MERS (mild encephalopathy/encephalitis with a reversible splenial lesion) is a reversible clinical-radiological syndrome associated with neurological signs and symptoms and restricted diffusion in the splenium on magnetic resonance imaging (MRI) studies. The precise pathophysiology remains unknown but the condition is mainly associated with infections.
We present the case of a 16-year-old girl with no relevant history who was assessed due to prostration and paraesthesia affecting the lower third of the right leg, progressing for 2 hours.
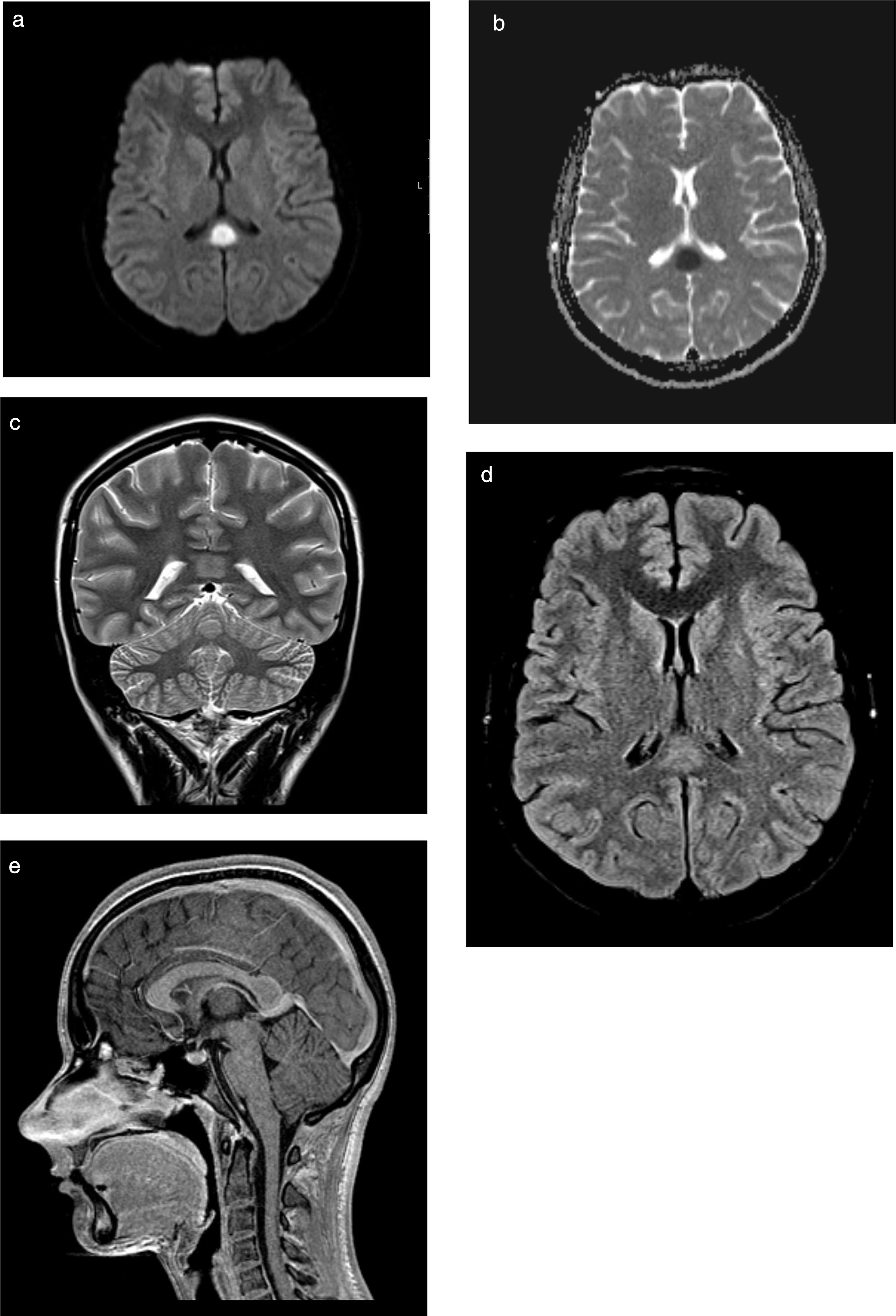
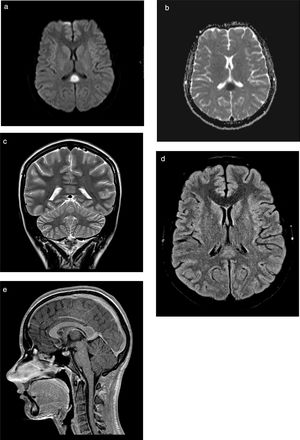
The previous day she had been attended due to 24-hour history of fever and dysuria, which was treated with antibiotics (oral cefuroxime). Physical examination revealed that the patient was oriented but unable to leave bed and showed psychomotor retardation (Glasgow Coma Scale [GCS] score of 14), with normal cranial nerve findings and no motor or sensory deficits in the upper limbs; we also observed monoparesis (grade 4+) and painful tactile hypoaesthesia in the distal part of the right leg. Deep tendon reflexes were normal and symmetrical and plantar reflexes were flexor. Cerebellar examination yielded normal results, and we observed no meningeal signs. A blood count revealed leukocytosis (20080cells/μL) with neutrophilia (17630cells/μL), normal biochemistry and electrolyte study findings (sodium 135mmol/L), and increased inflammatory parameters (erythrocyte sedimentation rate 40mm/h; C-reactive protein 24.66mg/dL). Urine culture revealed growth of Escherichia coli. A lumbar puncture revealed clear, colourless cerebrospinal fluid with normal pressure, 6leukocytes/μL (17% polymorphonuclear and 83% mononuclear), and normal biochemistry results; bacteriological, mycological, and mycobacteriological cultures, virus molecular biology (enterovirus, varicella zoster virus, and herpes simplex virus 1 and 2), and Mycoplasma culture yielded negative results. The polymerase chain reaction test for E. coli was not performed. Renal ultrasonography revealed an enlarged, globular left kidney, parenchymal hyperechogenicity, and perirenal fluid; these signs are suggestive of pyelonephritis. A head CT scan revealed no alterations. We started empirical treatment with intravenous ceftriaxone and aciclovir. A spinal and brain MRI scan (Fig. 1A-E) performed on the third day after admission showed an ovoid tumefactive lesion in the centre of the splenium, showing diffusion restriction. At that time, we observed complete resolution of neurological symptoms: GCS score of 15 and normal strength and sensitivity.
MRI study. Ovoid tumefactive lesion located in the splenium of the corpus callosum, with diffusion restriction. A) Axial diffusion-weighted imaging at b1000; B) axial diffusion-weighted sequence with ADC mapping; C) coronal T2-weighted sequence; D) axial T2-weighted FLAIR sequence; (E) sagittal gadolinium-enhanced T1-weighted sequence.
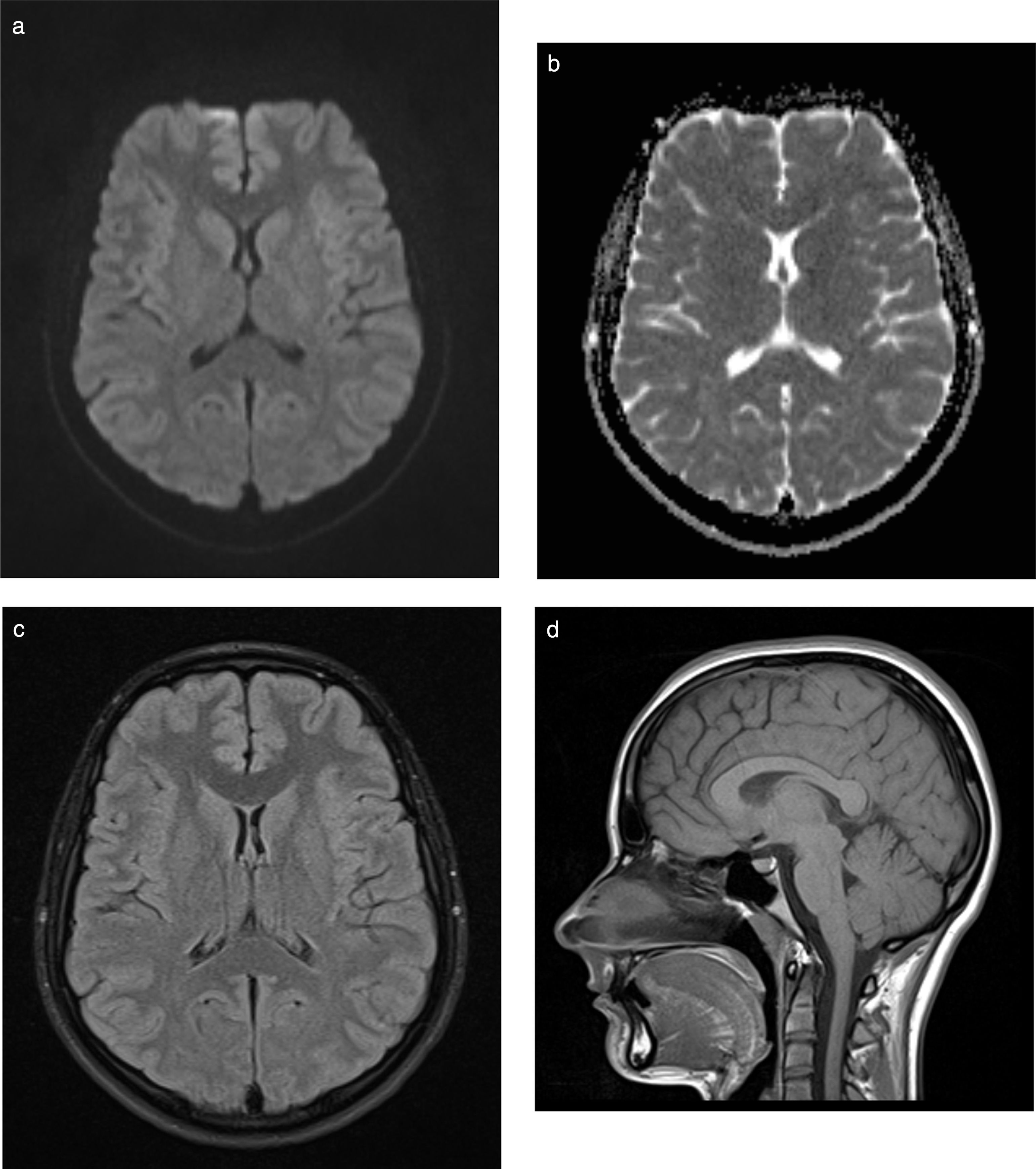
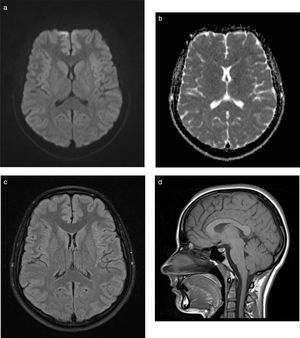
Nine weeks later, the patient is asymptomatic with normal neurological examination findings and resolution of the lesion on the MRI sequence (Fig. 2A-D), and presents no sequelae.
Neurological symptoms typically appear in the first 7 days of fever and are highly variable, ranging from altered level of consciousness to seizures, blindness, ataxia, tremor, hallucinations,1 acute urinary retention,2 and sensory alterations.3
MRI usually shows an ovoid lesion in the splenium with diffusion restriction, tumefactive appearance, and signal hyperintensity on T2-weighted sequences. MERS type II was described more recently, and is characterised by the involvement of white matter adjacent to the corpus callosum.4–6 In most cases, the lesion resolves within approximately one month.
Initially, this syndrome has been mainly associated with viral (influenza, adenovirus, mumps virus,1 varicella zoster virus,2 rotavirus,7 and cytomegalovirus4,8) and bacterial infections (E. coli O157:H7,1Legionella pneumophila,2Klebsiella pneumoniae,9Salmonella,4Streptococcus pneumoniae, and Mycoplasma pneumoniae6,10). More recent reports include cases associated with other autoimmune diseases (Kawasaki disease,11 systemic lupus erythematosus12), Amanita phalloides intoxication,13 antiepileptic drugs,12,14 and metabolic disorders (for example, vitamin B12 deficiency12).
The most widely accepted pathophysiological hypothesis points to intramyelinic oedema, probably associated with hyponatraemia and/or local infiltration by proinflammatory cytokines.1,15,16
Treatment of the underlying disease is considered adequate, although intravenous corticosteroid and immunoglobulin treatment has been tried, with unclear benefits.1,2,4,7,9,17
Most of the earliest cases of MERS were described in Asia,1–3,5–7,9–11,16,17 and more specifically in China and Japan. Our case presents several peculiarities; specifically, it is one of the few cases reported in Europe and is associated with urinary tract infection9,17 (specifically due to non-enterohaemorrhagic E. coli3) in the absence of hyponatraemia.15 The shape and location of the lesion rule out other diagnoses that are more common in paediatric patients, such as acute disseminated encephalomyelitis and posterior reversible encephalopathy syndrome. Furthermore, the reversible nature of the lesion suggests intramyelinic oedema rather than cytotoxic oedema, especially related to ischaemia.
Despite its low incidence and its frequent association with viral infections, MERS syndrome should be considered in the event of neurological symptom onset in patients with bacterial infections, especially acute pyelonephritis. It is essential to recognise this entity due to the good prognosis after treatment of the underlying condition. Although diagnosis can only be confirmed subsequently (reversible character of the lesion), the imaging (radiological) signs in this age group and clinical context are sufficiently characteristic to consider this the most probable hypothesis.
FundingThis study has received no specific funding from public agencies, pharmaceutical companies, or non-profit organisations.
Please cite this article as: Miranda J, Pereira I, Nunes J, Santos F. Encefalitis/encefalopatía leve con lesión reversible del esplenio del cuerpo calloso asociada a pielonefritis aguda; a propósito de un caso clínico. Neurología. 2020;35:530–534.