars); this represented 73% of total costs. Similar costs were found in our series. In our setting, Navarrete-Navarro32 estimated the indirect costs of spontaneous cerebral haemorrhage at €31 108 per patient (in 2007 euros). In 2014, Parés-Badell et al.21 analysed the indirect costs of different neurological diseases in Spain. According to these authors’ data, the indirect costs of brain tumours are the fourth highest (€6826/year), with only neuromuscular diseases (€14185/year), multiple sclerosis (€12160/year), and Parkinson's disease (€9612/year) representing larger amounts (costs expressed in 2014 euros). Although the mean age in our series was 61 years, 58.58% of patients were active workers at the time of death, which explains the high indirect costs. According to our results, the indirect costs of GB would be higher than those of other neurological diseases. These results must be interpreted with caution, since the costs reported correspond to the years analysed and the calculation method may vary to a degree between studies. Given the retrospective nature of our study, we calculated indirect costs only after the date of death, disregarding the survival period after diagnosis, surgery, and oncological treatment.
Study limitationsOur study included only those patients with an anatomical pathology diagnosis of GB. Some patients displayed radiological findings of high-grade glioma, but neither received oncological treatment nor underwent surgery for a number of reasons (poor clinical status, advanced age, patient decision, etc.). These patients were not included in our study since they lacked an anatomical pathology diagnosis of GB. Estimating indirect costs requires a salary forecast; this may change depending on the model used, which limits the possibility of comparison between studies.
ConclusionsDespite the low incidence of GB, healthcare costs associated with the condition are extremely high. Indirect costs represent a considerable part of the total cost of GB. At present, there is no consensus on the most appropriate method for estimating indirect costs. In our series, 58.58% of the patients were of working age, resulting in a mean indirect cost of €111926.90 per patient.
FundingThis study has received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank Andoni Mozo, Maite Iñarra, Ana González, and the healthcare professionals at our unit at Hospital Universitario Donostia's Aranzazu building for their constant assistance and their dedication to our patients.
Please cite this article as: Undabeitia J, Torres-Bayona S, Samprón N, Arrázola M, Bollar A, Armendariz M, et al. Costes indirectos asociados al glioblastoma. Experiencia en un centro. Neurología. 2018;33:85–91.