To characterize Hirayama disease in female patients, and increase awareness among clinicians regarding the specifics of this disease.
MethodsBaseline data, clinical manifestations, characteristics of cervical-flexion magnetic resonance imaging, and electromyography were collected and compared among females and males with Hirayama disease. In addition, the literature on Hirayama disease in females up to October, 2021 was searched in PubMed and the relevant data were compared with the data from our study.
ResultsTwenty female and 40 male patients were included in this study. The average ages of onset and menarche were 14.65 and 12.75 years old. All patients suffered from muscular weakness and atrophy of the upper limb(s), with flattening and/or atrophy of the lower cervical spinal cords in cervical-flexion magnetic resonance imaging, and neurogenic patterns in the atrophic muscles as determined using electromyography. The age of onset in females was about 2 years later than the age of menarche, and the age of onset in females was 2 years earlier than that in males. There were no obvious differences in clinical presentation between males and females.
DiscussionAlthough females presented with Hirayama disease two years earlier than males, no other clinical differences were observed. Hirayama disease is likely associated with growth and development in puberty, and early identification, regardless of whether patients are male or female, is critical to optimizing prognosis.
Para detallar la enfermedad de Hirayama en pacientes femeninos y promover la conciencia entre los médicos con respecto a los detalles específicos de esta enfermedad.
MetodologíaDatos de referencia, manifestaciones clínicas, características de las imágenes de resonancia magnética de flexión cervical y la electromiografía fueron recopilados y comparados entre mujeres y hombres con la enfermedad de Hirayama. Aunado a esto, la literatura sobre la enfermedad de Hirayama en mujeres hasta octubre de 2021 fue revisada en PubMed y la información relevante fue comparada con los datos de nuestro estudio.
Resultado20 pacientes femeninos y 40 pacientes masculinos fueron incluidos en este estudio. Las edades promedio de inicio y menarquia fueron 14, 65 y 12, 75 años. Todos los pacientes padecían debilidad muscular y atrofia de las extremidades superiores, con aplanamiento y/o atrofia de la médula espinal cervical inferior en imágenes de resonancia magnética de flexión cervical y patrones neurogénicos en los músculos atrofiados como fue determinado usando electromiografía. La edad de inicio en las mujeres fue aproximadamente 2 años más tarde que la edad de la menarquia, y la edad de inicio en las mujeres fue 2 años antes que en los hombres. No hubo diferencias obvias en la presentación clínica entre hombres y mujeres.
DiscusiónAunque las mujeres presentaron la enfermedad de Hirayama dos años antes que los hombres, no se observaron otras diferencias clínicas. Es probable que la enfermedad de Hirayama sea asociada con el crecimiento y desarrollo en la pubertad, y la identificación temprana, independientemente de si los pacientes son hombres o mujeres, es fundamental para optimizar el pronóstico médico.
Hirayama disease (HD), also known as juvenile benign muscular atrophy of the upper extremities, or monomelic amyotrophy (MMA), is characterized by muscular atrophy in the upper extremities, and predominantly affects unilateral or asymmetric bilateral distal upper limb(s). Hirayama disease typically progresses for 3 to 5 years, then stabilizes. The pathogenesis of Hirayama disease has not been characterized. However, a leading hypothesis is that ischemic injuries in anterior horn cells (AHCs) caused by spinal cord compression on the posterior edge of the vertebral bodies and/or spinal vasospasm during neck flexion because of an imbalance of spinal cord and dura mater during juvenile growth spurts may cause Hirayama disease.1 Therefore, the disease is viewed as self-limiting once growth stops.
Hirayama disease is rare, and the reported incidence is likely inaccurate.2. However, the number of patients with HD has increased in the past two decades. Diagnosis of HD has been more common in Asia, but some cases have been reported in other regions of the world.3,4 Clinical manifestations of HD appeared between ages 15 and 17 in 53.4% of patients.2 In addition, the reported male:female ratio of HD varies as follows: 8.3:1 and 20:1 in Japan; 2.8:1 in India; and 11:1 and 31.6:1 in China.1,2,5,6However, the finding that males were more likely to be diagnosed with HD than females was consistent across these studies.
Most clinicians are not aware of differential presentation of HD between males and females. Therefore, diagnosis of HD might be delayed, or the disease might be misdiagnosed, in females, resulting in poor prognosis. In such cases of delayed or incorrect diagnosis, symptoms such as spastic gait could remain despite proper treatment.7 In recent years, there have been case reports of female patients with HD in many countries and regions,8–10 which suggests that HD may not be as rare in females as previously believed. However, differences between male and female HD cases have not been reported in the literature.
The first two female cases of HD were reported in 2001.11 Since these reports, few case reports focused on female patients with HD have been published, and no clinical research has been performed. Our center is currently the largest center for the diagnosis and treatment of HD, and has taken the lead in formulating international guidelines, proposing new clinical diagnostic criteria and the first clinical classification.12Therefore, we performed a cross-sectional study with the largest sample of female cases in the world using our clinical progression diagnostic criteria, and it provided important information about HD in female and male patients, including clinical manifestations, medical imaging and electrophysiological characteristics. The aim of this study was to elucidate the clinical characteristics of HD in females and to increase awareness of the specifics of this disease in females to aid in early and accurate diagnosis.
MethodsSubjectsFemale patients diagnosed with HD between September 2007 and September 2021 in the Department of Orthopedics, Huashan Hospital, Fudan University were included in this study, and two male patients with HD that visited at the same time as the females were also included. The inclusion criteria were as follows: 1) clear clinical diagnosis of HD, as determined by muscular atrophy of an upper limb as the main clinical manifestation, according to the diagnostic criteria,6 2) availability of information regarding clinical manifestations, cervical-flexion magnetic resonance imaging (MRI), and electromyography (EMG), and 3) cross-sectional study. Exclusion criteria were as follows: 1) previous history of cervical spine trauma and surgery, 2) imaging evidence of retrograde degeneration of the cervical spine and obvious skeletal malformations, 3) neoplastic disease, and 4) infectious disease. The project was reviewed and approved by the Institutional Review Board, Huashan Hospital, Fudan University, China, and the requirement for individual consent was waived because the study was observational and the data were anonymized.
Collected dataWe recorded baseline data, clinical manifestations, cervical-flexion MRI, and EMG from patients with HD. Baseline data included sex, age of onset, and age of onset of menarche (females only). Clinical manifestations included initial symptoms (including the exact location of atrophy or weakness), affected side(s), deep tendon reflex (characterized as absent, decreased, normal, brisk, and hyperactive), and pathological reflex (presence of Hoffmann sign, Babinski sign, Oppenheim sign, Gordon sign, or Romberg sign). Cervical-flexion MRI data showing anterior-shifting, flattening, and atrophy of the cervical spinal cord, loss of attachment (LOA) between the posterior dural sac and the subjacent lamina (presence of a crescent-shaped high-intensity mass), and high signal intensity at AHCs on T2-weighted imaging (T2WI). Electromyography data showing affected side(s), muscles, and segments, with waves presenting with one of the following: fibrillation potentials, positive sharp waves, increased amplitude of motor unit potentials (MUPs), or pathologic interference patterns.
Literature searchPubMed was searched for articles published in English through September 21, 2021 using “female” AND (“Hirayama disease” OR “juvenile muscular atrophy of distal upper extremity” OR “juvenile amyotrophy of the distal upper extremity” OR “monomelic amyotrophy” OR “benign focal amyotrophy” OR “juvenile asymmetric segmental muscular atrophy” OR “cervical flexion induced myelopathy”) as key words without article type restrictions. Exclusion criteria were as follow: duplication, other diseases, and unavailability of the full text.
The following data were extracted from the literature sources: baseline data, clinical manifestations, cervical-flexion MRI, and EMG. Baseline data included publication date, age of onset, and nationality. Clinical manifestations were the same as those listed in the Collected data section. Cervical-flexion MRI data included LOA and high signal intensity located at AHCs on T2WI. Electromyography data included the affected segments.
Statistical analysisNormally distributed quantitative variables are presented as the mean and standard deviation (SD), and were analyzed using t tests after excluding missing data. Qualitative and ordinal data were presented as percentages, and were analyzed using χ2 tests, adjusted χ2 tests, and Fisher’s exact tests, as appropriate, after excluding missing data. Statistical analysis was performed using SPSS (Statistical Product and Service Solutions) for Windows (version 26.0; SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
ResultsData of female patientsFrom September 2007 to September 2021, there were 633 patients diagnosed with HD in our department. Among these, 613 were males and 20 were females, resulting in a male-to-female ratio of 30.65:1. For the 20 female patients, the age of onset ranged from 11 to 21 (14.65 ± 2.250) (Fig. 1), and age of menarche ranged from 10 to 14.5 (12.75 ± 1.278), with the exception of 4 patients for whom the age of menarche was unknown (Table 1).
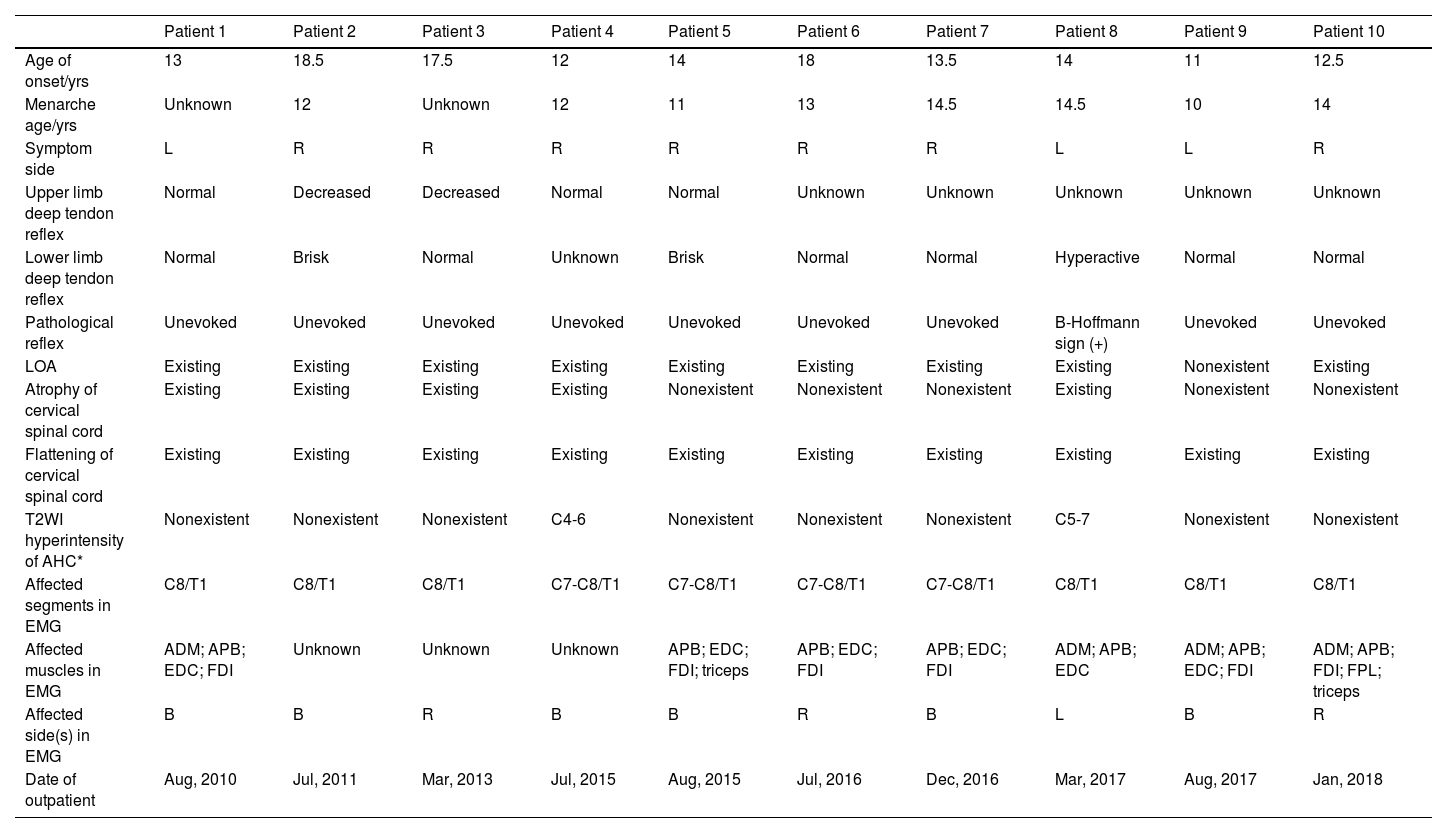
The detailed information about female Hirayama disease patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age of onset/yrs | 13 | 18.5 | 17.5 | 12 | 14 | 18 | 13.5 | 14 | 11 | 12.5 |
| Menarche age/yrs | Unknown | 12 | Unknown | 12 | 11 | 13 | 14.5 | 14.5 | 10 | 14 |
| Symptom side | L | R | R | R | R | R | R | L | L | R |
| Upper limb deep tendon reflex | Normal | Decreased | Decreased | Normal | Normal | Unknown | Unknown | Unknown | Unknown | Unknown |
| Lower limb deep tendon reflex | Normal | Brisk | Normal | Unknown | Brisk | Normal | Normal | Hyperactive | Normal | Normal |
| Pathological reflex | Unevoked | Unevoked | Unevoked | Unevoked | Unevoked | Unevoked | Unevoked | B-Hoffmann sign (+) | Unevoked | Unevoked |
| LOA | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Nonexistent | Existing |
| Atrophy of cervical spinal cord | Existing | Existing | Existing | Existing | Nonexistent | Nonexistent | Nonexistent | Existing | Nonexistent | Nonexistent |
| Flattening of cervical spinal cord | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing |
| T2WI hyperintensity of AHC* | Nonexistent | Nonexistent | Nonexistent | C4-6 | Nonexistent | Nonexistent | Nonexistent | C5-7 | Nonexistent | Nonexistent |
| Affected segments in EMG | C8/T1 | C8/T1 | C8/T1 | C7-C8/T1 | C7-C8/T1 | C7-C8/T1 | C7-C8/T1 | C8/T1 | C8/T1 | C8/T1 |
| Affected muscles in EMG | ADM; APB; EDC; FDI | Unknown | Unknown | Unknown | APB; EDC; FDI; triceps | APB; EDC; FDI | APB; EDC; FDI | ADM; APB; EDC | ADM; APB; EDC; FDI | ADM; APB; FDI; FPL; triceps |
| Affected side(s) in EMG | B | B | R | B | B | R | B | L | B | R |
| Date of outpatient | Aug, 2010 | Jul, 2011 | Mar, 2013 | Jul, 2015 | Aug, 2015 | Jul, 2016 | Dec, 2016 | Mar, 2017 | Aug, 2017 | Jan, 2018 |
| Patient 11 | Patient 12 | Patient 13 | Patient 14 | Patient 15 | Patient 16 | Patient 17 | Patient 18 | Patient 19 | Patient 20 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age of onset/yrs | 13 | 13 | 16 | 15 | 21 | 17 | 13 | 14 | 14 | 13 |
| Menarche age/yrs | 13 | 12.5 | Unknown | Unknown | 13.5 | 12.5 | 12 | 12 | 14.5 | 13 |
| Symptom side | L | L | L | R | R | R | B | L | B | R |
| Upper limb deep tendon reflex | Hyperactive | Unknown | Normal | Normal | Unknown | Normal | Normal | Normal | Brisk | Unknown |
| Lower limb deep tendon reflex | Hyperactive | Hyperactive | Normal | Normal | Normal | Unknown | Brisk | Unknown | Hyperactive | Normal |
| Pathological reflex | Unevoked | Unevoked | Unevoked | Unevoked | B-Hoffmann sign (+) | B-Hoffmann sign (+) | B-Hoffmann sign (+) | Unevoked | B-Hoffmann sign (+) | Unevoked |
| LOA | Nonexistent | Existing | Existing | Nonexistent | Nonexistent | Nonexistent | Nonexistent | Existing | Existing | Existing |
| Atrophy of cervical spinal cord | Nonexistent | Nonexistent | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing |
| Flattening of cervical spinal cord | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing | Existing |
| T2WI hyperintensity of AHC* | Nonexistent | Nonexistent | C5/6 | C5/6 | C5/6 | C4-5 | Nonexistent | Nonexistent | C5 | Nonexistent |
| Affected segments in EMG | C8/T1 | C8/T1 | C7-8 | C8/T1 | C7-C8/T1 | C7-8 | C7-C8/T1 | C7-8 | C7-C8/T1 | C7-C8/T1 |
| Affected muscles in EMG | ADM; APB; EIP; FDI | EIP; FDI | EDC; EPL | ADM; APB; EPL; FDI; triceps | ADM; APB; EIP; FCR; FCU; FDI; FPL; triceps | ADM; FDI | EDC; FCR; FDI; triceps | EDC; FCR; FDI; | ADM; APB; EDC; FCR; FDI; triceps | ADM; APB; EDC; FCR; FDI |
| Affected side(s) in EMG | L | L | L | R | B | R | B | B | B | R |
| Date of outpatient | Aug, 2018 | Dec, 2018 | Jul, 2019 | Aug, 2019 | Jun, 2020 | Jun, 2020 | Sept, 2020 | Jan, 2021 | Jun, 2021 | Sept, 2021 |
ADM: abductor digiti minimi; APB: abductor pollicis brevis; AHC: snterior horn cell; B: bilateral; EDC: extensor digitorum communis; EIP: extensor indicis proprius; EMG: electromyography; EPL: extensor pollicis longus; FCR: flexor carpi radialis; FCU: flexor carpi ulnaris; FDI: first dorsal interossei; FPL: flexor pollicis longus; L: left; LOA: loss of attachment; R: right; T2WI: T2-weighted imaging.
All female patients with HD presented with muscular weakness and atrophy of the upper limb(s), sparing the brachioradialis muscles (oblique atrophy), and cold paralysis. No patients complained of sensory deficits. Seven patients (35.0%) reported symptoms on the left side, 11 (55.0%) reported symptoms on the right side, and 2 (10.0%) reported bilateral symptoms. Eight patients did not have information regarding upper limb deep tendon reflex. In the remaining patients, 8 (66.7%) had normal upper limb deep tendon reflex, 2 (16.7%) showed a decrease, 1 (8.3%) showed a brisk reflex, and 1 (8.3%) showed a hyperactive reflex. Information regarding lower limb deep tendon reflex was unknown for 3 patients, and 10 (58.9%), 3 (17.6%), and 4 (23.5%) had normal, brisk, and hyperactive reflexes, respectively. Finally, Hoffmann sign was elicited in 5 of the 20 female patients with HD (25.0%).
Cervical-flexion MRIEach female patient underwent a cervical-flexion MRI examination, and the imaging showed flattening and/or atrophy of the lower cervical spinal cords of all of the female patients. Loss of attachment was observed in 14 of the 20 patients (70.0%) (Fig. 2). Furthermore, 7 of 20 (35.0%) showed high signal intensity at AHCs on T2WI.
ElectromyographyElectromyography data was collected for each of the 20 female patients, all of whom showed neurogenic patterns in the atrophic muscles. Ten of 20 patients (50.0%) showed neurogenic changes in the bilateral sides in EMG, but 8 (40.0%) only complained of unilateral symptoms. In the other 2 patients with bilateral symptoms, the side with more severe symptoms was consistent with the side with more severe damage in EMG. The affected cervical spinal cord segments in EMG were C7-8/T1. The numbers of patients affected at C7, C8, and T1 were 9 (45.0%), 20 (100%), and 17 (85.0%), respectively. Finally, 3 patients did not have detailed information on the affected muscles. In the remaining 17 patients, neurogenic changes were observed in the following 10 muscles: first dorsal interossei in 15 patients (88.2%); abductor pollicis brevis in 12 (70.6%); extensor digitorum communis in 11 (64.7%); abductor digiti minimi in 10 (58.8%); triceps in 6 (35.3%); flexor carpi radialis in 5 (29.4%); extensor indicis proprius in 3 (17.6%); extensor pollicis longus in 2 (11.8%); flexor pollicis longus in 2 (11.8%); and flexor carpi ulnaris in 1 (5.9%).
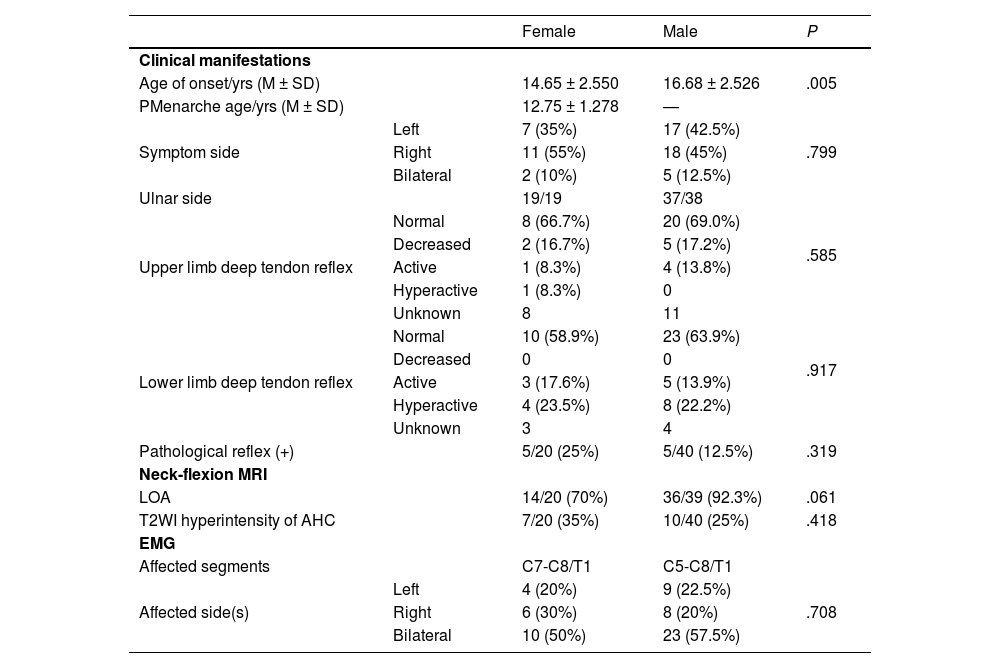
Comparison of male and female casesWe also collected data from male patients with HD, and compared females to males at a 1:2 ratio (Table 2). There was a significant difference in age of onset between males and females (P = .005; Fig. 1). In contrast, there were no significant differences in symptom side(s) (P = .799), upper limb tendon reflex (P = .585), lower limb deep tendon reflex (P = .917), pathological reflex (P = .319), LOA (P = .061), hyperintensity of AHC on T2WI (P = .418), or affected segments in EMG (P = .708) (Table 3).
Comparison of male and female Hirayama disease patients.
| Female | Male | P | ||
|---|---|---|---|---|
| Clinical manifestations | ||||
| Age of onset/yrs (M ± SD) | 14.65 ± 2.550 | 16.68 ± 2.526 | .005 | |
| PMenarche age/yrs (M ± SD) | 12.75 ± 1.278 | — | ||
| Symptom side | Left | 7 (35%) | 17 (42.5%) | .799 |
| Right | 11 (55%) | 18 (45%) | ||
| Bilateral | 2 (10%) | 5 (12.5%) | ||
| Ulnar side | 19/19 | 37/38 | ||
| Upper limb deep tendon reflex | Normal | 8 (66.7%) | 20 (69.0%) | .585 |
| Decreased | 2 (16.7%) | 5 (17.2%) | ||
| Active | 1 (8.3%) | 4 (13.8%) | ||
| Hyperactive | 1 (8.3%) | 0 | ||
| Unknown | 8 | 11 | ||
| Lower limb deep tendon reflex | Normal | 10 (58.9%) | 23 (63.9%) | .917 |
| Decreased | 0 | 0 | ||
| Active | 3 (17.6%) | 5 (13.9%) | ||
| Hyperactive | 4 (23.5%) | 8 (22.2%) | ||
| Unknown | 3 | 4 | ||
| Pathological reflex (+) | 5/20 (25%) | 5/40 (12.5%) | .319 | |
| Neck-flexion MRI | ||||
| LOA | 14/20 (70%) | 36/39 (92.3%) | .061 | |
| T2WI hyperintensity of AHC | 7/20 (35%) | 10/40 (25%) | .418 | |
| EMG | ||||
| Affected segments | C7-C8/T1 | C5-C8/T1 | ||
| Affected side(s) | Left | 4 (20%) | 9 (22.5%) | .708 |
| Right | 6 (30%) | 8 (20%) | ||
| Bilateral | 10 (50%) | 23 (57.5%) | ||
AHC: Anterior horn cell; EMG: Electromyography; LOA: Loss of attachment; MRI: Magnetic resonance imaging; M ± SD: Mean ± Standard deviation; T2WI: T2-weighted imaging.
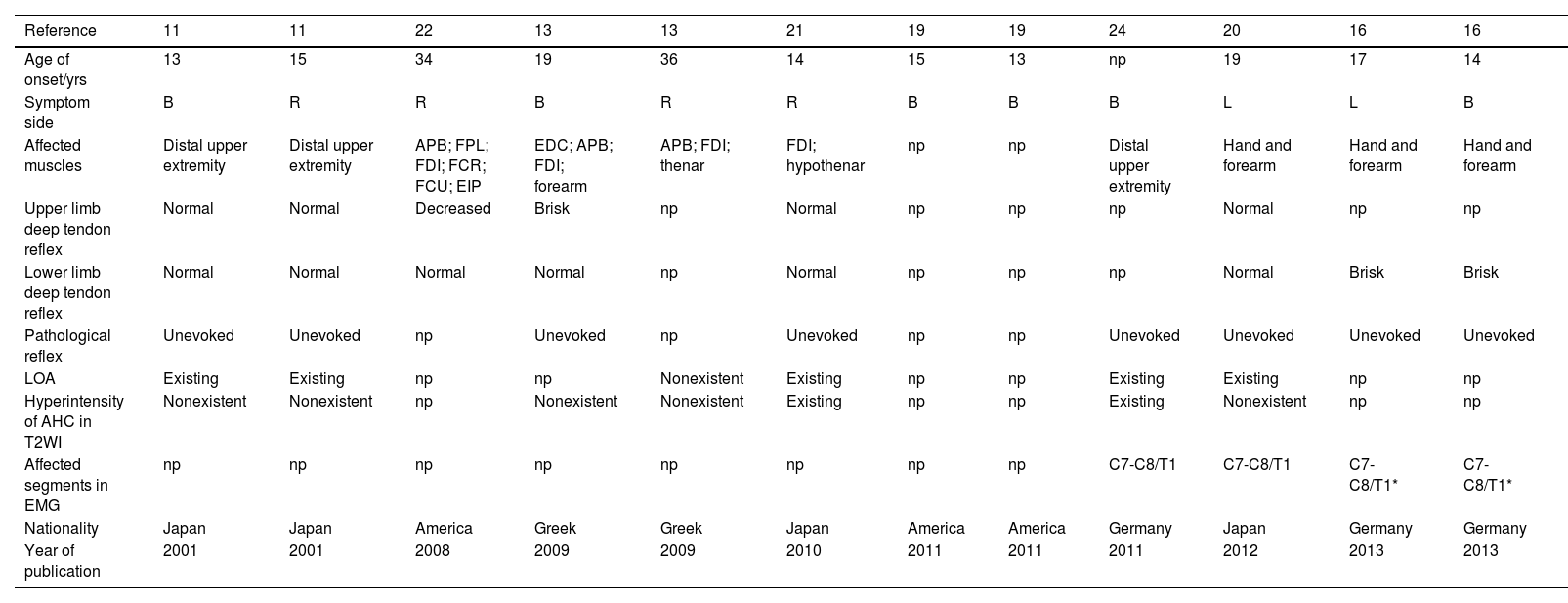
Review of the literatures of females suffered from Hirayama Disease.
| Reference | 11 | 11 | 22 | 13 | 13 | 21 | 19 | 19 | 24 | 20 | 16 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age of onset/yrs | 13 | 15 | 34 | 19 | 36 | 14 | 15 | 13 | np | 19 | 17 | 14 |
| Symptom side | B | R | R | B | R | R | B | B | B | L | L | B |
| Affected muscles | Distal upper extremity | Distal upper extremity | APB; FPL; FDI; FCR; FCU; EIP | EDC; APB; FDI; forearm | APB; FDI; thenar | FDI; hypothenar | np | np | Distal upper extremity | Hand and forearm | Hand and forearm | Hand and forearm |
| Upper limb deep tendon reflex | Normal | Normal | Decreased | Brisk | np | Normal | np | np | np | Normal | np | np |
| Lower limb deep tendon reflex | Normal | Normal | Normal | Normal | np | Normal | np | np | np | Normal | Brisk | Brisk |
| Pathological reflex | Unevoked | Unevoked | np | Unevoked | np | Unevoked | np | np | Unevoked | Unevoked | Unevoked | Unevoked |
| LOA | Existing | Existing | np | np | Nonexistent | Existing | np | np | Existing | Existing | np | np |
| Hyperintensity of AHC in T2WI | Nonexistent | Nonexistent | np | Nonexistent | Nonexistent | Existing | np | np | Existing | Nonexistent | np | np |
| Affected segments in EMG | np | np | np | np | np | np | np | np | C7-C8/T1 | C7-C8/T1 | C7-C8/T1* | C7-C8/T1* |
| Nationality | Japan | Japan | America | Greek | Greek | Japan | America | America | Germany | Japan | Germany | Germany |
| Year of publication | 2001 | 2001 | 2008 | 2009 | 2009 | 2010 | 2011 | 2011 | 2011 | 2012 | 2013 | 2013 |
| Reference | 18 | 18 | 15 | 14 | 7 | 17 | 23 | 23 | 9 | 9 | 10 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age of onset/yrs | 16.5 | 12.5 | 10.5 | 17 | 12 | 15 | 16 | 17 | 17.5 | 18 | 28 | 28 |
| Symptom side | R | Unilateral | B | R | R | R | Unknown | Unknown | L | Unknown | B | B |
| Affected muscles | Distal upper extremity | Distal upper extremity | Thenar; hypothenar; intrinsic hand muscles | Hand | Intrinsic hand muscles; forearm | Distal upper extremity | np | np | FDI; ADM | np | Thenar; hypothenar; intrinsic hand muscles | Hand and the dorsal face of the forearm |
| Upper limb deep tendon reflex | Normal | Decreased | Normal | np | Decreased | Decreased | np | np | Normal | np | Decreased | Absent radial stylus |
| Lower limb deep tendon reflex | Normal | np | Normal | np | Hyper-active | Brisk | np | np | Normal | np | Brisk | Absent pronator cubit |
| Pathological reflex | Unevoked | Unevoked | Unevoked | np | Romberg and Babinski(+) | np | np | np | np | np | Hoffmann sign (+) | Unevoked |
| LOA | np | np | Nonexistent | Nonexistent | Existing | Existing | Existing | Existing | Existing | np | Existing | Nonexistent |
| Hyperintensity of AHC in T2WI | np | np | Existing | Nonexistent | Existing | Existing | Nonexistent | Existing | Nonexistent | np | Existing | Nonexistent |
| Affected segments in EMG | np | np | C7-C8/T1 | np | C5-C8/T1 | np | np | np | C7-C8/T1 | C7-C8/T1 | np | C7-C8/T1 |
| Nationality | Austria | Austria | Austria | Turkey | Japan | Japan | Russia | Russia | India | India | China | Albnia |
| Year of publication | 2013 | 2013 | 2017 | 2017 | 2017 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2020 |
ADM: Abductor digiti minimi;APB: Abductor pollicis brevis; AHC: Anterior horn cell; B: Bilateral; EDC: Extensor digitorum communis; EIP: Extensor indicis proprius; EMG: Electromyography; FCR: Flexor carpi radialis; FCU: Flexor carpi ulnaris; FDI: First dorsal interossei; FPL: Flexor pollicis longus; L: Left; LOA: Loss of attachment; np: not applicated; R: Right; T2WI: T2-weighted imaging.
We reviewed literature regarding females with HD with detailed information published in English through September 2021. Seventeen7–11,13–24 studies reported 24 female cases of HD from Asia, Europe, America, and Oceania. The detailed information from these studies is summarized in Table 3.
The median age of onset was 16.5 years of age, the range was not normally distributed, and after excluding 4 outliers and 1 patient with unknown age of onset the ages ranged from 10.5 to 19 (15.32 ± 2.43). These results were similar to those in our study (P = .410). The clinical manifestations reported were wasting and weakness of the distal upper limb(s), which was also consistent with our study. Four cases did not indicate which side was affected, 2 of 20 (10.0%) reported that the left side was affected, 8 (40.0%) reported that the right side was affected, and 10 (50.0%) reported bilateral symptoms in the upper extremities. These results were different from those in our study (P = .014). Upper limb deep tendon reflex was not reported for 10 patients, and 7 of 14 patients (50.0%) showed a normal reflex, 6 patients (42.9%) showed decreased or absent reflex, 1 (7.1%) showed brisk reflex, and no patients showed a hyperactive reflex. Lower limb deep tendon reflex was not reported in 9 patients, and 9 (65.0%), 1 (6.7%), 4 (26.6%), and 1 (6.7%) of 15 patients showed normal, decreased or absent, brisk, and hyperactive reflexes, respectively. Furthermore, pathological reflexes were observed in 2 of 14 patients (14.3%), and information was not provided for 10 patients. There were no significant differences in upper limb deep tendon reflex (P = .534), lower limb deep tendon reflex (P = .497), or pathological reflex (P = .676) between these previous studies and our study.
In cervical-flexion MRI, LOA was observed in 11 of 15 patients (73.3%), and information was not available for 9 patients. High signal intensity at AHCs on T2WI was observed in 7 of 16 patients (43.75%), and information was not available for 8 patients. There were no significant differences in these two parameters between previous studies and our study (P = 1.000 and P = .734). Finally, fifteen patients did not have EMG data. In the remaining 9 patients, the numbers of patients with affected cervical spinal segments at C5, C6, C7, C8, and T1 were 1 (11.1%), 1 (11.1%), 9 (100%), 9 (100%), and 9 (100%). Only the number of patients with C7 affected was significantly different from ours (P = .027), whilst the others were similar (C5/C6: P = .310, C8: P = 1.000, and T1: P = .532).
DiscussionHirayama disease was first reported in 12 cases by Hirayama25 in 1959, and was initially called juvenile muscular atrophy of unilateral upper extremity. Most patients with HD were juvenile males from Asia who presented with muscular weakness and atrophy of the distal unilateral upper limb, without sensory deficits. The disease was observed to be self-limiting after a progression of 3-5 years. The clinical characteristics in female patients with HD are similar to those in males, but age of onset in females is 2 years earlier than in males. No series of reports has previously characterized HD in females. Our study was a cross-sectional study with the largest number of reported cases of females with HD.
The average age of onset of HD in females is 2 years later than the average age of menarche. Menstruation marks the beginning of puberty, during which growth accelerates, which may correlate with onset of HD in female patients. Cross-innervation of spinal nerve segments may also explain why the onset of HD is approximately 2 years after menarche.
Later age of onset of HD in males compared to females requires further study. On average, adult females are shorter than males, which indicates that growth may occur at a slower overall rate in females. This may also explain why the incidence in females is lower than that in males. According to Li and Ji et al.26, girls grow fastest from 9 to 12 years old, while boys grow fastest from 11 to 14 years old. Therefore, Chinese girls experience fastest growth approximately two years earlier than Chinese boys. In addition, a study by Ding et al.27 showed the heights of 60 patients with HD increased by 7.1 ± 1.8 cm in the year prior to disease onset. This finding suggested that the onset of HD might correlate with growth in puberty, and further supported the hypothesis that cervical spinal cord compression was an underlying cause of HD for the disproportionate lengths of the spinal cord and canal.28–31 However, the vast majority of adolescents who undergo growth spurts do not suffer from HD. Therefore, pathogenesis of HD is likely more complex than spinal compression during growth, such as immune factors,32 dysplasia of the spinal venous plexus,33 and structural abnormalities of the spinal ligaments.34 The results of our study were different from those in a nationwide survey of HD in Japan.2 The onset of HD was earlier (mean 17.6 years) in males than that in females (mean 19.3 years), but the difference in age of onset was not significantly different between males and females. They concluded that these sex-related differences in age of onset might have been due to differential effects of sex hormones on motor neurons.
There were no significant differences between males and females in symptom side(s), upper limb tendon reflex, lower limb deep tendon reflex, pathological reflex, “sand-watch’’ spinal cord,35 LOA, hyperintensity of AHC on T2WI, or affected segments in EMG. Therefore, there were no differences in clinical presentation between males and females, and all patients met the same diagnostic criteria.
In our study, both sides of the body were affected equally, which was not consistent with previous studies.2 A possible reason for unilateral involvement to be common may be due to asymmetric compression of the cervical spinal cord due to unequal left-to-right distribution of posterior epidural ligaments between the ligamentum flavum and the posterior dura.36 What needs attention is that it is common for symptoms affected both sides; the number of patients suffering from bilateral symptoms were more than stereotype in clinicians’ mind possibly. Furthermore, the numbers of patients with unilateral or bilateral symptoms may not be reflected in EMG results. The number of patients with bilateral changes in EMG was greater than the number of patients who complained of unilateral weakness and atrophy (Fig. 3). This discrepancy may indicate that changes in EMG might precede onset of clinical symptoms of HD. Therefore, failure to intervene early in patients with changes in EMG only might result in increased risk of bilateral weakness and atrophy, and poorer prognosis. Although HD is considered a self-limiting disease, early intervention is critical to avoiding poor prognosis.37 and delays in diagnosis or misdiagnosis can directly result in greater severity of HD.38
Prior to our study, only 24 cases of females with HD had been described in publications. In these publications, the results were similar to those in our study. The differences were that more patients presented with bilateral atrophy and more patients showed C7 injury in EMG in literature review. This difference may have indicated that symptoms had progressed to collateral side and adjacent segment in cervical spinal cord due to later diagnosis. Therefore, the characteristics of females with HD were similar in Asia as in the rest of the world, which further indicated that HD was not a disease only found in Asia. Furthermore, the low rate of diagnosis might be a result of lack of awareness of HD.39 It is possible that the incidence of HD has been underestimated,6,40 and HD is likely not limited to Asia.3
There were some limitations to our study. First, this was a single-center clinical study, which limited generalization of our conclusions. Furthermore, small sample size may have contributed to bias. However, our study described the largest sample of females with HD to date, which indicates that our study may have suffered from less bias than other previous studies. Finally, the cross-sectional nature of our study did not allow for determination of a causal relationship. However, because growth during adolescence occurs in one direction and occurs in every individual, we were able to make strong estimates of the causal relationship between growth and onset of HD.
The clinical characteristics of HD, including clinical manifestations, cervical-flexion MRI, and EMG were similar among all patients. The major difference observed in our study was that female patients experienced onset of HD 2 years earlier than male patients. Hirayama disease likely results from compression of the lower cervical spinal cord caused by abnormal development of the cervical spinal cord during adolescence. When adolescent females aged 13-18, particularly 2 years after menarche, present with unilateral or asymmetrical localized muscle atrophy of the upper limb without cranial nerve and lower extremity muscle involvement, clinicians should consider the possibility of HD and perform MRI in the flexed neck position and EMG to allow for early diagnosis and early intervention. A diagnosis should be made as early as possible, regardless of whether the patient is male or female, to improve prognosis and to limit disabilities.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Study fundingClinical Research Plan of SHDC (No.SHDC2020CR4030), Clinical Technology Innovation Project of SHDC (No. SHDC12019X26),National Natural Science Foundation of China (No.82072488) and AO Spine National Research Grant 2020 [No. AOSCN(R)2020-9]
Author contributionsHW Wang, W Lei and HL Wang designed research. HW Wang, W Lei contributed unpublished data and performed research. Y Tian, JW Wu and HW Wang did the literature search. HW Wang analyzed data, and wrote original draft. XL Xia, XS Ma, FZ Lyu, JY Jiang, and HL Wang were responsible for supervision.