Carboplatin is a chemotherapeutic agent used for the treatment of lung, head and neck, kidney, ovarian, and breast cancer; it is better tolerated than cisplatin and is associated with a low incidence of non-haematological complications. The most frequent adverse reactions result from myelosuppression: anaemia, thrombocytopaenia, and neutropaenia. Neurotoxicity is usually associated with peripheral neuropathy and ototoxicity; ocular toxicity, a frequent adverse reaction to cisplatin, is rare in patients treated with carboplatin. Adverse reactions also depend on the route of administration1; intracarotid administration may cause palpebral oedema and erythema, ptosis, conjunctival hyperaemia, chemosis and corneal oedema of the eye ipsilateral to the injection, acute glaucoma, optic neuropathy, macular pigment changes, and exudative retinal detachment.2–5 The literature also reports cases of orbital pseudotumour ipsilateral to the site of intracarotid infusion of carboplatin in patients with brain tumours.6 Adverse reactions to intravenous carboplatin, as administered in the case presented here, include cortical blindness, eye pain, blurred vision, chorioretinitis, and optic neuritis.
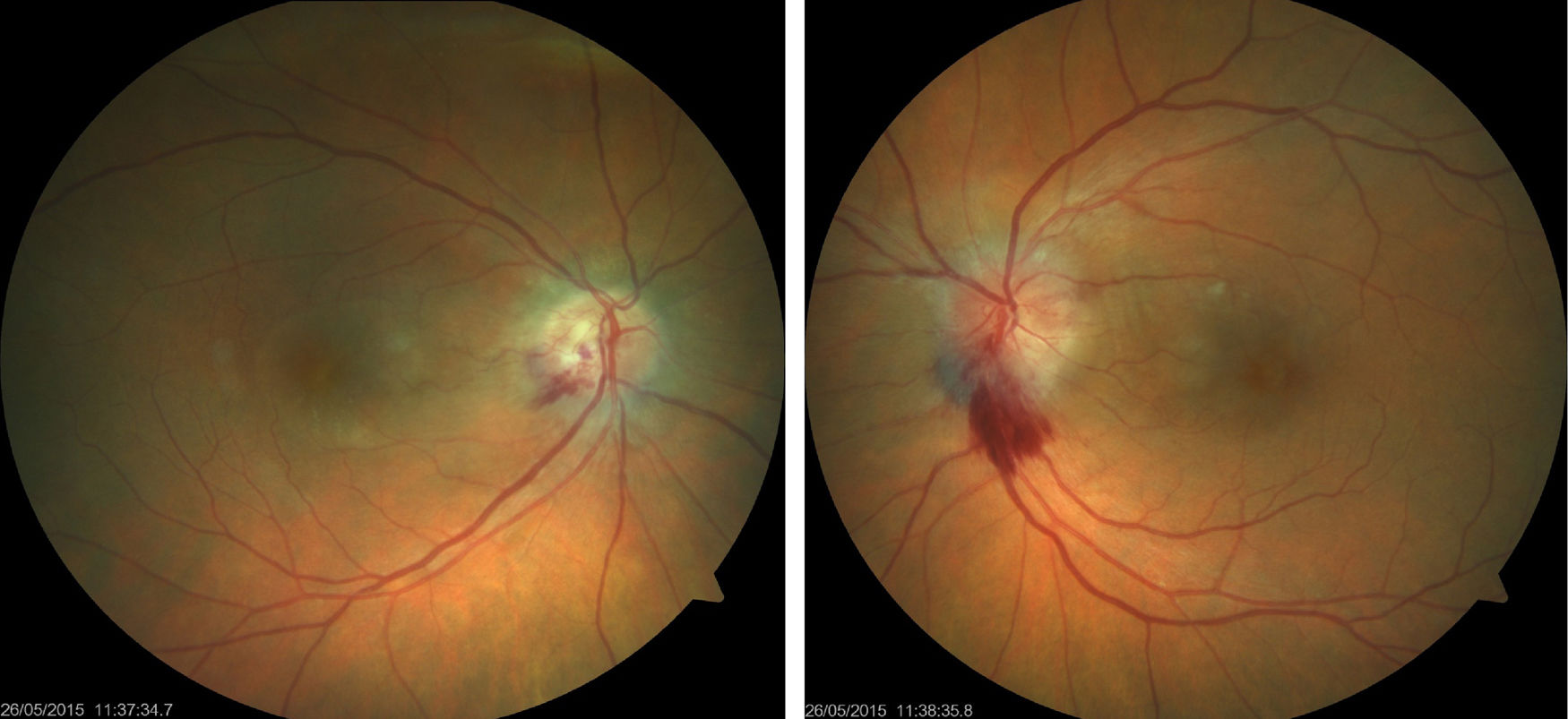
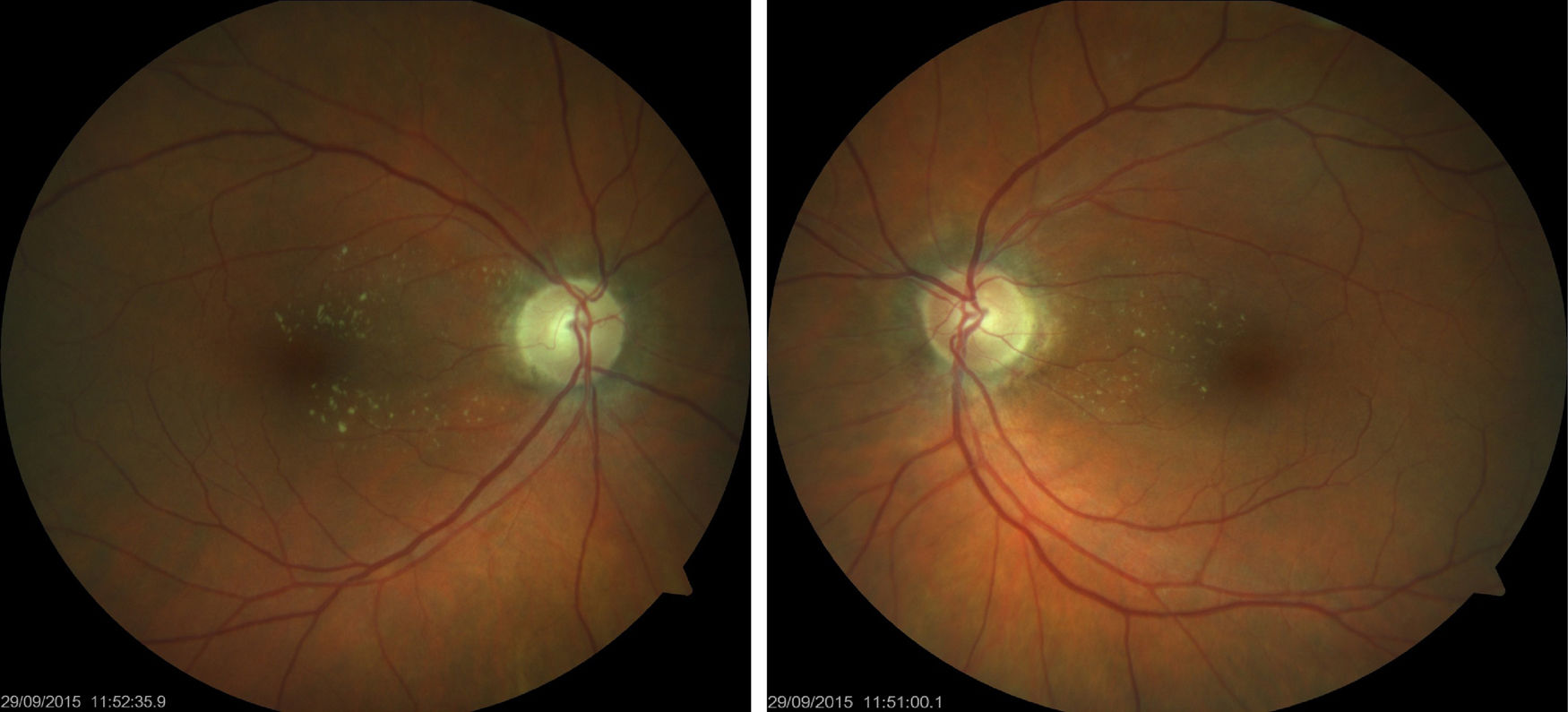
We present the case of a 61-year-old woman diagnosed with invasive ductal breast carcinoma. She had received chemotherapy and radiotherapy (doxorubicin-cyclophosphamide, capecitabine-bevacizumab); an initially good response was followed by tumour progression and metastasis. She was finally treated with 4 cycles of carboplatin at standard doses (carboplatin area under the curve of 6 every 21 days), showing good tolerance with the exception of anaemia and thrombocytopaenia. She was referred to the neuro-ophthalmology department due to a one-month history of vision loss in the right eye, coinciding with the end of the last cycle of treatment. Visual acuity was 0.5 in the right eye and 0.8 in the left. Biomicroscopy revealed no abnormalities; intraocular pressure was normal bilaterally. The eye fundus examination revealed bilateral papilloedema with a haemorrhagic component, predominantly in the left eye (Fig. 1). The 24-2 Humphrey visual field test revealed inferior field loss, which was deeper in the right eye. Optical coherence tomography (Heidelberg Engineering Inc., Heidelberg, Germany) showed increased nerve fibre layer thickness, especially in the left eye, and increased macular thickness in both eyes, predominantly in the right. Complementary test results were normal (lumbar puncture, complete blood count, serology study). MRI showed residual punctiform lesions from previously treated brain metastases, radiotherapy-induced leukoencephalopathy, and chronic thrombosis of the right transverse and sigmoid venous sinuses, which had been observed previously and showed no changes from previous MRI studies; we ruled out differential diagnosis of bilateral tumoural infiltration of the optic nerve and primary or secondary intracranial hypertension. The oncologist discontinued carboplatin; papilloedema disappeared progressively over the following 4 months (Fig. 2), leaving papillary pallor in the right eye and atrophy in the posterior pole of both eyes. The patient displayed visual acuity of 0.8 in the right eye and 1.0 in the left; the 24-2 Humphrey visual field test showed mild improvements in the depth and extension of the inferior field defects. Optical coherence tomography revealed decreased nerve fibre layer thickness in the superior quadrant of both eyes and improvements in macular thickness in the right eye.
The literature includes few cases of papilloedema secondary to carboplatin; the cases reported are usually associated with high-dose treatment and kidney failure. In 1992, O’Brien et al.7 described 2 cases of cortical blindness in patients with ovarian cancer treated with high-dose carboplatin (720 and 900mg, respectively). According to the authors, CNS toxicity may result from carboplatin crossing the blood–brain barrier combined with impaired renal excretion. Until that time, pharmaceutical companies marketing carboplatin had reported only 10 cases of ocular toxicity (eye pain, blurred vision, chorioretinitis, optic neuritis, and eye alterations), but no cases of cortical blindness had been described. Both cases showed nearly complete recovery.
In 1993, Rankin and Pitts8 presented the cases of 2 patients with ovarian cancer treated with carboplatin who developed maculopathy (one also developed bilateral optic atrophy), which may suggest previous papilloedema. The patients recovered partially; improvements were more marked in the eyes with no macular involvement. In a 1997 case report, Caraceni et al.9 presented the case of a patient receiving cisplatin and carboplatin for ovarian cancer who developed papilloedema 13 weeks after completing the last cycle. She did not recover until a year later, displaying partial recovery in the right eye and complete recovery in the left. The authors attributed the adverse effects to the cisplatin. A more recent article by Fischer et al.10 describes a case of bilateral papilloedema after 4 cycles of carboplatin at standard doses. Papilloedema had resolved fully 2 years after treatment was completed, but the patient was left with optic nerve fibre atrophy. She received empirical treatment with systemic corticosteroids.
In 2014, Lewis et al.11 published the case of a patient with high-grade endometrioid ovarian cancer who developed unilateral papilloedema with bilateral intense late disc leakage after 4 cycles of carboplatin. The patient was receiving standard doses and had normal renal function. Five months after treatment discontinuation, she developed left optic disc atrophy and decreased visual acuity, while the contralateral eye remained asymptomatic.
Few cases of carboplatin-induced CNS toxicity have been described; most published cases are attributed to cisplatin.12 Most patients had impaired renal function or poorly controlled arterial hypertension, or were receiving high doses of the chemotherapeutic drugs. This is not always the case, however, as shown by the article by Lewis et al.11 and our own patient. The toxicity mechanisms of carboplatin are poorly understood; however, toxicity seems to be due to carboplatin accumulation in the CSF after crossing the blood-brain barrier. Damage to the optic nerve and other structures may be associated with mechanisms of cell death: alkylation of nucleic acids, double-strand breaks in DNA, and failed DNA replication and RNA transcription.11 Patients may recover months to years after treatment discontinuation; sequelae include nerve fibre layer atrophy. No treatment has been demonstrated to be effective, although oral corticosteroids are used empirically in some cases.
In conclusion, optic nerve lesions should be suspected in patients receiving carboplatin and reporting decreased visual acuity or any other visual symptom. Symptoms resolve with carboplatin discontinuation, although patients may be left with sequelae secondary to optic nerve atrophy.
Please cite this article as: Santos-Bueso E, Muñoz-Hernández AM, Porta-Etessam J, Benítez-del-Castillo JM. Edema de papila bilateral hemorrágico secundario a carboplatino. Neurología. 2019;34:614–616.