The Frontotemporal Dementia Rating Scale (FTD-FRS) is a tool designed to aid with clinical staging and assessment of the progression of frontotemporal dementia (FTD-FRS).
ObjectivePresent a multicentre adaptation and validation study of a Spanish version of the FRS.
MethodologyThe adapted version was created using 2 translation-back translation processes (English to Spanish, Spanish to English) and verified by the scale's original authors. We validated the adapted version in a sample of consecutive patients diagnosed with FTD. The procedure included evaluating internal consistency, testing unidimensionality with the Rasch model, analysing construct validity and discriminant validity, and calculating the degree of agreement between the Clinical Dementia Rating scale (CDR) and FTD-FRS for FTD cases.
ResultsThe study included 60 patients with DFT. The mean score on the FRS was 12.1 points (SD=6.5; range, 2-25) with inter-group differences (F=120.3; df=3; P<.001). Cronbach's alpha was 0.897 and principal component analysis of residuals delivered an acceptable eigenvalue for 5 contrasts (1.6-2.7) and 36.1% raw variance. FRS was correlated with the Mini-mental State Examination (r=0.572; P<.001) and functional capacity (DAD; r=0.790; P<.001). FTD-FRS also showed a significant correlation with CDR (r=−0.641; P<.001), but we did observe variability in the severity levels; cases appeared to be less severe according to the CDR than when measured with the FTD-FRS (kappa=0.055).
ConclusionsThis process of validating the Spanish translation of the FTD-FRS yielded satisfactory results for validity and unidimensionality (severity) in the assessment of patients with FTD.
La Frontotemporal Dementia Rating Scale (FTD-FRS) es una escala diseñada para facilitar la estadificación clínica y la evaluación de la progresión de pacientes con demencia frontotemporal (DFT).
ObjetivoRealizar un estudio multicéntrico de adaptación y validación al castellano de la FTD-FRS.
MetodologíaLa adaptación se realizó mediante 2 procesos de traducción y retrotraducción inglés-español español-inglés y se verificó con los autores originales. El proceso de validación se llevó a cabo en una muestra consecutiva de pacientes diagnosticados de DFT. Se evaluó la consistencia interna, se determinó la unidimensionalidad con el método Rasch, se analizaron la validez de constructo y la validez discriminante, y se calculó el grado de acuerdo entre la Clinical Dementia Rating scale y la FTD-FRS para los casos con DFT.
ResultadosSe incluyeron 60 pacientes con DFT. La puntuación media de la FTD-FRS fue de 12,1 puntos (DE=6,5; rango=2-25) mostrando diferencias intergrupos (F=120,3; gl=3; p<0,001). El α de Cronbach=0,897, el análisis de componentes principales de los residuos produjo un aceptable autovalor para 5 contrastes (1,6-2,7) y una varianza respecto al origen del 36,1%. La FTD-FRS correlacionó con el Mini-mental test (r=0,572; p<0,001) y capacidad funcional (DAD; r=0,790; p<0,001). La FTD-FRS correlacionó significativamente con la Clinical Dementia Rating scale (r=−0,641; p<0,001) pero se observó variabilidad entre la distribución de la gravedad, siendo valorados como más leves según la Clinical Dementia Rating scale que con la FTD-FRS (kappa=0,055).
ConclusionesEl estudio de traducción y validación al español mostró resultados de validez y unidimensionalidad (gravedad) satisfactorios para el uso de la FTD-FRS en el estudio de la gravedad en pacientes con DFT.
Frontotemporal dementia (FTD) represents 5% of the cases diagnosed with dementia at autopsy in case series studies. Its incidence is estimated at 9.4 cases per 100000 person-years in subjects younger than 65, and it is the second most frequent neurodegenerative dementia in these subjects.1–6 Neuropathology studies of the brains of patients with FTD show frontal and temporal atrophy as well as abnormal protein aggregates in neurons and/or glia, which could be considered frontotemporal lobar degeneration.7 However, histopathological, biochemical and genetic findings are heterogeneous. Clinically, FTD is characterised by a progressive change in personality and behaviour, early language impairment and executive dysfunctions with relative preservation of episodic memory and visuospatial functions during the earliest stages.1,2,8 Clinical, behavioural, and cognitive symptoms present insidiously and progressively; however, the initially predominating symptoms depend on the subarea that is affected.9,10
Functional impairment is one of the criteria for a diagnosis of clinically probable FTD. However, early impairment of behaviour and executive dysfunction, and especially, the absence of a predominant memory alteration make it difficult to use such common scales and tools as the Clinical Dementia Rating scale (CDR) or the Geriatric Deterioration Scale to assess disease severity. These scales were designed to assess severity of Alzheimer disease (AD), the most prevalent dementia subtype, and applying them to cases of FTD raises validity problems.1,2,11–14
In clinical terms, mean time between symptom onset and diagnosis is longer in FTD than in AD; also, patients with FTD take more psychodrugs (antipsychotics and anxiolytics) and anti-dementia drugs (which are not indicated for treating FTD).15–17 Therefore, having valid tools to measure the clinical stage or severity is essential for developing a standardised way of evaluating disease progression in patients with FTD for clinical or research purposes.18
Mioshi et al.19 developed and validated a specific scale to establish the clinical stage and severity of FTD: the Frontotemporal Dementia Rating Scale (FTD-FRS). This scale classifies patients on a 6-point scale of severity (from very mild to profound) and by frequency of occurrence (never, sometimes, all the time). It assesses 30 signs and symptoms selected for their validity, unidimensionality, and good internal reliability in patients with FTD. In the original study, the FTD-FRS showed an internal consistency of Cronbach α=0.93 and an intraclass correlation coefficient of 0.994. The FTD-FRS has been shown to be a useful tool for establishing the stage and assessing longitudinal progression in patients with FTD. The aim of our study was to adapt and validate the Spanish-language version of the FTD-FRS.
MethodsDesignThis is a multi-centre, cross-sectional and analytical study.
Study populationPatients diagnosed with FTD in specialised care units.
SampleConvenience sample of patients with FTD recruited from the 3 hospitals participating in the study: Hospital Clínic in Barcelona, Hospital San Jorge in Huesca, and Hospital Santa Caterina in Girona. The study was approved by the clinical research ethics committees at each location and included those participants meeting criteria for probable or possible FTD.1,3 A family member or carer had to be present to answer questions about the patient's condition and sign informed consent (forms were signed by patients and their legal guardians/carers, where applicable). We also recruited patients with a clinical diagnosis of AD to form a control group.20
ProcedureThis study included 2 stages: the first stage consisting of translating and adapting the scale to produce the Spanish version, and a second stage for clinical validation of the scale. The adaptation process was performed following the standard procedures of synthesis of 2 translations with back-translation.21
In addition to generating the final consensus version in Spanish of the FTD-FRS, the validation protocol included recording patients’ and carers’ sociodemographic data and such clinical data as progression time to symptom onset/diagnosis, cognitive function, functional capacity, frequency and severity of psychological and behavioural symptoms, and severity of dementia.
Instruments- •
Severity of dementia: We used 2 scales:
- ∘
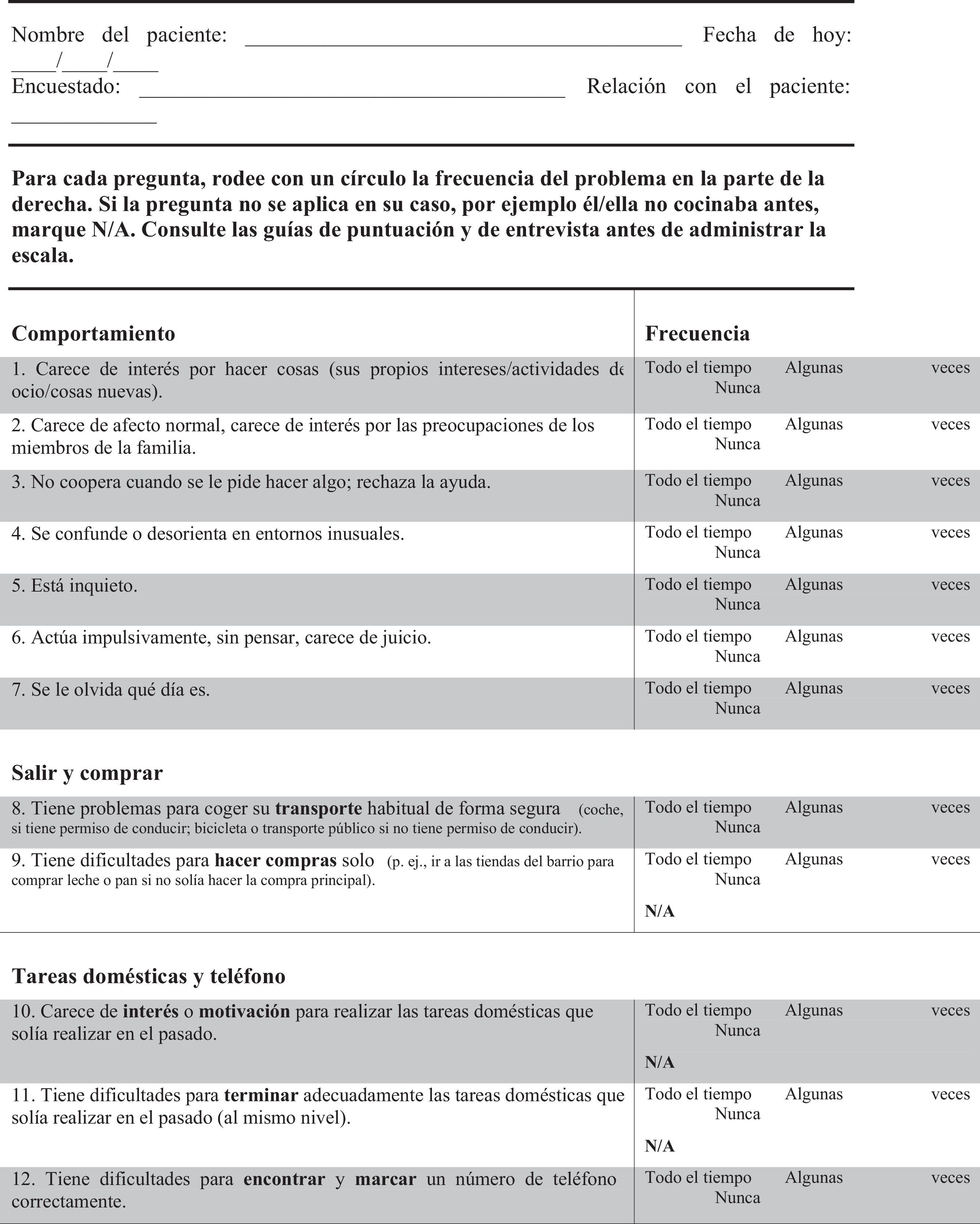
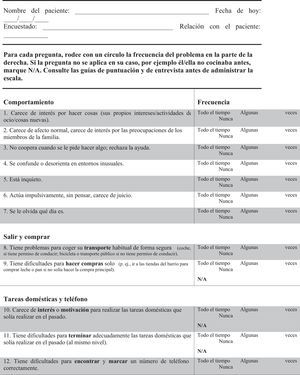
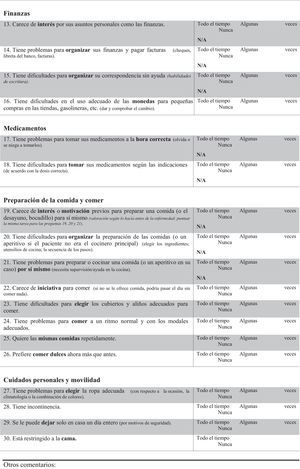
FTD-FRS19: This scale, administered to the carer, assesses 30 items with 3 possible answers regarding frequency (never, sometimes, and all the time). Items are divided into 8 dimensions (behaviour, outing and shopping, household chores and telephone, finances, medications, meal preparation and eating, and self-care and mobility). This scale classifies dementia in 6 severity stages (very mild, mild, moderate, severe, very severe, and profound).
- ∘
CDR11: clinical scale classifying dementia into 5 stages of cognitive and functional impairment. Scores of 0 indicate no impairment, 0.5 very mild dementia, 1 mild dementia, 2 moderate dementia, and 3 severe dementia. Information is obtained through a semi-structured interview of the carers and a quick assessment of the patient. It evaluates memory, orientation, judgement and problem solving, community affairs, home and hobbies, and personal care.
- ∘
- •
Administrative and sociodemographic data: age, sex, place of residence, age of carer, sex of carer, relationship with the participant, date of diagnosis, and date of consultation.
- •
Cognitive function: we used Addenbrooke's Cognitive Examination-Revised22 to assess patents’ cognitive function. The test assesses 5 domains (attention, memory, verbal fluency, language, and visuospatial abilities) with a maximum score of 100 points. The Mini-Mental State Examination (MMSE)23 was also used.
- •
Functional disability: we used the Disability Assessment in Dementia (DAD), a scale which assesses the percentage of preserved functional autonomy. It includes 40 items addressing the performance of basic and instrumental activities of daily living in the preceding 15 days.24
- •
Psychological and behavioural symptoms associated with dementia: we used the Neuropsychiatric Inventory to assess the frequency and severity of such diverse neuropsychiatric symptoms as delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behaviour, sleep disturbances, and changes in appetite. Scores on each subscale range from 0 to 12, and the maximum total score is 44; higher scores indicate a greater presence of evaluated disorders.25
The descriptive study of the data was conducted using absolute and relative frequencies for qualitative variables and measures of central tendency and dispersion for quantitative variables. Normal distribution was assessed with the Kolmogorov–Smirnov test.
For the adaptation and validation process, we assessed internal consistency with Cronbach α and determined scale composition by following the parameters of the original scale. To this end, we used infit and outfit statistics to check that there were no items outside these parameters (MNSQ=0.60 to 1.49, and Z-scores=2 to 2). Principal component analysis of residuals was replicated using the Rasch method to determine the unidimensionality of the Spanish version of the FTD-FRS.26,27 For the concurrent validity analysis, we correlated scores on the FTD-FRS with scores for cognitive and functional impairment. For the discriminant validity analysis, we calculated ANOVA applying Bonferroni corrections for the differences in the mean scores on FTD-FRS and in the variables of cognitive and functional capacity. Likewise, we calculated the degree of agreement between the CDR and the FTD-FRS using the kappa index for cases with FTD.
Statistical analysis was performed using SPSS v.19.0 for Windows (SPSS Inc., Chicago) and Winsteps 3.90.0 for Windows.24
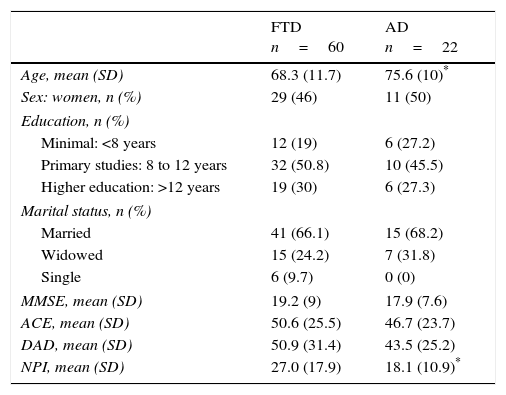
ResultsDemographic characteristics of participantsThe final study sample included 60 patients with FTD, with a mean age of 68.1 years (SD=12.1; range, 45.0-95.7) and 54.2% were men. Mean time of disease progression was 7.3 years (SD=5.6; range, 0.5-22.0 years). Of the total FTD cases, 71.4% lived in their own homes, 14.3% in a relative's house, and the remaining 14.3% were institutionalised. Fifty-six per cent of the carers were patients’ partners, 32% were their sons or daughters, and 12% were other relatives. Of the 29 cases with AD, 44.8% were men and the mean age was 75.5 years (SD=9; range, 56.1-90.4). Mean time of disease progression was 5.2 years (SD=4.5; range, 0.5-15 years). At the time of the study, 72% lived in their own homes, 16% in a relative's house, and the remaining 12% were institutionalised. The main carer was a daughter or son for 52.6% of these patients, followed by partners for 36.8%; patients were looked after by other relatives or carers in the remaining cases. Patients’ clinical characteristics are listed in Table 1.
Overall characteristics of the sample and stratification by diagnosis.
| FTD n=60 | AD n=22 | |
|---|---|---|
| Age, mean (SD) | 68.3 (11.7) | 75.6 (10)* |
| Sex: women, n (%) | 29 (46) | 11 (50) |
| Education, n (%) | ||
| Minimal: <8 years | 12 (19) | 6 (27.2) |
| Primary studies: 8 to 12 years | 32 (50.8) | 10 (45.5) |
| Higher education: >12 years | 19 (30) | 6 (27.3) |
| Marital status, n (%) | ||
| Married | 41 (66.1) | 15 (68.2) |
| Widowed | 15 (24.2) | 7 (31.8) |
| Single | 6 (9.7) | 0 (0) |
| MMSE, mean (SD) | 19.2 (9) | 17.9 (7.6) |
| ACE, mean (SD) | 50.6 (25.5) | 46.7 (23.7) |
| DAD, mean (SD) | 50.9 (31.4) | 43.5 (25.2) |
| NPI, mean (SD) | 27.0 (17.9) | 18.1 (10.9)* |
ACE: Addenbrooke's Cognitive Examination; DAD: Disability Assessment in Dementia; SD: standard deviation; FTD: frontotemporal dementia; AD: Alzheimer disease; MMSE: Mini-Mental State Examination; NPI: Neuropsychiatric Inventory.
Translators elaborated 2 independent translations and adaptations from English into Spanish. Texts were subsequently back-translated by external professionals specialising in scientific translation. Both versions were reviewed and accepted by the authors of the original scale. Lastly, a consensus process delivered a single scale in Spanish that proceeded to the validation stage (Appendix A).
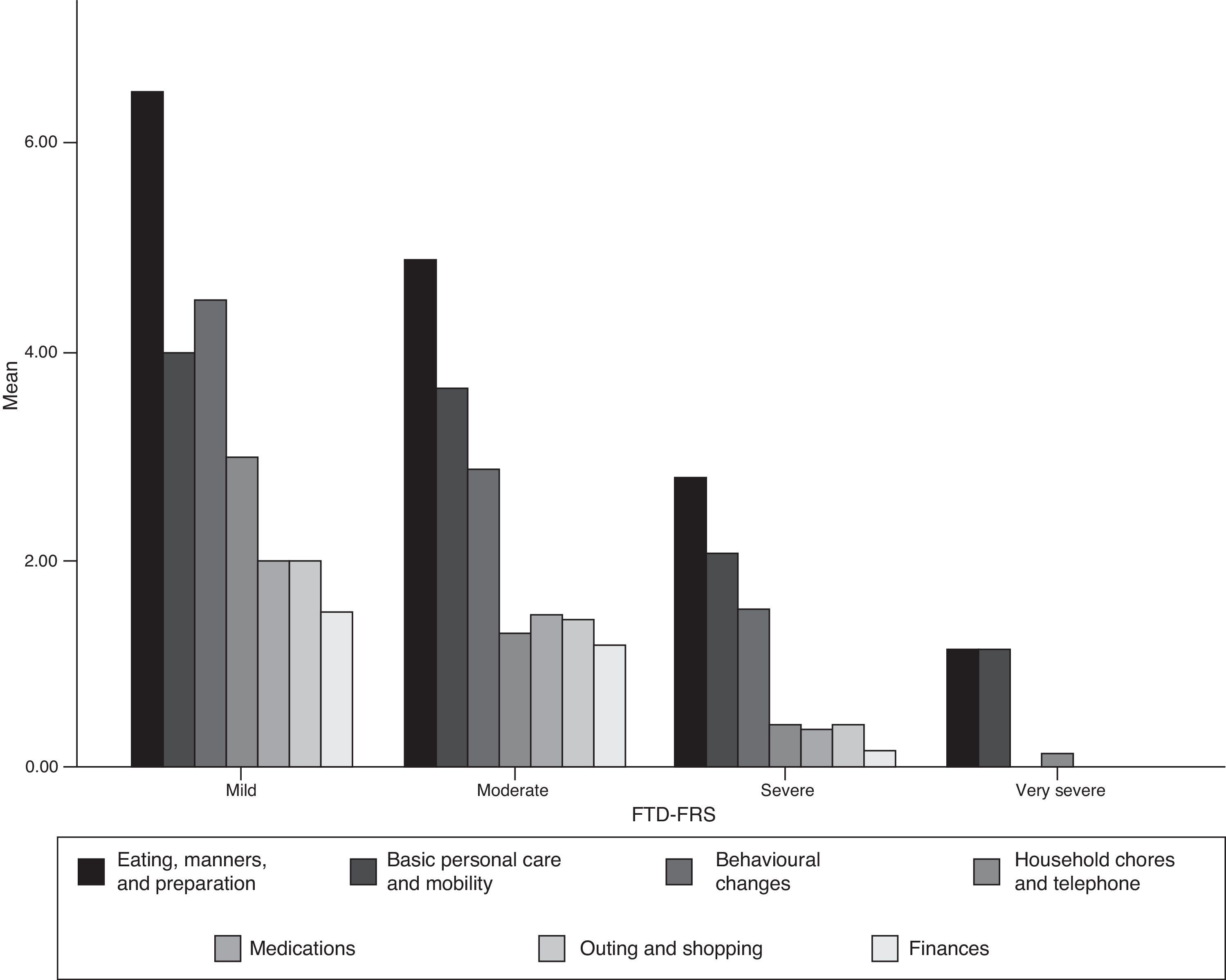
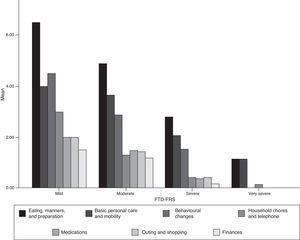
Acceptability and internal consistency of the Spanish version of the Frontotemporal Dementia Rating ScaleThe FTD-FRS was administered to the carers of patients who were willing to participate in the study. We observed no difficulties in understanding the instructions or any of the 30 items. Table 2 shows the percentage of scores of 1 (never) for each item; Fig. 1 shows results by FTD-FRS domain.
Percentage of negative responses (never).
| Items | Never (%) |
|---|---|
| 1. Lacks interest in doing things - their own interests/leisure activities/new things | 15 |
| 2. Lacks normal affection, lacks interest in family members’ worries | 28.3 |
| 3. Is uncooperative when asked to do something; refuses help | 31.7 |
| 4. Becomes confused or muddled in unusual surroundings | 41.7 |
| 5. Is restless | 26.7 |
| 6. Acts impulsively without thinking, lacks judgement | 31.7 |
| 7. Forgets what day it is | 38.3 |
| 8. Has problems taking his/her usual transportation safely (car if has a driver licence; bike or public transport if does not have a driver licence) | 46.7 |
| 9. Has difficulties shopping on their own (e.g. to go to the local shops to get milk and bread if did not use to do the main shopping) | 32.1a |
| 10. Lacks interest or motivation to perform household chores that he/she used to perform in the past | 20.7b |
| 11. Has difficulties completing household chores adequately that he/she used to perform in the past (to the same level) | 23.7b |
| 12. Has difficulty finding and dialling a telephone number correctly | 45.8c |
| 13. Lacks interest in his/her personal affairs such as finances | 22.2c |
| 14. Has problems organising his/her finances and to pay bills (cheques, bankbook, bills) | 11.3c |
| 15. Has difficulties organising his/her correspondence without help (writing skills) | 23.2c |
| 16. Has problems handling adequately cash in shops, petrol stations, etc. (give and check change) | 45.8c |
| 17. Has problems taking his/her medications at the correct time (forgets or refuses to take them) | 34.8d |
| 18. Has difficulties taking his/her medications as prescribed (according to the right dosage) | 42.4d |
| 19. Lacks previous interest or motivation to prepare a meal (or breakfast, sandwich) for himself/herself (rating based pre-morbid functioning; score same task for questions 19, 20, and 21) | 36.8 |
| 20. Has difficulties organising the preparation of meals (or a snack if patient was not the main cook) (choosing ingredients; cookware; sequence of steps) | 37.5 |
| 21. Has problems preparing or cooking a meal (or snack if applicable) on their own (needs supervision/help in kitchen) | 31.5 |
| 22. Lacks initiative to eat (if not offered food, might spend the day without eating anything at all) | 73.3 |
| 23. Has difficulties choosing appropriate utensils and seasonings when eating | 75 |
| 24. Has problems eating meals at a normal pace and with appropriate manners | 46.7 |
| 25. Wants to eat the same foods repeatedly | 53.3 |
| 26. Prefers sweet foods more than before | 40 |
| 27. Has problems choosing appropriate clothing (with regard to the occasion, the weather, or colour combination) | 55 |
| 28. Is incontinent | 63.3 |
| 29. Cannot be left at home by himself/herself for a whole day (for safety reasons) | 53.3 |
| 30. Is restricted to the bed | 95 |
Mean score on the FTD-FRS in cases with FTD was 1.21 points (SD=6.5; range, 2-25) and the distribution was normal (Kolmogorov–Smirnov=0.183). Considering the original criteria, 5% of the cases presented mild dementia with a mean score on the FTD-FRS of 25.2 points (SD=1.3; range, 24-26.7); 41.7% moderate dementia, with a mean score of 17.8 points (SD=2.8; range, 13-23); 41.7% severe dementia with a mean score of 8.5 points (SD=2.4; range, 4-11); and the remaining 11.7% showed very severe dementia, with a mean score on the FTD-FRS of 2.4 points (SD=0.5; range, 2-3). No very severe or profound cases of FTD were observed.
Internal consistency analysis yielded good results (Cronbach α=0.897) and Rasch analysis results were within the recommended limits for the infit and outfit statistics (MNSQ=1, Z-score=0, P=.21; and MNSQ=0.96, Z-score=−0.1, P=.31; respectively). The principal component analysis of residuals yielded acceptable eigenvalues for 5 contrasts (1.6-2.7) and variance from origin of 36.1%.
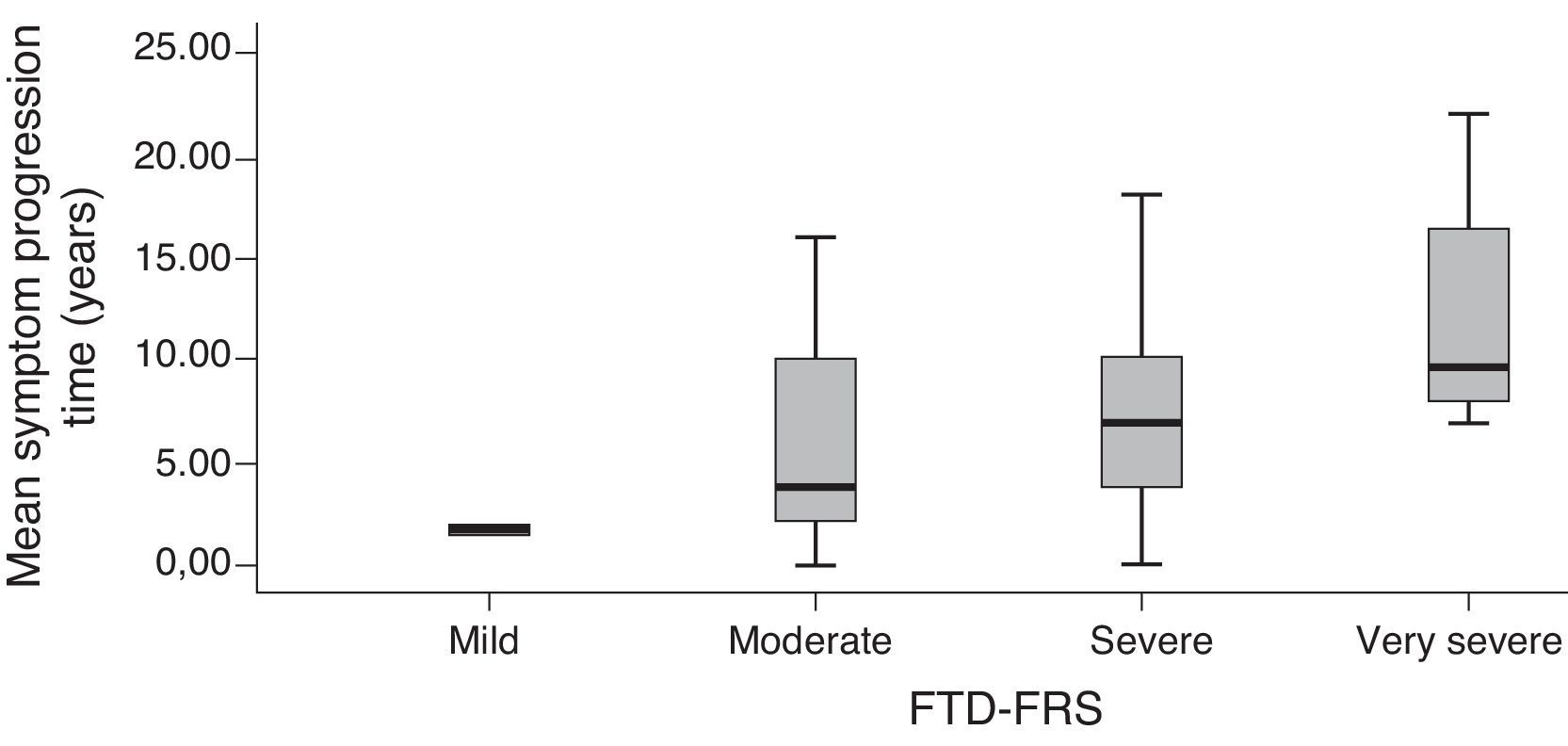
Validity criteria of the Frontotemporal Dementia Rating ScaleFTD-FRS scores were moderately correlated with MMSE scores (r=0.572; P<.001), and scores on Addenbrooke's Cognitive Examination-Revised (ACE-R) (r=0.534; P<.001). This scale showed a greater correlation with the DAD (r=0.790; P<.001); no correlation was observed with scores on the Neuropsychiatric Inventory (r=−0.241; P=.101). Approximate progression time of symptoms presented a statistically significant inverse correlation with FTD-FRS scores (the longer the time, the poorer the capacity) (r=−0.554; P<.001). The distribution of mean years of progression and the different FTD-FRS stages was also statistically significant (F=3.3; df=3; P=.017).
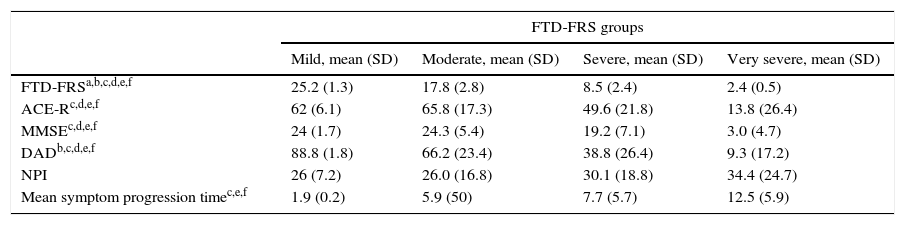
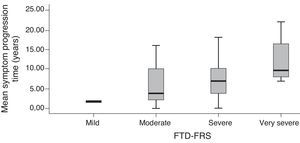
Grouping scores by stage on the FTD-FRS revealed intergroup differences with continuous scores (F=130.4; df=3; P<.001), as well as intragroup differences according to Bonferroni comparisons. We observed differences between cognitive function scores measured with the ACE-R (F=5.0; df=3; P=.001) and with MMSE (F=5.9; df=3; P<.001), and functional capacity scores measured with the DAD (F=19.9; df=3; P<.001). These differences were especially pronounced between mild and more severe cases (Table 3). We observed differences between FTD-FRS stages and symptom progression times, as can be seen in Fig. 2 (F=3.4; df=3; P=.026).
Description of clinical assessments of patients with FTD stratified by degree of severity on the FTD-FRS.
| FTD-FRS groups | ||||
|---|---|---|---|---|
| Mild, mean (SD) | Moderate, mean (SD) | Severe, mean (SD) | Very severe, mean (SD) | |
| FTD-FRSa,b,c,d,e,f | 25.2 (1.3) | 17.8 (2.8) | 8.5 (2.4) | 2.4 (0.5) |
| ACE-Rc,d,e,f | 62 (6.1) | 65.8 (17.3) | 49.6 (21.8) | 13.8 (26.4) |
| MMSEc,d,e,f | 24 (1.7) | 24.3 (5.4) | 19.2 (7.1) | 3.0 (4.7) |
| DADb,c,d,e,f | 88.8 (1.8) | 66.2 (23.4) | 38.8 (26.4) | 9.3 (17.2) |
| NPI | 26 (7.2) | 26.0 (16.8) | 30.1 (18.8) | 34.4 (24.7) |
| Mean symptom progression timec,e,f | 1.9 (0.2) | 5.9 (50) | 7.7 (5.7) | 12.5 (5.9) |
ACE-R: Addenbrooke's Cognitive Examination-Revised; DAD: Disability Assessment in Dementia; FTD: frontotemporal dementia; FRD-FRS: Frontotemporal Rating Scale; MMSE: Mini-Mental State Examination; NPI: Neuropsychiatric Inventory.
ANOVA with Bonferroni correction, P<.05: a) Mild * Moderate; b) Mild * Severe; c) Mild * Very severe; d) Moderate * Severe; e) Moderate * Very severe; f) Severe * Very severe.
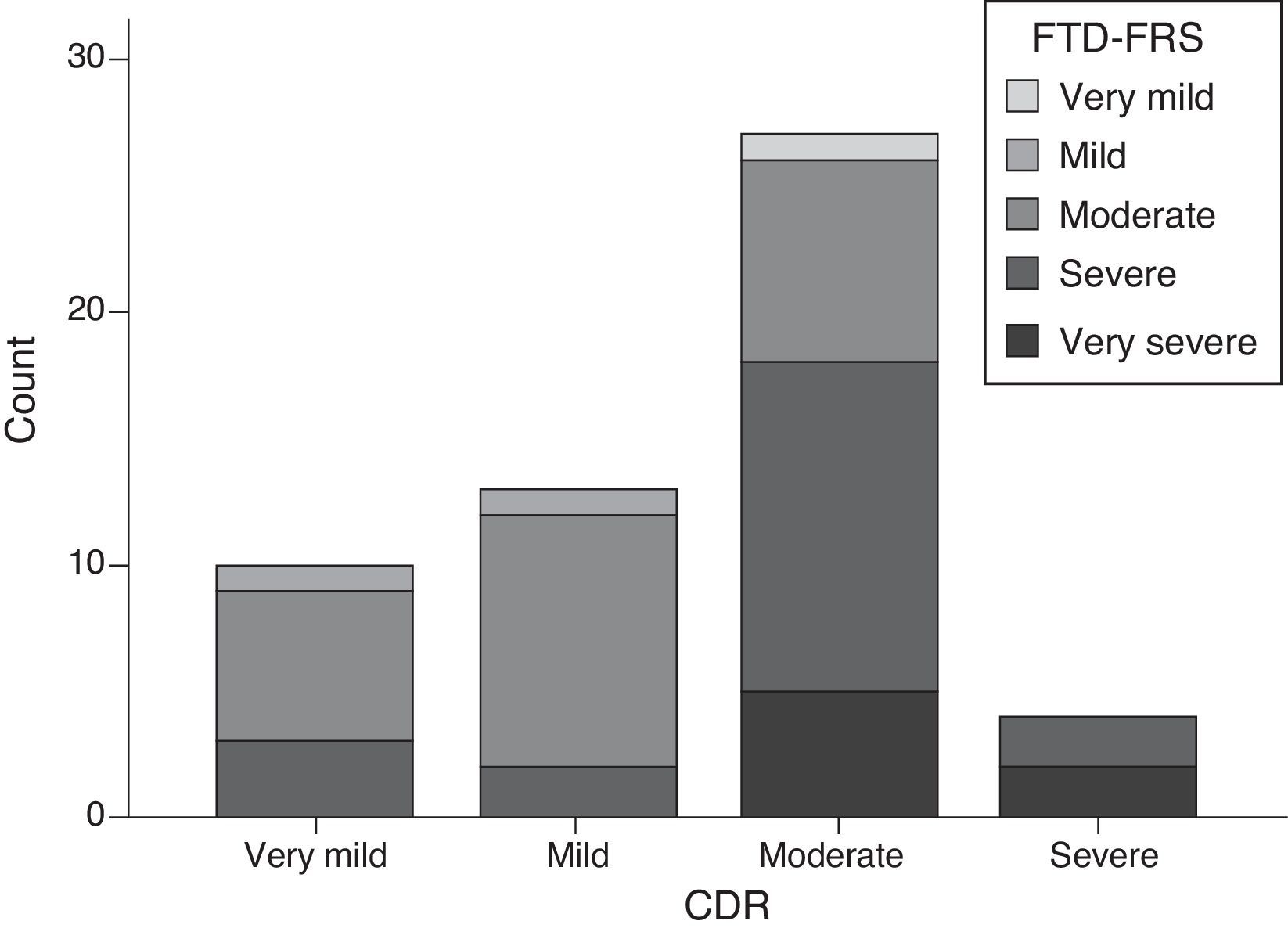
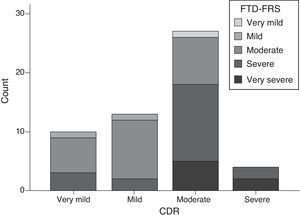
Scores on the FTD-FRS showed a moderate inverse correlation with CDR scores (r=−0.641; P<.001); however, if we stratify by FTD-FRS severity stages, there are discrepancies with the CDR severity groups (χ2=20.2; df=9; P=.017) and a low degree of agreement (kappa=0.055). Cases with moderate and severe FTD present the highest variability, and may even be classified as mild on the CDR (Fig. 3).
Mean scores on the FTD-FRS did not differ significantly between cases with FTD and cases with AD (t=−0.769; df=79; P=.444), and neither did distribution of severity (χ2=0.985; df=3; P=.811). If we compare correlation results on the FTD-FRS with those from cases with AD, we observe equally significant although moderately lower scores on both the MMSE (r=0.402; P=.034) and ACE (r=0.400; P=.035). No correlations with symptom progression time were observed (r=−0.382; P=.145). In contrast, we observed a high degree of correlation with the DAD (r=0.933; P<.001). In patients with AD, we observed a sizeable inverse correlation between CDR and the MMSE (r=−0.560; P<.001) and ACE-R (r=−0.434; P=.002), as well as between the CDR and DAD (r=0.723; P<.001). The comparative analysis between symptom progression time and severity on the FTD-FRS did not reveal differences among cases with AD (F=2.8; df=2; P=.102), unlike in cases with FTD. This finding reflects the longer time to diagnosis in FTD.
DiscussionThe aim of this study was to adapt and validate a Spanish-language version of the FTD-FRS. The results obtained show that the Spanish version is equivalent to the original English version and applicable in our setting, and that it classifies stages of FTD severity correctly. This scale helps identify mild cases in the initial stage, and also differentiate between severe, very severe, or profound cases. Both ends of the scale provide information of great clinical relevance, which is favourable to both early diagnosis and to adapting guidelines for carers and palliative treatments during the most advanced stages.28,29
Frontotemporal dementia is more prevalent in young patients (<65 years), but paradoxically, it shows a longer diagnostic delay compared to other forms of dementia; patients often have to consult multiple healthcare units to receive a diagnosis.30 One of the difficulties of performing the first assessment in these patients is that many of the instruments developed for a quick screening and/or classification of dementia severity, such as the CDR or the Reisberg's Global Deterioration Scale, do not consider the typical features of this disease, such as changes in social behaviour or habits. However, the CDR and the Geriatric Deterioration Scale are both commonly used to follow up on FTD cases and classify them in terms of severity. Although several adaptations have been made, assessments carried out with poorly suited instruments may pose various disadvantages for both patients and carers.31,32
For example, scores on the FTD-FRS showed a good level of correlation with the functional disability scale, but also a moderate inverse correlation with CDR scores. We also observed differences in the way stages are stratified, such that CDR usually classified patients as having milder dementia than did the FTD-FRS (Fig. 3). Assessment of severity should take into account the issues of functional disability specific to each disease.
Despite the advances made in characterising FTD, diagnosis remains closely linked to clinical symptoms and previous studies have shown that the Neary et al. criteria display low sensitivity for cases in milder stages.33 Our study did not include patients in extreme stages, and this is one of its main limitations. Detecting FTD in young adults with behavioural disturbances or insidious mood changes is not always easy, as is shown by the longer progression times in FTD than in AD.17 In many cases, they are only diagnosed after consulting with multiple primary care or psychiatric units.30,34,35 The lack of profound cases in our study may be the result of assessing cases in an outpatient environment.
This validation process returned some differences with respect to the original study, and these should also be regarded as limitations. The instrument was originally validated in patients with behavioural disorders, progressive nonfluent aphasia, and semantic dementia. In our study, we analysed only those cases with behavioural presentation, which is the most prevalent in the clinical setting.31 Future studies will enable expansion of the validation process by means of an inter-rater reliability test and a re-test test. We should also stress that this translated Spanish-language version might not be directly applicable to all Spanish-speaking populations worldwide. It would therefore be interesting to expand the validation of this scale in the future, and adapt it to the linguistic characteristics of other Spanish-speaking communities.
This study provides clinicians and researchers with a valid instrument with which to classify and follow up on patients diagnosed with FTD. The drafting of a severity scale adapted to the disorders typical of FTD may facilitate early identification of these patients and reduce delays between symptom onset and symptom detection. It would also favour both proper selection and timely use of drug treatments, and promote providing carers with up-to-date information and advice. The FTD-FRS is a tool able to improve clinical attention to patients (and their family members), whether in the initial or terminal stages of FTD.
FundingThis study was partially financed by the Agrupació de Ciències Mèdiques de Girona (second prize in the 2012 grant awards) and TV3's Fundació Marató (20143810).
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the ReDeGi members Margarita Flaqué, Marta Hernández, Glòria Mas, Marta Linares, and Isabel Casas for their contributions to the study.
Please cite this article as: Turró-Garriga O, Hermoso Contreras C, Olives Cladera J, Mioshi E, Pelegrín Valero C, Olivera Pueyo J, et al. Adaptación y validación de la Frontotemporal Dementia Rating Scale (FTD-FRS) al castellano. Neurología. 2017;32:290–299.