We propose a protocol for study of complex regional pain syndrome (CRPS) based on a battery of quantitative measures (skin thermography, electrochemical skin conductance and sensory thresholds) and apply such protocol to 5 representative cases of CRPS.
Patients and methods5 CPRS cases (2 women/3 men) that met the Budapest criteria for the diagnosis of CRPS.
ResultsAll patients showed spontaneous pain and allodynia. Two cases correspond to a stage I, in both the resting basal temperature was increased in the affected limb. Three cases reflect more advanced stages with a decrease in resting temperature and a delay in the recovery of the temperature when compared to contralateral limb.
DiscussionThese non-invasive quantitative functional tests not only improve the diagnostic accuracy of CRPS but also, they help us to stratify and understand the pathological processes of the disease.
Proponemos un protocolo para el estudio del síndrome de dolor regional complejo (SDRC) basado en una batería de medidas cuantitativas (termografía cutánea, conductancia electroquímica cutánea y umbrales sensoriales en la prueba sensorial cuantitativa [QST]) y aplicamos dicho protocolo a cinco casos representativos de SDRC.
Pacientes y métodosSe presentan cinco casos de SDRC (dos mujeres/tres hombres) que cumplieron con los criterios de Budapest para el diagnóstico de SDRC.
ResultadosTodos los pacientes presentaron dolor espontáneo y alodinia. Dos casos corresponden a un estadio I, en ambos, la temperatura basal de reposo se incrementó en el miembro afectado. Tres casos muestran estadios más avanzados con disminución de la temperatura de reposo y retraso en la recuperación de la temperatura, en comparación con la extremidad contralateral, que reflejan fases más avanzadas de la enfermedad.
DiscusiónEstas pruebas funcionales cuantitativas no invasivas no solo mejoran la precisión diagnóstica del SDRC sino que también nos ayudan a estratificar las diferentes fases y comprender los procesos patológicos de la enfermedad.
The term complex regional painful syndrome (CRPS) refers to a particular type of chronic pain of neuropathic origin usually affecting distal regions of the extremities which, unlike posttraumatic neuralgia, is characteristically associated to circumscribed skin edema, sweating and vasomotor abnormalities, limited mobility and bone demineralization. The pain is usually intense, continuous and disproportionately persistent in time in relation to the initial injury or trauma. The pain does not follow a specific dermatome and its associated features (sensory, motor, sudomotor, vasomotor and trophic abnormalities) exceed the initial area of traumatic damage. In a small percentage (around 10% of cases) there is no clear trigger.1 There have been numerous postulates to explain the mechanisms related to the etiology of CRPS, with evidence of inflammatory mechanisms, neurogenic inflammation and central pain perception changes.2 In most cases, these phenomena are due to a dysfunction or degeneration of the innervation of the small fibers of the blood vessels.1,3 Vasomotor instability (microvasculopathy) seems to be the primary cause of the edema, abnormalities in coloration and temperature of the skin. In 1994 the International Association for the Study of Pain (IASP) defined CRPS and classified it into two subtypes: Type I (formerly known as “reflex sympathetic dystrophy”), in which no peripheral nerve injury is observed and represents 90% of cases, and type II (formerly known as “causalgia”), in which objective peripheral nerve injury is detected (10% of cases). Additionally, some researchers differentiate two CRPS subtypes based on regional skin temperature changes at the onset of symptoms: warm subtype (“warm”), with increased skin temperature, and cold subtype (“cold”), with decreased skin temperature.4,5 The current diagnosis and clinical follow-up of CRPS is largely based on the identification of a set of qualitative clinical features, being the Budapest consensus criteria the most accepted diagnostic approach today.4,5 There is a unanimous agreement that an early diagnosis and treatment of pain and rehabilitation improve the functional prognosis in CRPS. However, especially in such early stages, the diagnosis of CRPS is challenging because of the wide variability in clinical manifestations, frequently overlapping with features caused by trauma or surgical intervention. The implementation of objectively quantifiable functional measures may be extremely helpful for early diagnosis, severity classification and treatment response monitoring of CRPS. However, with the exception of electromyography, which is used to rule out associated nerve injury and to support differential diagnosis in CRPS, few ancillary diagnostic and monitoring tools have been incorporated to improve clinical practice in CRPS. New technological approaches potentially allow non-invasive functional quantification of core autonomic and sensorial features of CRPS, such as changes in skin temperature (thermographic cameras), sweating (electrochemical conductance sensors) and sensitivity thresholds (quantitative sensory testing). In the present work through five clinical cases we illustrate the usefulness of implementing such functional quantification tools in the protocol for diagnosis and clinical severity classification of CRPS.
Material and methodsWe performed a cross-sectional study of five CRPS patients fulfilling Budapest diagnostic criteria (Table 1)4 that were prospectively selected in the clinical routine of the Neurology Department of Cruces University Hospital and Autonomic Center of “San Juan deDios” hospital between the years 2015 and 2019. Evaluations were part of the standard assessments for patients with suspected CRPS or small fiber neuropathies in the Dysautonomia Laboratory of both centers. Study procedures were approved by the local Clinical Research Ethics Committee. Prior to their participation in the study, all participants gave informed consent for clinical evaluations and for the use of anonymized recorded data, in accordance to tenets of Declaration of Helsinki. For each participant, we registered age, gender, clinical background, cause of CRPS and clinical signs of CRPS, and we conducted a non-invasive quantitative evaluation of sudomotor function, skin temperature and sensory thresholds.
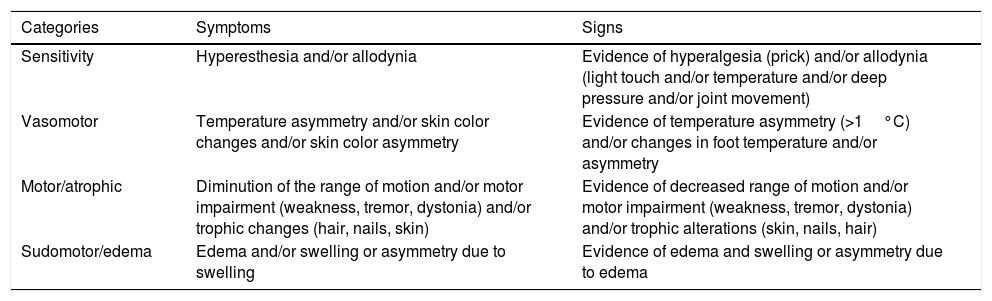
Budapest consensus criteria for the diagnosis of complex regional pain syndrome.4
| Categories | Symptoms | Signs |
|---|---|---|
| Sensitivity | Hyperesthesia and/or allodynia | Evidence of hyperalgesia (prick) and/or allodynia (light touch and/or temperature and/or deep pressure and/or joint movement) |
| Vasomotor | Temperature asymmetry and/or skin color changes and/or skin color asymmetry | Evidence of temperature asymmetry (>1°C) and/or changes in foot temperature and/or asymmetry |
| Motor/atrophic | Diminution of the range of motion and/or motor impairment (weakness, tremor, dystonia) and/or trophic changes (hair, nails, skin) | Evidence of decreased range of motion and/or motor impairment (weakness, tremor, dystonia) and/or trophic alterations (skin, nails, hair) |
| Sudomotor/edema | Edema and/or swelling or asymmetry due to swelling | Evidence of edema and swelling or asymmetry due to edema |
Complex regional pain syndrome is defined as continuous pain, which is disproportionate to the initial event. For the diagnosis of CRPS, the patient must report at least one symptom in three of the four categories within the table (the strictest criteria require at least one symptom of the four categories). For the clinical diagnosis of CRPS, the patient must present at least one sign at the time of the evaluation in two of the four categories (the strictest criteria require at least one symptom of the four categories).
Sudomotor function of the skin was measured by means of Sudoscan® (Impeto Médica ©, Paris France). Sudoscan® uses reverse iontophoresis to quantify objectively and non-invasively electrochemical skin conductance (ESC) by means of two pairs of large-area stainless steel plate electrodes were hands and feet are placed.6,7 ESC, expressed in micro- Siemens (μS), is a surrogate measure of functional integrity of autonomic cholinergic sympathetic nervous fibers innervating sweat glands, being typically decreased compared to age-equivalent healthy controls in diseases causing small fiber neuropathies such as diabetes mellitus. The in-built software of Sudoscan contains a normative database with ESC values from 1350 generally healthy study participants providing reference ESC values with respect to age-matched reference population for any newly acquired measurement. In early stages of CRPS, due to sudomotor hyperactivity, there is a characteristic increase in sweating of the affected limb, while in later CRPS stages, due to atrophy of the glands, decreased sweating is the usual finding. Thus, in the course of CRPS dynamic changes in ESC are expected in the affected as compared to the contralateral limb, with an increase in ESC in early stages and with a decrease in ESC in later stages. During Sudoscan testing, subjects were required to be in resting situation for 5min before placing their hands and feet on electrode plates for approximately 2min. We obtained separate average ESC measures for the affected limb and for the equivalent un-affected contralateral limb and compared them with respect to reference values of age-matched normative data.
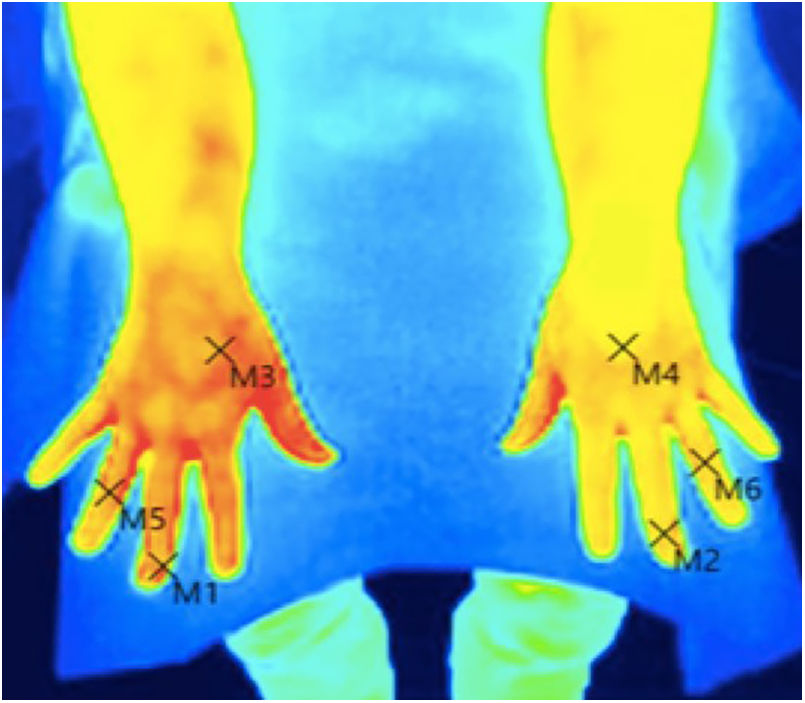
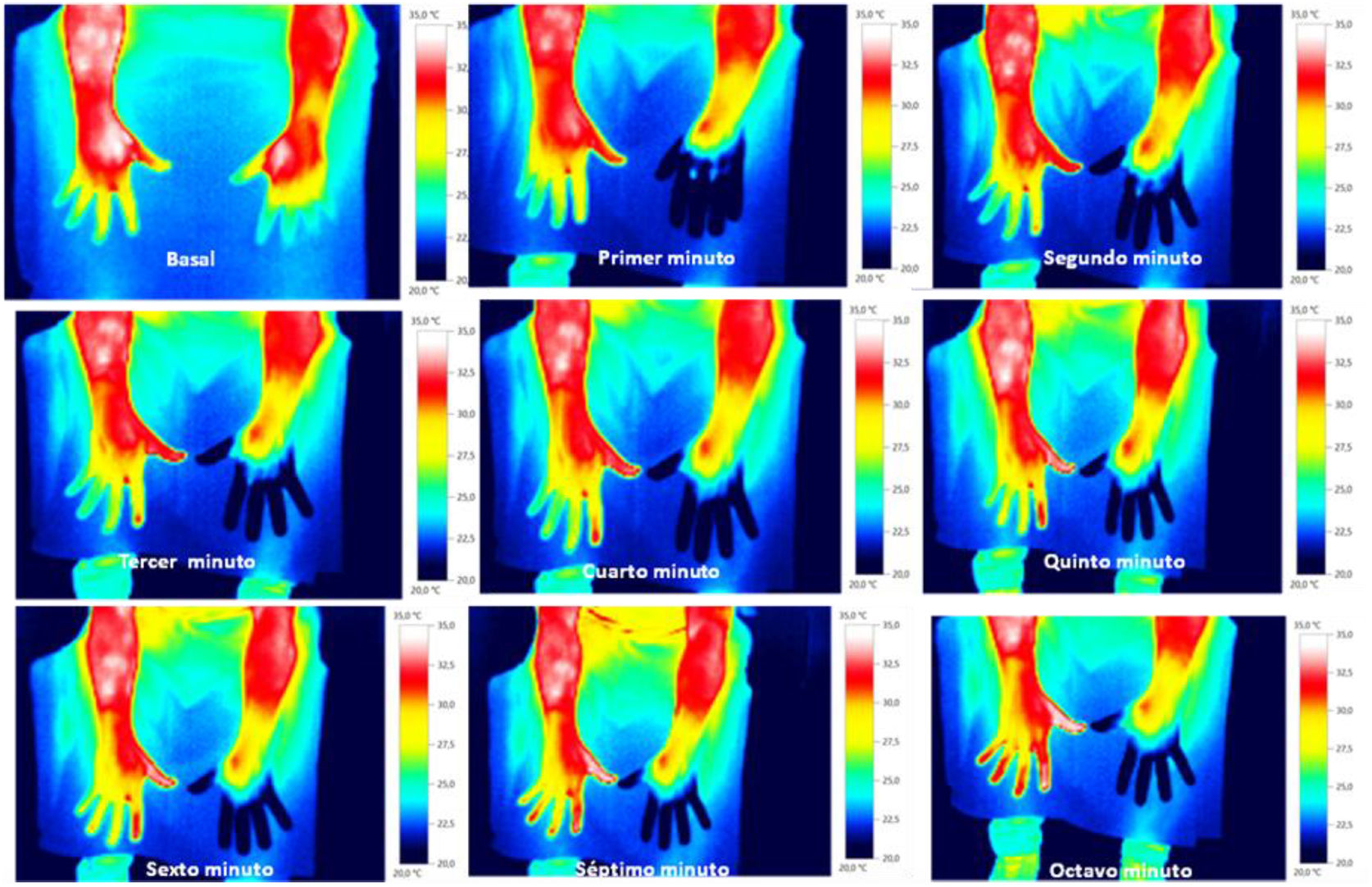
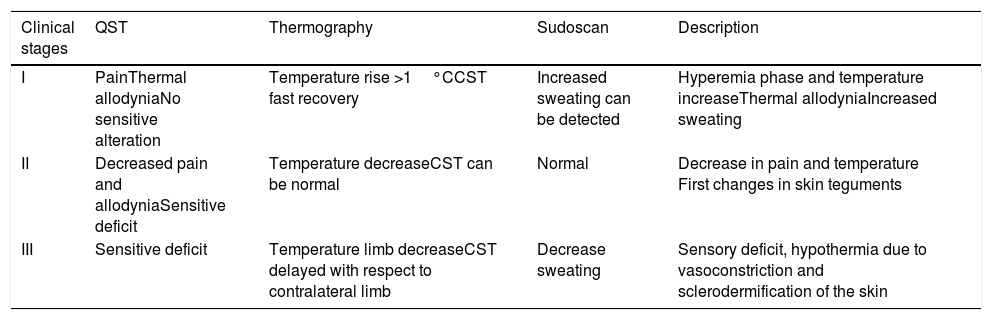
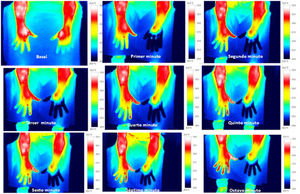
Skin temperature of limbs, a measure of functional integrity of autonomic noradrenergic sympathetic nervous system, was evaluated with serial infrared thermal images of limbs that were recorded with a Testo 875 Thermal imager® (Testo SE & Co, Lenzkirch, Germany). Thermal images were obtained in two conditions: in resting situation and in response to immersion of affected limb in cold water (Cold Stress Test8,9). Image acquisition and analysis was performed following the guidelines of thermal imaging in medicine10 and the protocol proposed by Antonio Rubio et all.8 In brief, the study was performed in a room with a stable ambient temperature, between 22–23°C. The contact of the extremities with any surface that could modify the temperature was avoided. Skin temperature was calculated separately after acquisition of images in the affected limb and in the contralateral limb for two regions of interest (ROIs), one positioned in the base of the third phalanx (distal) (1.75cm2) and the other at the center of the carpus/tarsus (proximal) (5cm2) (Fig. 1). Resting temperature of the affected limb was obtained by comparing it with the contralateral one. Differences in distal-proximal limb temperature and temperature asymmetries between limbs under study were recorded. Subsequently, cold stress test was conducted, which consisted in an immersion of the affected limb in cold water for 2min at a temperature of 0 to 2°C, followed after resting for 15min by the same procedure in the un-affected contralateral limb (reference) (see Fig. 2 as an example with images from cold stress test for case #4). Cold stress test measures the cutaneous vasomotor response, which depends on the integrity of noradrenergic sympathetic innervation of blood vessels (postganglionic unmyelinated C fibers), being part of the adaptive autonomic mechanisms modulating peripheral temperature through vasoconstriction or vasodilation. According to the well-stablished clinical signs of CRPS, right after cold stress a rapid recovery of limb temperature is expected for patients at early stages of the syndrome while in those at advanced stages of CRPS, due to the abnormally persistent reactive vasoconstriction, a delay in the normalization of limb skin temperature is expected.
Lastly, sensory thresholds of affected limbs were quantified by means of NerveCheck Master® (Phi Med Europe©. Barcelona, Spain). NerveCheck, a device for quantitative sensory testing system, was designed to assess vibration, cold, warm perception and heat pain thresholds.11–13 It applies a series of predefined stimuli over a broad range of intensities to the skin using the method of levels. For each stimulus, the subject reports whether the stimulus was perceived or not or whether it was painful or not. As with the Sudoscan, NerveCheck Master includes an in-built normative dataset that provides reference values for acquired thresholds. For our study evaluations, we recorded thresholds for the affected limb and for the contralateral un-affected limb including sensory modalities of specific types of unmyelinated nerve fibers: heat (C fibers), vibration (A beta fibers), cold (A delta fibers). The thresholds for thermal pain and in particular the presence of thermal allodynia was also assessed.
ResultsStudy participants included 3 men and 2 women, with a median age of 38 years (range between 33 and 62). For 3 out of 5 cases CRPS occurred in an upper extremity and for the remaining two in a lower limb, being in all cases trauma and/or orthopedic surgery the identified triggers for CRPS. Only in one patient (case #1) there was a history of a painful syndrome prior to trauma/surgery, which met the diagnostic criteria for fibromyalgia. She had a compressive discopathy with cervical canal stenosis that required surgical treatment and immobilization for a long time that triggered CRPS. The patient showed a significant increase in temperature in the affected limb at the distal level. In the QST, the presence of a discrete loss of vibratory sensitivity with normal non-painful thermal thresholds stands out. The dermatological inspection was normal Trophic changes of the skin were detectable by visual inspection in 3 out of 5 cases. All patients showed spontaneous pain and allodynia. Table 2 summarizes demographics, clinical characteristics and the results of quantitative functional tests for all patients. According to findings on autonomic quantitative functional tests for skin temperature, sweating and sensory thresholds, we were able to classify for all 5 cases the severity of CRPS in 3 clinical stages (Table 3). Cases #1 and #5 corresponded to a stage I, where resting temperature at baseline was increased in the affected limb as compared to the contralateral un-affected limb (Fig. 1). In both cases there were no trophic alterations in the skin or in teguments and the sudomotor function was normal. When performing the thermography after exposure to cold (cold stress test), an early recovery time was observed for both stage I cases. In case #1, there was a history of fibromyalgia, while in case #5 there was no history of neuropathic pain, previous neuropathies or history of any condition potentially influencing on pain thresholds. Cases #2, #3 and #4 corresponded to more advanced stages of the disease as documented by functional assessments: in all three patients there was a decrease in skin resting temperature, a delay in the recovery of the temperature in cold stress test (Fig. 2) and cutaneous trophic changes, which all together define stages II to III of the disease (Table 3). Case #3 is a 33 year old man. For the last 6 years, he abruptly presents with intense pain, with burning, allodynia in the right thigh in relation to a muscle-tendon rupture that was observed in an MRI. The pain has not improved over time. He describes disorders of sensitivity (hyperpathy and allodynia), vasomotor (coldness, edema) and vegetative (increased sweating and coldness) not only limited to the affected limb. Case # 4 is a 44-year-old patient who presented trauma in his right foot. Surgical amputation of several phalanges was performed. Months later he presents significant pain, sweating disorders (increased), circulatory problems and cold tolerance. The symptoms are not limited to the traumatic area but rather radiate in proximity to the rest of the foot. The examination shows a decrease in temperature, a sclerodermiform pattern in the areas adjacent to the scar, with hair loss and changes in skin color.
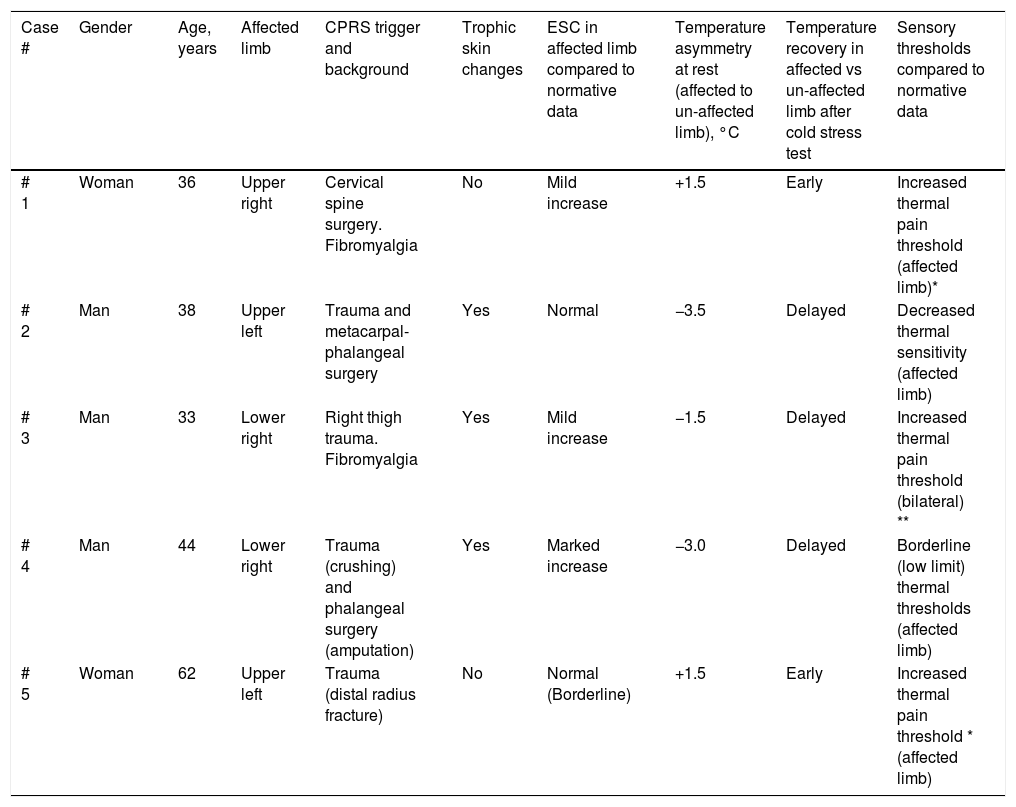
Demographics, clinical characteristics and results from quantitative functional assessments in CPRS patients.
| Case # | Gender | Age, years | Affected limb | CPRS trigger and background | Trophic skin changes | ESC in affected limb compared to normative data | Temperature asymmetry at rest (affected to un-affected limb), °C | Temperature recovery in affected vs un-affected limb after cold stress test | Sensory thresholds compared to normative data |
|---|---|---|---|---|---|---|---|---|---|
| # 1 | Woman | 36 | Upper right | Cervical spine surgery. Fibromyalgia | No | Mild increase | +1.5 | Early | Increased thermal pain threshold (affected limb)* |
| # 2 | Man | 38 | Upper left | Trauma and metacarpal-phalangeal surgery | Yes | Normal | −3.5 | Delayed | Decreased thermal sensitivity (affected limb) |
| # 3 | Man | 33 | Lower right | Right thigh trauma. Fibromyalgia | Yes | Mild increase | −1.5 | Delayed | Increased thermal pain threshold (bilateral) ** |
| # 4 | Man | 44 | Lower right | Trauma (crushing) and phalangeal surgery (amputation) | Yes | Marked increase | −3.0 | Delayed | Borderline (low limit) thermal thresholds (affected limb) |
| # 5 | Woman | 62 | Upper left | Trauma (distal radius fracture) | No | Normal (Borderline) | +1.5 | Early | Increased thermal pain threshold * (affected limb) |
* High thermal thresholds are found in patients taking analgesics.
Different phases of the CRPS and in relation to findings in complementary explorations.
| Clinical stages | QST | Thermography | Sudoscan | Description |
|---|---|---|---|---|
| I | PainThermal allodyniaNo sensitive alteration | Temperature rise >1°CCST fast recovery | Increased sweating can be detected | Hyperemia phase and temperature increaseThermal allodyniaIncreased sweating |
| II | Decreased pain and allodyniaSensitive deficit | Temperature decreaseCST can be normal | Normal | Decrease in pain and temperature First changes in skin teguments |
| III | Sensitive deficit | Temperature limb decreaseCST delayed with respect to contralateral limb | Decrease sweating | Sensory deficit, hypothermia due to vasoconstriction and sclerodermification of the skin |
QST: quantitative sensory test. CST: Cold stress test (thermography).
Intriguingly, none of the 5 cases showed decreased ESC in sudomotor testing. Patients #1 and #5 had values at the high limit of normality, reflecting an increase in sweating in the affected limb but they did not exceed the pathological range. The increase of pain thresholds in QST observed in most cases could be partially explained by the presence of spontaneous pain and allodynia (with regular use of NSAID in all cases). Except for cases #2 and #5, no follow up clinical data was available at the moment of the preparation of this manuscript. Cases #2 is a 38-year-old man who suffers trauma with a fracture in the metacarpal phalangeal joints of the left upper extremity. After surgery, the pain worsens, and regional vasomotor alterations appear with edema and redness. In the successive annual visits 3 years after CRPS onset, clinical records describe associated trophic cutaneous disorders such as loss of skin hair, increased skin thickness and coldness of the affected limb, compatible with phase III features of CPRS (Table 3). A bone densitometry was performed in which osteopenia was observed in the distal region of the ulna and radius. Case #5 was a 62-year-old patient who had an accidental distal radius fracture. She required immobilization of the arm for two months. A few months later, she began with spontaneous pain, allodynia, and an increase in temperature in that extremity. The patient is evaluated one year later. In this second consultation, dystrophic changes in the skin begin to be seen with a slight decrease in sweating. In this time it has evolved from a CPRS phase I to a phase II.
DiscussionBy means of implementing a combination of non-invasive quantitative measures in the clinical assessment of five cases with CPRS, including skin temperature determined with thermography, ESC with Sudoscan® and skin sensory thresholds with QST, in the present study we illustrate the potential usefulness of such functional parameters in the diagnosis and severity stratification of patients with CPRS. Moreover, the use of skin thermography allowed us to show its potential applicability as a biomarker of the mechanisms underlying abnormalities in microvasculature adaptability in CPRS, which in healthy conditions is regulated by noradrenergic innervation. On one hand, the findings observed in initial phases of CRPS consisting in an increase of baseline resting temperature with early correction of the temperature with respect to the contralateral limb after the cold stress test (see Table 3) orient to think about a permanent opening of the arteriovenous shunts (AVS). A dense innervation, fundamentally sympathetic, maintains a tonic contraction of the smooth muscle of vessels to keep the AVS closed and allowing the direct passage of blood to the capillary bed. If such sympathetic nerves degenerate, the AVS opens allowing the blood to avoid the capillaries. In such circumstances the limb may appear hyperemic and reddened but ischemic or hypoxic and acidic, as demonstrated in tests of nuclear medicine imaging in muscle.14 The altered perfusion causes chronic edema and changes in skin color and temperature and leaves the tissues without adequate oxygenation and nutrition. On the other hand, in chronic phases of CPRS, the findings consisting of a decrease in baseline resting temperature with a delay in the correction after the stimulation with cold stress test lead to an inability of the distal microvasculature to control the temperature or to an inability to respond to stimuli. These alterations already correspond to trophic changes of the skin and cutaneous annexes leading to a more evolved phase of CPRS.
Thermography can be a good tool to quantify thermal changes in the affected limb, taking into account the changes that are observed in skin temperature in the different phases. Some studies emphasize the importance of cold stress tests to differentiate from controls.15 In other studies, they do not find differences in baseline situation, one of the possible explanations is that they include patients in different stages of the disease.16,17 The thermography itself can be used not only for diagnostic purposes but also in assess treatment efficacy in CRPS over time.18
With regard to the inconclusive findings in sudomotor function quantification of our study, it is important to state that previous literature has also yielded discordant results in this respect.19–21 In our assessments, we quantified ESC as a surrogate marker of sweating, a method that has proven useful, especially in the study of autonomic neuropathies.13,22,23 Sweating alterations can also be subclinical and some patients may have areas of hypo or hyperhidrosis according to different situations.24 A partially denervated gland may sweat insufficiently from direct nerve stimulation but excessively in response to circulating catecholamines.3 In our study, a tendency to hyperhidrosis was detected in stage I of the disease. Lastly, regarding threshold measurements for different sensory modalities, to date no specific pattern has been observed for CPRS. QST is highly dependent on patient's collaboration. In our study, the results of QST revealed increased sensitivity thresholds, a characteristic finding in patients taking analgesics. In addition, we observed in two cases a decrease in the thresholds of thermal sensitivity, which may reflect damage of unmyelinated nerve fibers (C-fibers). Some authors have shown that when patients are urged to delimit the epicenter of pain, the latter is usually more restricted in the area of a specific nerve.3 However, since not all sensory nerves are accessible to nerve conduction studies, the involvement of the clinician is necessary to delimit the anatomical area.1,3 Microneurography studies have shown that nerve fibers with smaller diameters (unmyelinated C- and A-delta fibers) have wider sensory fields and more widespread innervation in adjacent bones and vascular Trees.20 In addition, the terminals of fine fiber axons can communicate with adjacent axons, which facilitates overlapping of territories. Lastly, skin pathological examinations of affected limbs in CPRS have shown a loss of epidermal nerve fibers, which innervate sweat glands and the wall of blood vessels, and a loss of integrity and hypertrophy of the vascular endothelium CPRS, when compared to unaffected areas.25
Several pathophysiological mechanisms have been proposed for CRPS26: changes produced after post-traumatic inflammation; peripheral vasomotor dysfunction; central sensitization phenomenon, with functional and structural changes in the central nervous system secondary to poor adjustment to chronic pain and alteration of the immune system, which would cause excessive and pathological activation of dendritic cells after the initial injury. All these mechanisms have one feature in common, hyperactivity of the sympathetic nervous system after trauma. The results of the complementary tests illustrate these pathophysiological changes in the CPRS. The hyperactivity of the sympathetic nervous system produces the first vasomotor changes with increased sweating and temperature seen in phase I. Maintained vasoconstriction produces chronic ischemic changes in the skin, integuments and bone, leading to trophic changes in the advanced phases (phase III such as osteoporosis, hyposudation, scleroderma, hair loss). The alteration of type C amyelinic fibers occurs in advanced stages in which alterations in thermal thresholds and pain are observed in the QST.25,27
The main limitation of the study is the sample size, but it is intended to serve as a pilot study for a larger future series. The methods mix quantitative (thermography) with qualitative (QST) examinations, but it provides a method of study of this syndrome where to date, we only used clinical valuation with radiological (bone desitometry, scintigraphy, etc.)
As a conclusion, through the analysis of five illustrative cases, our study shows that the implementation of non-invasive quantitative measures of the skin (electrochemical conductance, thermography and sensory thresholds) is potentially useful in the clinical evaluation of CPRS. In particular, our analysis suggests that the study with cutaneous thermography and cold response (cold stress test) can be especially helpful to stratify the phase of the disease. The damage of the unmyelinated nerve fibers can be objectified with QST, especially in its late phases. Moreover, we observed that quantitative functional tests not only help in the diagnosis of CRPS but also in the classification of CPRS (Table 3) and the understanding of the pathological processes underlying different phases of the disease.
Authors’ statementWe confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
FundingThis study has not received any type of funding.
Conflict of interestsNone of the authors have any conflicts of interest to report.