To study the relationship between sleep quality and memory in healthy ageing.
MethodsThe study included 99 people older than 50 years (69 women and 30 men; mean age, 68.74 ± 7.18 years) with no associated diseases. Patients completed digital versions of the Word Learning (WL) and Visual Paired Associates (VPA) tests and the Pittsburgh Sleep Quality Index (PSQI) questionnaire to assess the quality of sleep.
ResultsPSQI score was negatively correlated with VPA and WL test performance. Performance in these 2 memory tests decreased in line with sleep quality. In addition, performance in VPA test was negatively correlated with subjective sleep quality, duration, and sleep disturbances. Performance on the WL test was negatively correlated with subjective sleep quality and efficiency. Participants’ sex showed a weak effect on VPA performance and sleep latency.
ConclusionsMedical professionals working with elderly patients should take into consideration the effect of poor sleep quality on memory. Cognitive impairment in these patients may be a manifestation of a neuroendocrine imbalance due to a disrupted circadian rhythm. More research is needed to prove this hypothesis.
Se estudió la relación entre la calidad del sueño y la memoria en el envejecimiento sano.
MétodoParticiparon 99 personas mayores de 50 años, 69 mujeres y 30 hombres, con una media de edad de 68,74 años (DT = 7,18) y sin patologías asociadas. Se aplicaron las pruebas Aprendizaje de Palabras (AP) y Pares Visuales Asociados (PVA) mediante versiones computarizadas así como el cuestionario PSQI para evaluar la calidad del sueño.
ResultadosLos resultados mostraron que el índice global de calidad de sueño (PSQI) estaba relacionado negativamente con el rendimiento en las pruebas de pares visuales asociados y de aprendizaje de palabras. A peor calidad del sueño peor rendimiento en estas dos pruebas de memoria. Además, el rendimiento en la prueba de pares visuales asociados estuvo negativamente relacionado con la calidad subjetiva de sueño, la duración y las perturbaciones del sueño. El rendimiento en la prueba de aprendizaje de palabras estuvo relacionado negativamente con la calidad subjetiva del sueño y la eficiencia. Se encontró un efecto débil del género de los participantes sobre el rendimiento en pares asociados y la latencia de sueño.
ConclusionesLos profesionales a cargo de personas de la tercera edad deberían tomar en consideración el efecto negativo de la baja calidad del sueño sobre la memoria. El deterioro de la cognición y en estos pacientes pueden ser manifestaciones del desequilibrio neuroendocrino asociado a la ruptura de los ritmos circadianos. Sería necesaria más investigación para probar esta hipótesis.
Increased life expectancy is associated with greater prevalence of neurodegenerative diseases. One challenge for applied neuroscience is the development of fast, simple systems for the early diagnosis of cognitive disorders. However, the study of age-related cognitive changes presents methodological complexities due to the confounding effect of nervous system disorders as a comorbidity during ageing.
Healthy ageing is associated with greater variability of cognitive function between individuals; a broad range of variables, including education, genetic factors, medical history, lifestyle, working history, and sociocultural factors, are known to influence different cognitive capacities over an individual’s life, and determine their functional status in old age.1–4
Healthy ageing involves neural changes that do not necessarily manifest as functional disabilities, and age-related cognitive changes are known to present a relatively stable progression, rarely progressing to dementia.5 These changes mainly affect attentional performance, working memory, learning, long-term memory, language, and visuospatial and executive functions.1
Long-term memory presents distinctive characteristics during ageing, and its decline may be explained by alterations to processes of encoding, storage, and recovery, or disorders in the interaction between encoding and recovery.6–9 Ageing can also be associated with deficits in the attentional processing of the information used to solve tasks10–12 and deficits in executive function,13 which may be responsible for the impairment of working memory.14
While they are distinct constructs, it is methodologically challenging to separate the evaluation of attention and working memory. The latter concept refers to the conscious representation and temporary manipulation of the information needed to perform complex cognitive operations, such as learning, language comprehension, and reasoning.15 There is overlap between the neural systems involved. The prefrontal cortex and hippocampus are connected by various circuits, which must remain intact to guarantee cognitively healthy ageing. With age, reduced postsynaptic density is observed in the hippocampus, which may indicate the presence of silent, non-functional synapses16; gene transcription aberrations have also been detected that affect the brain’s capacity for learning and memory.17 However, prefrontal systems age more rapidly than the temporal lobes, showing synapse loss and regression of apical dendrites,18 which would explain the cognitive pattern observed in elderly individuals, in whom processes of working memory, attention, and cognitive flexibility are affected first.19
Sleep in old ageSleep patterns and circadian rhythms are modulated by sex steroids, which have receptors in the suprachiasmatic nucleus of the hypothalamus. Plasma concentrations of these steroids change in accordance with the period of development (childhood, puberty, old age), affecting sleep.20–22
Prevalence of poor sleep quality is reported in the adult population, and age is directly correlated with poorer sleep quality and quantity.23,24 These alterations particularly affect women due to the age-related decrease in sex steroid levels.22,25
Extensive reorganisation of the central nervous system occurs during sleep,24 and while it is unclear whether sleep quality influences memory, sleep disorders have been shown to affect alertness, concentration, and individual performance. Both REM and non-REM sleep maintain neuronal integrity, and the REM phase is known to be necessary to prenatal brain development and postnatal maintenance of brain functions.23,24
As much of the available information is from studies with standardised conditions in clinical populations, there is a lack of data on memory task performance and perceived sleep quality in healthy older individuals in a community setting.
This study aims to address this issue.
MethodsParticipantsThe study sample comprised 99 healthy individuals aged over 50; 69 (69.7%) were women and 30 (30.3%) were men; all participants were users of the El Parque and Concepción Arenal centres for older people in Rivas-Vaciamadrid (Spain). Participants were aged between 52 and 87 years, with a mean (standard deviation [SD]) age of 68.74 years (7.18). Regarding level of schooling, 53.5% had completed primary education only, 29.3% had completed secondary education, and 16.2% had completed post-secondary education; one participant (1%) did not report the level of education completed. All participants were assessed by a geriatrician and a neurologist to rule out concomitant diseases affecting cognition, refractory visual disorders and colour blindness, previous diagnoses of sleep disorders, and use of drugs that may interfere with performance on neuropsychological tests. As this study did not aim to diagnose neurocognitive disorders, no test was administered to screen for these. To ensure that instructions were understood and that the responses given reflected the true performance of the cognitive tasks and subjective perception of sleep quality, both tests were administered by a single researcher. Participants were evaluated in a private, distraction-free space. All evaluation sessions took place in the morning, and lasted approximately 35 minutes. Participation was voluntary and all participants signed informed consent forms. The study complied with the principles of the Declaration of Helsinki for research involving human subjects. Upon completion of the study, each participant was given a summary of their assessment results, with suggestions for their family doctor if any anomaly was detected.
ProcedureEvaluation of memory. The study used the VINCI 1.0 program,26 a specifically designed computer test battery that evaluates memory through 2 tests, Visual Paired Associates (VPA I and II) and Word Learning (WL), based on tasks from the Wechsler Memory Scale-Revised.27 The tests were administered in the following order: VPA I, WL, and VPA II.
- -
VPA. The test was performed in 2 phases. Phase I involved 3 trials. In each trial, 6 pairs of colours and abstract, black-and-white figures (125 × 80 pixels; 7 × 4.5 cm for each figure and colour) were presented consecutively, against a grey background; each pair was shown for 6000 ms, with 1000 ms interval between cues. Next, all 6 colours were presented simultaneously and continuously, while the 6 figures were successively shown in a random order, for 6000 ms per figure. Participants were then asked to identify which colour was associated with each figure. In phase II, 30 minutes later, participants were shown the 6 colours and a random succession of the abstract figures, and were asked to identify which colour was associated with each figure. The variables recorded were total correct responses in VPA I and total correct responses in VPA II. Overall performance was calculated by adding together the 2 values. The maximum possible score was 24 points.
- -
WL. The test was performed in 3 phases. In the first, 15 words were shown in succession (1500 ms per word); subsequently, a window appeared in which the subject or operator wrote the words the subject recalled. In the second phase, all 15 words were shown for 90 seconds. Next, a window appeared in which the subject or operator wrote the words the subject recalled. In the third phase, 30 words (15 from the original list and 15 new words) appeared in succession; subjects were asked to hit the Enter key each time a new word appeared and the space bar each time a word from the original list was shown. Overall performance on the WL task was calculated by adding together the words recalled in the first and second phases. The maximum possible score was 30 points.
Evaluation of sleep quality. Sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI),28 which was administered by the lead author (TC) to ensure comprehension and correct completion of the questionnaire. The questionnaire evaluates sleep quality and difficulties in the last month. It includes 19 self-administered items (only these are scored) and 5 that are rated by the bed partner or room mate. The 19 items assess 7 components of sleep, each of which is scored from 0 to 3 (with 0 indicating no difficulty and 3 indicating great difficulty). The components evaluated are: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of scores for the 7 components provides a global score between 0 and 21, with 0 indicating no difficulty and 21 indicating severe difficulty in all components. Global PSQI scores greater than 5 are considered to indicate poor sleep quality.
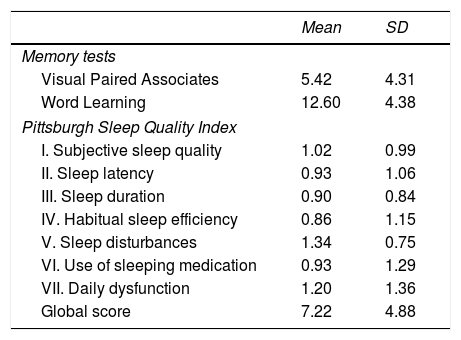
ResultsTable 1 shows the mean (SD) values for VPA, WL, and sleep components.
Means and standard deviations for each memory test and sleep quality component.
| Mean | SD | |
|---|---|---|
| Memory tests | ||
| Visual Paired Associates | 5.42 | 4.31 |
| Word Learning | 12.60 | 4.38 |
| Pittsburgh Sleep Quality Index | ||
| I. Subjective sleep quality | 1.02 | 0.99 |
| II. Sleep latency | 0.93 | 1.06 |
| III. Sleep duration | 0.90 | 0.84 |
| IV. Habitual sleep efficiency | 0.86 | 1.15 |
| V. Sleep disturbances | 1.34 | 0.75 |
| VI. Use of sleeping medication | 0.93 | 1.29 |
| VII. Daily dysfunction | 1.20 | 1.36 |
| Global score | 7.22 | 4.88 |
The mean VPA and WL scores (5.42 and 12.60, respectively) are both situated at the 50th percentile of the distribution of scores; therefore, performance can be considered average.
Mean sleep quality is poor (7.22 points). Mean scores for all sleep quality components evaluated were close to 1 point, the threshold value for sleep alterations; alterations in subjective sleep quality, sleep disturbances, and daytime dysfunction were most common.
Analysis of variance (ANOVA) was performed to determine the effect of sex and level of schooling on memory performance and PSQI components, and the Pearson correlation coefficient was calculated to determine the effect of age on these parameters.
SexSex affected performance in VPA (F[1, 98] = 10.610; P < .01; η2 = 0.103) and sleep latency (F[1, 98] = 7.533; P < .01; η2 = 0.076), with women scoring higher on the VPA test (mean [SD], 5.98 [4.72], vs 4.33 [2.88] for men) and presenting greater sleep latency (1.17 [1.14], vs 0.43 [0.63] for men). The effect size was small for both variables, indicating that sex has a weak effect. No significant differences were found between sexes for WL or the remaining PSQI components.
Level of schoolingLevel of schooling had a significant effect on VPA performance (F[2, 98] = 4.559; P < .05; η2 = 0.090). The mean score in the group of participants with primary education only (4.28 [3.43]) was lower than scores in the secondary (7 [4.89]) and post-secondary education subgroups (6.68 [4.77]), although the effect size was low, indicating a weak effect.
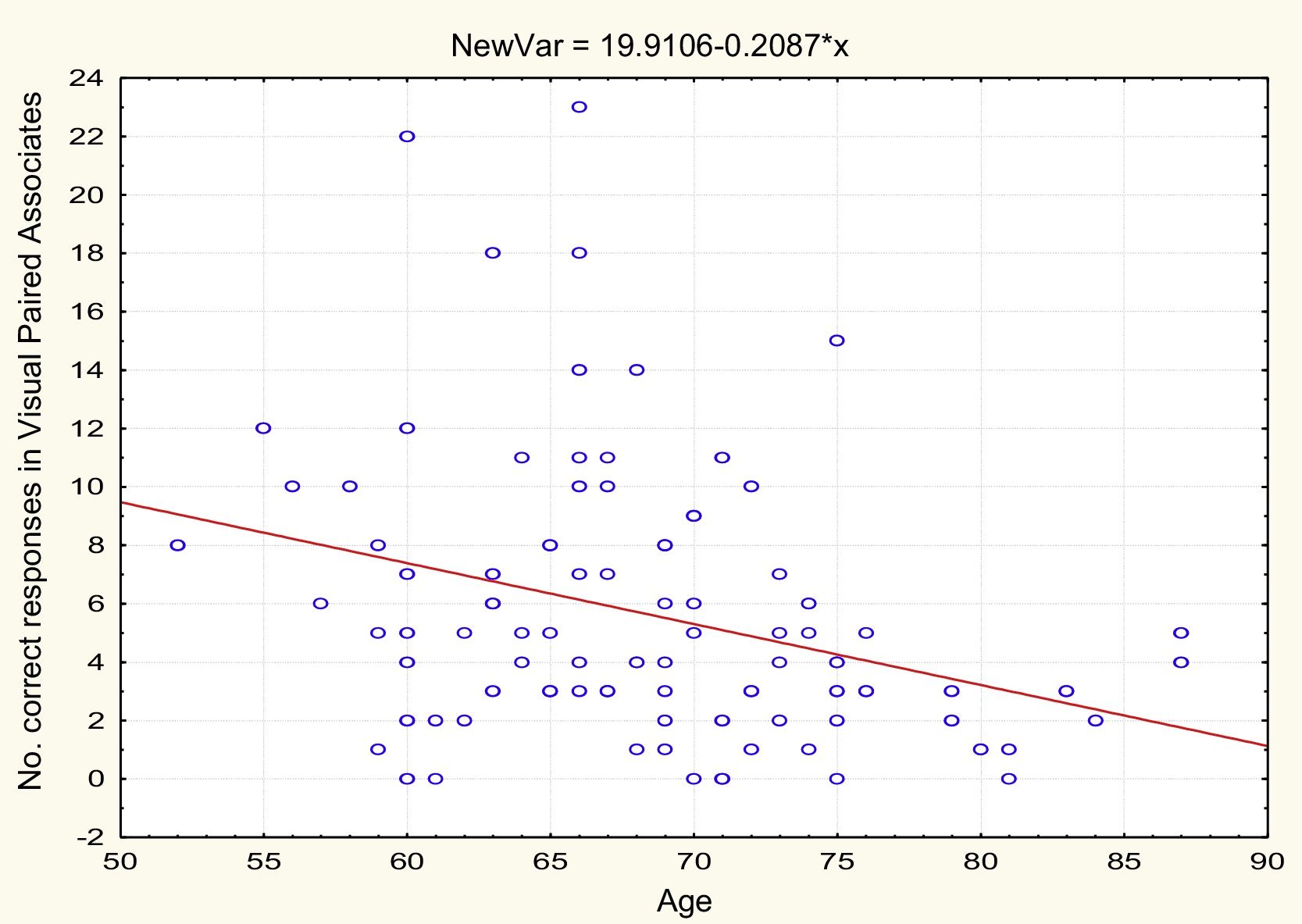
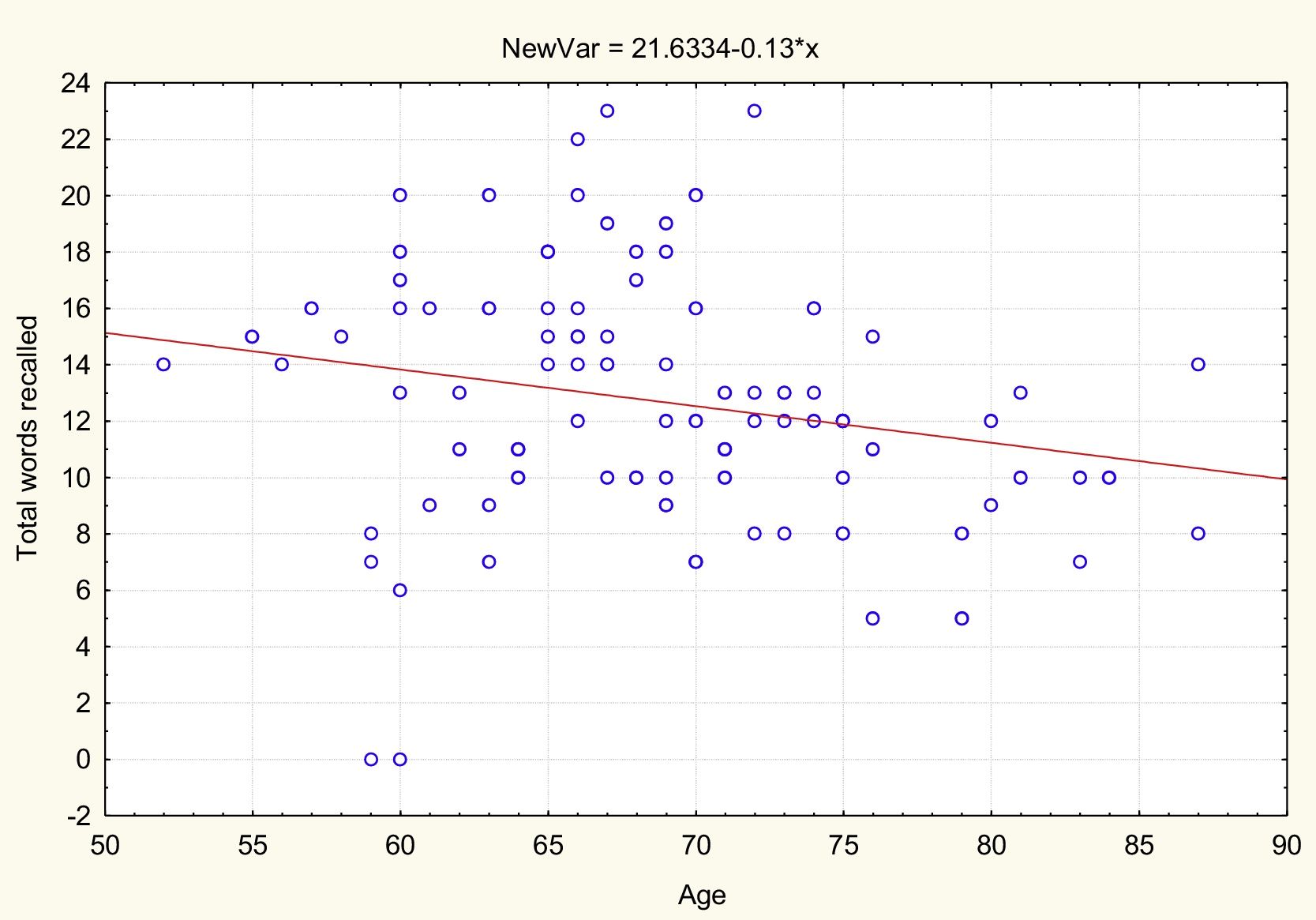
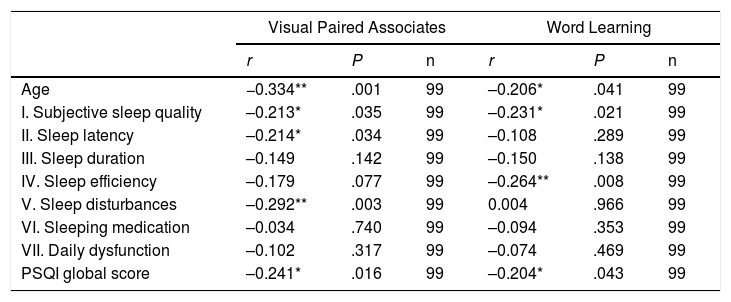
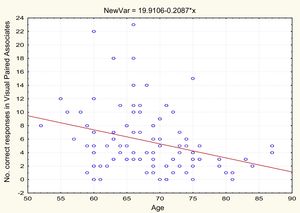
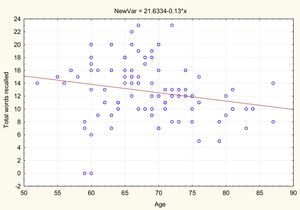
AgeTable 2 and Figs. 1 and 2 present the correlation analysis (2-tailed Pearson coefficient), which shows that age is inversely correlated with performance on both memory measures, VPA (r[99] = –0.334; P < .01) and WL (r[99] = –0.206; P < .05).
Correlations (2-tailed Pearson coefficient) between scores on memory tests and age and sleep quality components.
| Visual Paired Associates | Word Learning | |||||
|---|---|---|---|---|---|---|
| r | P | n | r | P | n | |
| Age | −0.334** | .001 | 99 | –0.206* | .041 | 99 |
| I. Subjective sleep quality | –0.213* | .035 | 99 | –0.231* | .021 | 99 |
| II. Sleep latency | –0.214* | .034 | 99 | –0.108 | .289 | 99 |
| III. Sleep duration | –0.149 | .142 | 99 | –0.150 | .138 | 99 |
| IV. Sleep efficiency | –0.179 | .077 | 99 | –0.264** | .008 | 99 |
| V. Sleep disturbances | –0.292** | .003 | 99 | 0.004 | .966 | 99 |
| VI. Sleeping medication | –0.034 | .740 | 99 | –0.094 | .353 | 99 |
| VII. Daily dysfunction | –0.102 | .317 | 99 | –0.074 | .469 | 99 |
| PSQI global score | –0.241* | .016 | 99 | –0.204* | .043 | 99 |
Age is not correlated with PSQI global score (r[99] = 0.070; P = .491) or any PSQI component, with the exception of habitual sleep efficiency (r[99] = 0.203; P < .05).
SleepAs shown in Table 2, PSQI global score is inversely correlated with performance in the VPA and WL tasks, with poorer sleep quality being associated with poorer performance in these memory tests. VPA performance was also inversely correlated with PSQI scores for subjective sleep quality, sleep duration, and sleep disturbances. WL performance was inversely correlated with PSQI scores for subjective sleep quality and habitual sleep efficiency.
DiscussionOur most noteworthy result was the negative effects of older age and poor sleep quality on VPA and AL performance, despite age only showing an association with habitual sleep efficiency.
Lower scores on these memory tests do not necessarily reflect clinical manifestations of cognitive impairment, as global cognitive performance is the end result of numerous neuronal networks that are continuously modified by plasticity mechanisms.
Another factor potentially involved in age-related memory impairment is the negative impact of slow-wave sleep. Pruning of synapses that accumulate during the day through experience occurs during slow-wave sleep.29 Synaptic density naturally decreases during ageing, with synaptic pruning during non-REM sleep being maintained; this has given rise to the theory that memory may be affected by an “over-pruning” phenomenon.30
The association between level of schooling and cognition has been studied extensively, with education showing a protective effect,31–33 promoting the formation of cognitive reserve.34–40 Higher level of education also favours better performance in complex tasks through increased synaptic activity, increased synaptic density in the neocortical association areas, and the development of alternative neural networks, enabling the creation of efficient circuits for the performance of cognitive functions through more flexible strategies.41 The complexity of the tasks evaluated requires an efficient neural substrate that may be influenced by schooling, particularly in tests involving executive function.33 The effect of level of schooling on memory tasks is partly explained by the executive component, which may determine better processing strategies.32,35,37,38,42
While we found sex to have a weak effect on memory, other studies have shown sex-related differences in performance in tasks evaluating verbal and visuospatial memory,43 with women performing better in tasks related to verbal information.44,45 Poor sleep quality among women is well documented, and its prevalence is known to increase with age, in comparison to men in the same age group.23,46–48 The most frequent problems are insomnia, increased sleep latency, and increased use of sleeping medication.23
The effect of sex on sleep quality may be explained by hormones and hormonal changes over the life cycle, as well as morphological differences in genes controlling the circadian rhythm.22,48–50 Sex steroids organise sleep-wake cycles through their action on the hypothalamus and the orexin-hypocretin system,49 with their receptors depending on the levels of the latter hormones in the blood.50 It has been reported that sleep is more sensitive to hormonal changes in women,46 whose circadian rhythm pattern is different to that of men.49,50
The association between memory and sleep has previously been reported in the literature. While there is consensus that poor sleep quality affects cognitive performance and working memory, few studies have addressed this association in older ages, and the methodologies and results vary greatly.43,51–55 The decrease in sleep quality and memory may be related to the age-related loss of cholinergic neurons in the nucleus basalis of Meynert. These neurons are responsible for sleep-wake cycles and participate in memory.56 Age-related sleep alterations have also been explained in relation to structural changes in the brain and decreased cortical volume.51 These modifications may also affect memory given the earlier ageing of prefrontal areas and their role in memory, as mentioned above.18,19,57
Consolidation of memory depends on sleep integrity52,58 and the activation of connections between the hippocampus and prefrontal cortex during slow-wave sleep. This activation is promoted by electric rhythms characteristic of sleep. During ageing, changes in sleep latency, more frequent awakenings, and decreased slow-wave sleep and REM/non-REM cycles may reduce cognitive performance and memory consolidation.52
ConclusionsThe cross-sectional design of this study prevents us from identifying the cause of the association between sleep quality and memory; however, the study does provide basic and applied information.
From a theoretical perspective, impairment in both areas (cognition and sleep) may reflect a neuro-endocrine imbalance associated with the disruption of circadian rhythms. If this hypothesis is correct, pharmacological and environmental interventions could be designed to optimise sleep-wake cycles and reduce age-related memory changes.
Professionals caring for older people should be mindful of the harmful effect of poor sleep quality on memory.
FundingThis study was partly funded by the Social Affairs department of the municipal council of Rivas-Vaciamadrid. It is part of Santander/Universidad Complutense de Madrid research project PR75/18-21661.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to the El Parque and Concepción Arenal centres in Rivas-Vaciamadrid for their collaboration, and to all the users who volunteered to participate in the study.
Please cite this article as: Cruz T, García L, Álvarez MA, Manzanero AL. Calidad del sueño y déficit de memoria en el envejecimiento sano. Neurología. 2022;37:31–37.