Aetiological diagnosis of adult-onset ataxia represents a diagnostic challenge, and ruling out potentially treatable causes must be a priority.1 Among these, we may highlight paraneoplastic neurological syndromes (PNS), given the significance of their association with an underlying tumour. However, misdiagnosis may lead to iatrogenic complications secondary to unnecessary immunosuppression. We present the case of a patient with late-onset progressive ataxia, which was initially diagnosed as a PNS but finally found to be of degenerative origin.
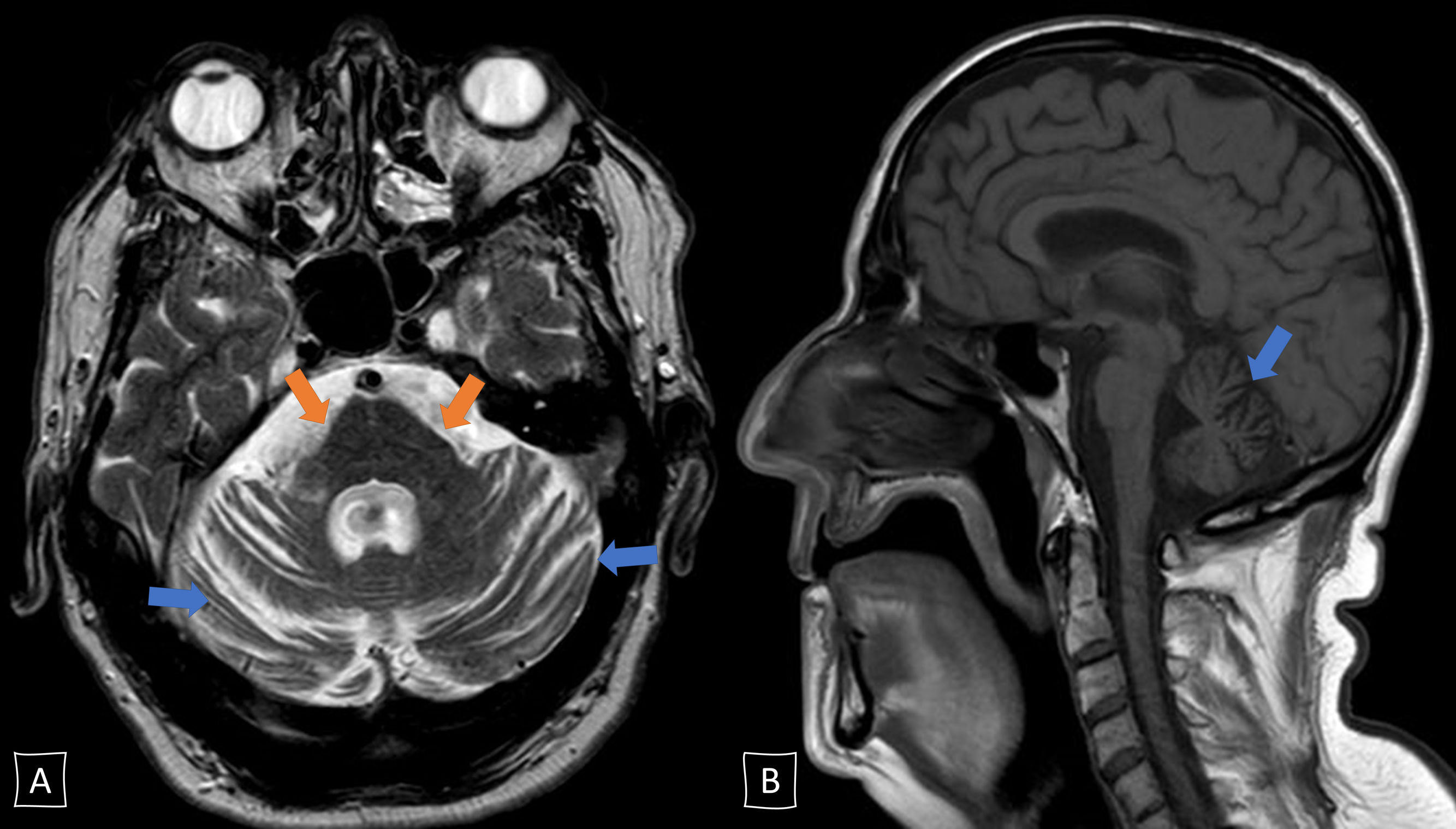
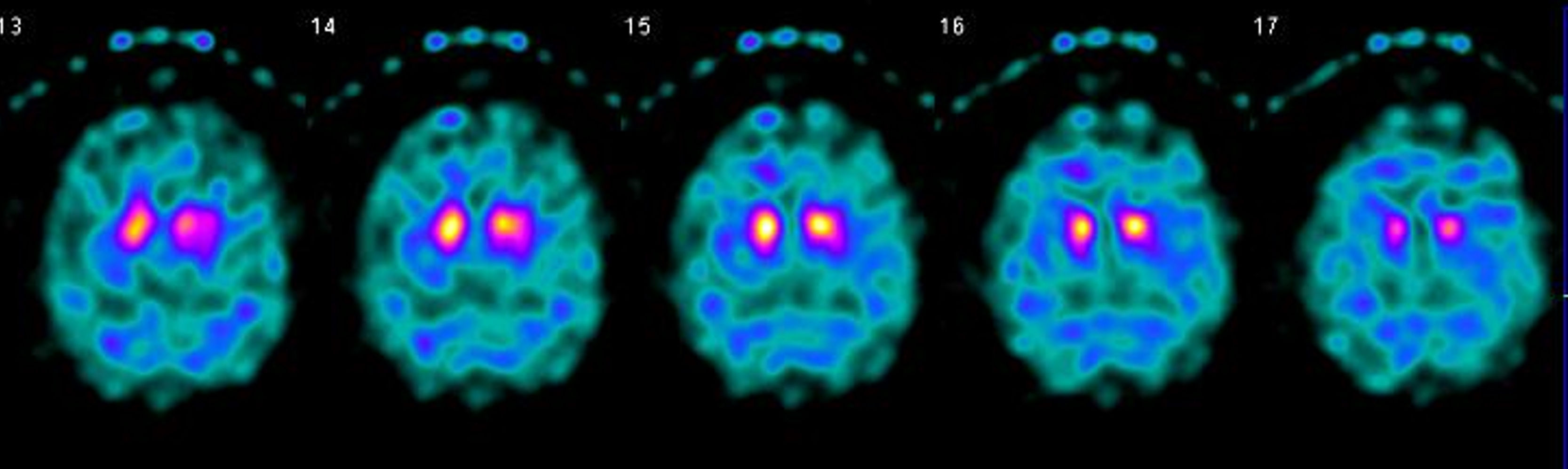
The patient was a 65-year-old man with no relevant family or personal history with the exception of smoking and dyslipidaemia, who consulted in 2017 due to a 2-year history of progressive gait disorder. The examination revealed scanning speech, fragmented saccades, bilateral appendicular dysmetria, mild truncal ataxia, and moderate gait ataxia. He reported no alcohol abuse or exposure to other toxic substances. A brain MRI scan revealed pontine and cerebellar atrophy with no other relevant findings (Fig. 1). Laboratory analysis returned positive results for anti-Ma2 antibodies in the serum (Immunoblot, EUROIMMUN AG) and slightly increased tumour markers (carcinoma embryonic antigen, 10.2ng/mL [normal range, 0-3.4]; squamous cell carcinoma antigen, 2.0ng/mL [0-1.5]); the remaining parameters, including autoimmunity, vitamins (B1, B12, and E), and cerebrospinal fluid analysis (including antineuronal antibody determination), yielded normal results. The patient was diagnosed with cerebellar ataxia of paraneoplastic aetiology, although test results for occult malignancy (cervical/thoracic/abdominal/pelvic CT scan, genitourinary ultrasound, colonoscopy, bronchoscopy, and PET) were negative throughout the 2 following years. We initially treated the patient with intravenous immunoglobulins (IVIg), and subsequently with a megadose of corticosteroids; as no benefit was observed, we started treatment with rituximab in June 2018. Despite this, his symptoms continued to worsen over the course of 2018: he needed walking aids and developed dysphagia, cognitive impairment, tremor, urinary incontinence, and REM sleep behaviour disorder. In early 2019, the patient showed signs of parkinsonism and autonomic dysfunction worsened (orthostatic hypotension and vasomotor changes in the hands). We decided to discontinue immune therapy and to reconsider his diagnosis; we requested a new determination of anti-Ma2 antibodies at our centre (Immunoblot, EUROIMMUN AG), once more returning weakly positive results, and simultaneously at a reference laboratory (immunohistochemistry on rat cerebellum; IDIBAPS, Hospital Clínic, Barcelona), which yielded negative results. Furthermore, we requested a brain SPECT study, which revealed bilateral dopaminergic hypometabolism (Fig. 2). All these data suggested a final diagnosis of cerebellar-type multiple system atrophy. The patient started symptomatic treatment with levodopa and physical therapy, with a poor response. Unfortunately, the patient died in May 2019. The family did not agree to an autopsy study.
Cerebellar degeneration is one of the most frequent PNS (up to 40% of cases),2 and usually presents a subacute course with rapid clinical deterioration over less than 12 weeks. Neuroimaging results may be normal or reveal mild cerebellar atrophy, and cerebrospinal fluid analysis usually indicates inflammation (pleocytosis, high protein level, or IgG oligoclonal bands). It has been reported in association with different tumours and onconeuronal antibodies targeting intracellular antigens, with anti-Ma antibodies rarely being reported.2 These cases of predominant cerebellar involvement occur in patients with simultaneous reactivity to both Ma1 and Ma2 antigens, whereas in patients with anti-Ma2 antibodies only, ataxia is part of a more complex group of symptoms with predominant limbic or brainstem involvement.3,4 Anti-Ma2 antibodies are associated with tumours in up to 75% of cases, with testicular cancer and non–small-cell lung cancer being most common3; their presence is therefore strongly associated with diagnosis of PNS. However, a small number of cases have been reported that presented no evidence of malignancy.3,4 Treatment of anti-Ma2-associated PNS is based on the identification and oncological management of the underlying tumour, as well as on immune therapy with corticosteroids, IVIg, cyclophosphamide, or rituximab.3 In the light of this, in the event of atypical clinical symptoms progressing for more than 6 months, low antibody titres, absence of malignant tumour, and lack of response to treatment, we must consider a false positive result for these antibodies.
We have not found other cases of false positive results for anti-Ma2 antibodies in the literature, but we did find cases for other antineuronal antibodies, especially when they were analysed with unsuitable techniques. It should be noted that treatment with commercial IVIg may lead to false positive results for such antibodies as anti-GAD or anti-aquaporin-4 when samples are analysed with ELISA.5 However, cellular techniques did not return these false positive results5; these techniques should be used preferentially when the pathogenic antibodies recognise conformational epitopes of proteins (extracellular antigens). Different studies reveal the difficulties faced by inexperienced laboratories when interpreting reactivity patterns of autoantibodies with commercial kits.6,7 Furthermore, onconeuronal antibodies may be positive in patients with tumours who present no neurological symptoms8; some autoantibodies are also positive at low titres in the healthy population.9 Given the importance of diagnosing PNS and the risks associated with immunosuppressive treatment, seropositivity for onconeuronal antibodies should be confirmed by experienced centres using 2 different techniques: ideally first by immunofluorescence on primate or rat nervous tissue, with subsequent confirmation by immunotransference or transfected cells techniques, depending on whether intracellular or extracellular antigens are used.10
Please cite this article as: Fernández Díaz E, Sánchez-Larsen Á, Redondo-Peñas I, Segura T. Ataxia cerebelosa progresiva con anticuerpos anti-Ma2 falsamente positivos. Neurología. 2021;36:334–336.
The early progression of this case was communicated in poster format at the 69th Annual Meeting of the Spanish Society of Neurology, held in Valencia from 21 to 25 November 2017, under the title “Chronic paraneoplastic cerebellar degeneration with positive anti-Ma2 antibodies.”