Persistence of measles infection in children and young adults can rarely lead to a fatal progressive neurodegenerative disorder known as subacute sclerosing panencephalitis (SSPE).1–4 Amongst the other known neurological manifestations of measles infection, SSPE is the rarest and most severe.1–4 However, measles infection is amenable to prevention by highly effective live vaccination.1–5 The latency period between measles infection and development of clinical features of SSPE is variable and can be up to 2–3 decades.1–4 Earlier affection and intrafamilial cases of SSPE have a shorter latency.1–4 The clinical course is characterized by subacute to chronic progressive cognitive impairment, behavioral abnormalities, movement disorders (especially myoclonus), seizures, visual disturbances, and ataxia.1–3 Although few reports of spontaneous remissions of SSPE have been documented,6,7 this disease is considered universally fatal. Diagnosis of SSPE is difficult in developed countries because of extreme rarity of the condition and atypical and non-specific initial presentation because the sufferers come rather early in the course of disease.1–3,8 On the contrary, in underdeveloped/developing countries, the delay in diagnosis occurs due to low health-related literacy in general population, significant lack of knowledge and training of primary care physicians regarding these disorders, inadequate health-seeking behavior of the general population, and inadequate investigational backup in peripheral health care setups .1–3,8
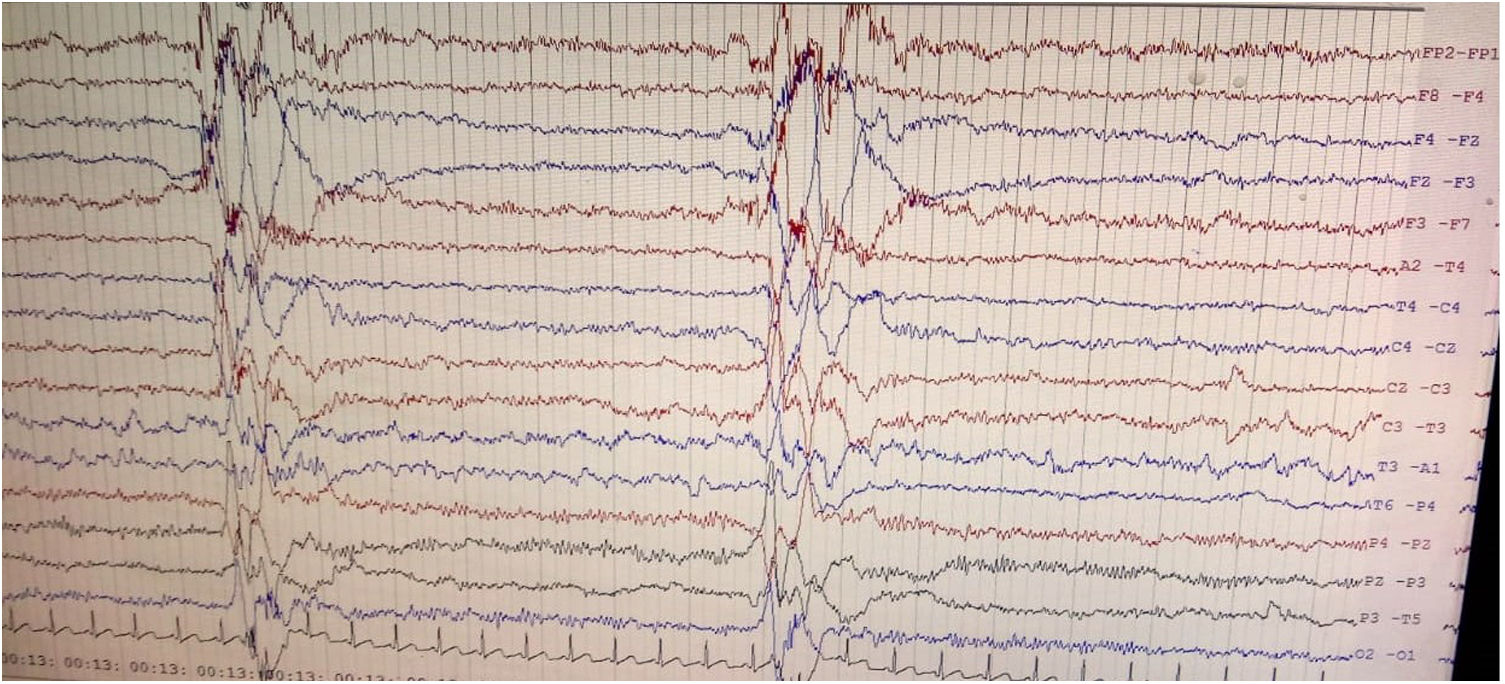
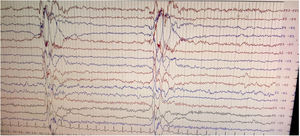
We herein report a case of SSPE in a 36-year-old previously healthy man with questionable vaccination status in childhood who presented with rapidly progressive early onset dementia and subcortical myoclonus. Neuroimaging revealed altered signals involving bilateral thalamus and brainstem widening the list of differential diagnoses; however, clinical history, examination findings, and high anti-measles IgG titers in serum and cerebrospinal fluid (CSF) clinched the diagnosis, which was supported by classical Radermecker's complex in electroencephalography (EEG).9
A 36-year-old previously healthy man from a middle socio-economic family of rural West Bengal (India) was brought to the out-patient department with complaints of rapidly progressive behavioral abnormalities (inattentiveness, forgetfulness, mood disturbances, aggressive behavior, lack of interest in surroundings, calculation difficulties, difficulties in recognizing relatives and common objects, and loosing ways during navigation) and visual difficulties for last 3–4 months. The family members also gave history of objects falling from his hands unknowingly, and gradually evolving abnormal involuntary jerky “shock-like” movements involving all the limbs and torso for the last 2 months. Past medical history and history of long-term drug exposure or addiction in any form were unyielding. The family members revealed that one of his brothers also had succumbed to a similar illness within 2 years of developing similar symptoms at the age of 18 years. On enquiry, his mother described an episode of “fever with confluent rash” with spontaneous recovery in all her children, in their childhood, over a period of 2 weeks suggestive of measles. Neurological examination revealed multi-domain cognitive impairments with subcortical myoclonus.
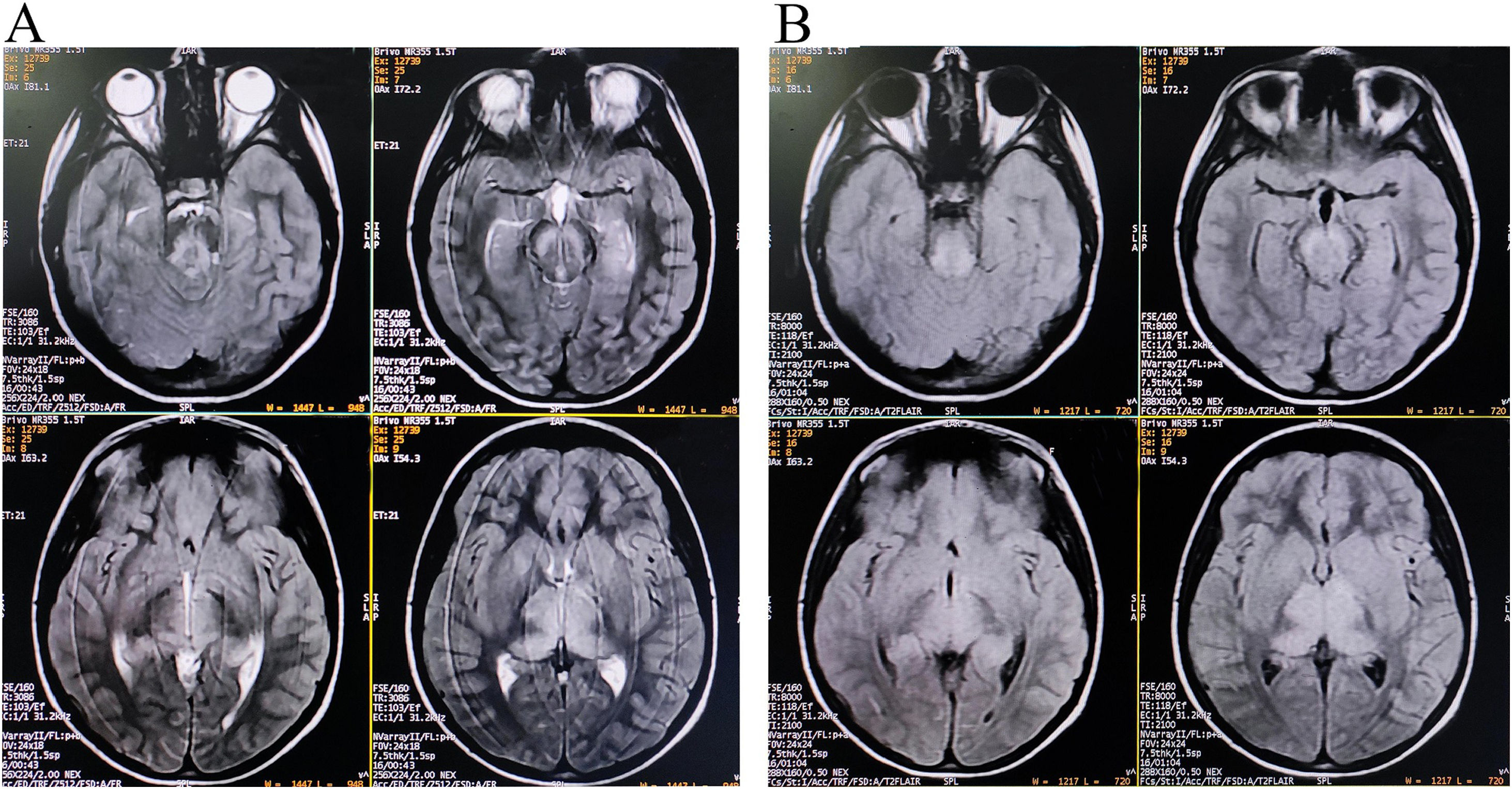
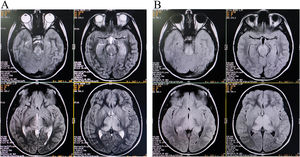
Brain magnetic resonance imaging (MRI) showed non-enhancing bilaterally symmetrical altered intensity lesions, hyperintense on T2-weighted imaging and FLAIR sequences, involving thalamus and brainstem (Fig. 1). EEG revealed periodic discharges suggestive of Radermecker complexes (Fig. 2). Contrast enhanced MR angiography and MR venography excluded common vascular insults. Accordingly, relevant tests were done, which excluded metabolic disorders (blood glucose, thyroid, liver and renal function tests, electrolytes, vitamin B1 and B12, arterial blood gas analysis, serum lactate, CSF lactate, lactate/pyruvate ratio, and anti-thyroperoxidase/thyroglobulin antibody were normal), toxic encephalopathy (toxicology screen), acute infectious encephalopathies (neuro-viruses panel, including SARS-CoV-2 and HIV 1, 2, were negative). Autoimmune/paraneoplastic encephalitis panel was negative. CSF study revealed lymphocyte-predominant pleocytosis (cell count: 25/μl), increased total protein (108mg/dL), and raised IgG index. CSF and serum were tested for anti-measles IgG antibody titers and they came out to be extremely high confirming the diagnosis of SSPE further (fulfillment of Dyken's criteria).8
Quite expectedly, resource-constrained countries with a high burden of measles encounter a higher incidence of SSPE.1–3 Besides, significant number of cases remain undiagnosed/misdiagnosed and unreported.1–3,8 However, measles vaccine has a preventive effect against SSPE by simply protecting against measles.5 Countries with high vaccination gap and recent outbreaks is associated with higher incidence of SSPE.1–5 Provided the potential lethality of long-term infection of measles, SSPE should be diagnosed early and reported.
Of note is that MRI patterns of SSPE may mimic metabolic disorders.10 Symmetrical thalamic and brainstem involvement without affection of periventricular sub-cortical white matter and cortex is extremely rare in SSPE.10–12 However, neuroimaging findings of SSPE have typically a dynamic and evolving course. And in this case, though the cortical involvement was not evident on conventional imaging, clinical picture suggested involvement of fronto-parietal and occipital cortices (inattentiveness, forgetfulness, mood disturbances, aggressive behavior, apathy, dyscalculia, prosopagnosia, and visual object agnosia). Cortical and sub-cortical white matter intensity changes are expected to be visualized in the follow-up scans. Symmetrical thalamic involvement made it difficult for us to diagnose the case as similar neuroimaging findings can be observed in many other neurological disorders.13
In closing, SSPE is almost universally fatal and even symptomatic treatment does not help much leading to a disastrous end of a young productive life. Measles vaccination is the only definite way to prevent SSPE until date hence all children and eligible adolescents should get it without hesitancy. SSPE should be kept in the list of differential diagnoses, while dealing a case of subacute-to-acute onset rapidly evolving cognitive decline with myoclonic jerks.
Ethics statementInformed written consent was obtained from the patient involved in this study.
DisclosuresR. Ghosh reports no disclosures relevant to the manuscript.
S. Dubey reports no disclosures relevant to the manuscript.
A .K. Mukherjee reports no disclosures relevant to the manuscript.
J. Benito-León reports no disclosures relevant to the manuscript.
FundingNone.
Conflict of interestThe authors have no conflict of interest.
J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451).