Intrauterine infection due to cytomegalovirus is the most common of the intrauterine viral/parasitic infections that affect the central nervous system (CNS) and cause permanent lesions in the cortex as well as the subcortical white matter. Studies using brain magnetic resonance imaging (MRI) are limited.
Material and methodsSix patients (4 females and 2 males) were studied in the first months of life in order to make a diagnosis of congenital cytomegalovirus, and identify the cortical and subcortical lesions using the necessary MRI sequences.
ResultsThe six patients showed malformations of cortical development (MCD) (schizencephaly, polymicrogyria or lissencephaly-pachygyria) from the neonatal period, and diffuse changes of the white matter, which remained with few changes during the first two years. They then began reducing in size in the form of high signal areas in T2, restricted to certain areas, and were evident for a few years more with little change.
ConclusionIntrauterine infection due to cytomegalovirus causes changes in the cortical grey matter, which consists of MCD, and in the subcortical white matter. The latter show a changing aspect as they appear as diffuse and wide areas of high signal intensity, which is usually due to delay in myelinisation, but could also be caused directly by the cytomegalovirus. These changes in the white matter are subjected to morphological changes throughout the first years of life, leading to brain atrophy. The neurological sequelae of these lesions left by these alterations are severe and chronic.
La infección intrauterina por citomegalovirus es la más frecuente de las viriasis/parasitosis intrauterinas que afectan al sistema nervioso central y causan lesiones permanentes tanto en el córtex como en la sustancia blanca subcortical. Son escasos los estudios de resonancia magnética (RM) cerebral.
Material y métodosSeis pacientes (4 M y 2 V) fueron estudiados desde los primeros meses de vida para hacer el diagnóstico de citomegalia congénita e identificar la presencia de lesiones corticales y subcorticales, utilizando las necesarias secuencias de RM.
ResultadosLos 6 pacientes mostraban malformaciones del desarrollo cortical (MDC) (esquisencefalia, polimicrogiria o lisencefalia-paquigiria) desde la época neonatal y alteraciones difusas de la sustancia blanca, que se mantuvieron con pocos cambios durante los dos primeros años y después se iban reduciendo de tamaño en forma de zonas de hiperseñal en T2, circunscritas a determinadas áreas y permanecían con pocos cambios durante algunos años más.
ConclusiónLa infección intrauterina por citomegalovirus causa lesiones en sustancia gris cortical, que consisten en MCD y en sustancia blanca subcortical. Estas últimas muestran aspecto cambiante, ya que aparecen como áreas difusas y amplias de hiperseñal, que se suelen interpretar como retraso en la mielinización, pero que también pueden ser causadas directamente por el virus de la citomegalia. Estas alteraciones de la sustancia blanca sufren cambios morfológicos a lo largo de los primeros años de vida, dejando atrofia cerebral. Las secuelas neurológicas que dejan estas alteraciones son severas y crónicas.
Malformations of cortical development (MCDs) are classified within a large group of neuronal migration and cortical organisation disorders. The most well-known entities of this type are schizencephaly, lissencephaly-pachygyria, polymicrogyria, cortical dysplasia, and heterotopia.1 Interest in these entities resides in the fact that each one is linked to neurological problems, which mainly include epileptic seizures, mental retardation, and motor or sensory alterations. MCDs arise from changes in neuronal migration, cortical organisation, and the histological structure of neurons due to disturbances in the normal differentiation processes for neurons and glial cells in the ependymal germinal matrices of the lateral ventricles.2,3 The formative process is followed by the processes by which brain cells undergo differentiation, migrate, and take up their correct locations as directed by specific genes.4,5 Some genes are more important than others; the reelin gene directs the process.4 However, the aetiologies of some types of MCDs cannot always be identified, and linking them to a specific gene is harder still. According to some authors, early onset exogenous lesions, such as hypoxia, infections during gestation, or perinatal trauma, may play a part in the development of certain types of MCDs.6–9 In an experimental model, cortical dysplasia was also induced in rats with altered neuronal morphology and cortical development caused by exposure to radiation.10
Cytomegaly is an enlargement of cells, particularly one caused by cytomegalovirus. This virus is the most common cause of intrauterine and perinatal viral infections in the world, and affects more than 40000 children yearly in the United States alone.11,12 The TORCH infections (toxoplasmosis, rubella, cytomegalovirus, and herpes) have not been listed among the typical intrauterine and perinatal infections for many years now. Differential diagnosis is used to rule them out, especially through use of analytical markers, which are mostly haematological, immunological, or biochemical. Even so, the term is still commonly used among paediatricians, especially neonatal paediatricians. Although diagnostic capacities have increased with the early application of new imaging methods using intrauterine techniques, such as computed tomography (CT) and MRI,11–14 many infants continue to be born with the sequelae of intrauterine cytomegalovirus. Their clinical symptoms include jaundice, thrombocytopenia, hepatomegaly, petechiae, purpura, and splenomegaly. Nearly half of all cases present complications of the CNS including microcephaly, uveitis, sensory hearing loss, intracranial calcifications, delayed psychomotor development, and seizures.15 It seems that only minor advances in treatment have been made. The vaccine which inspired so much hope more than 30 years ago now delivers better results than it once did, and is used in preventing mother-to-child transmission.16 However, gains from this treatment are still a topic for debate.17
Our study contained 6 patients whose mothers were infected with cytomegalovirus during pregnancy, causing MCD (schizencephaly, lissencephaly-pachygyria and polymicrogyria), in addition to changes in white matter signals accompanied by severe neurological symptoms in the fetuses. Patients therefore underwent neurological and radiological study after birth, and were subsequently treated.
Materials and methodsThe group of 6 patients examined by the paediatric neurology department at Hospital Universitario La Paz contained 4 females and 2 males. Age at time of examination was neonate (NN) to 9 months (mean age 3 months); patients were seen due to convulsions, microcephaly, spasticity, delayed psychomotor development, and deafness. All of the patients’ mothers had given birth for the first or second time and more than 50% had experienced fever, abdominal pain, or high erythrocyte sedimentation rate during pregnancy. All births were normal and full-term. Apgar scores at 1min were 9/10; infants weighed between 2700 and 2850g and had head circumferences of 32 to 33cm. One patient presented with a bilateral ear infection which required drainage until it resolved a few months later. In addition to analytical tests run to determine the link between the syndrome and infection with cytomegalovirus, postnatal MR studies were performed in 5 patients, using different sequences and cortical surface reconstruction. We performed these studies because lesions caused by the virus often lead to polymicrogyria, pachygyria, and schizencephaly. The only imaging study performed in one case was CT.
The MR studies performed on the mothers of 2 patients showed no abnormalities.
ResultsIncreases in head circumference were below the level of P<−2 SD. Among the 3 patients who do not have schizencephaly and are still seen periodically, this remained the case at the time of the last check-up, when these patients were between 8 and 12 years old. The other 3 patients presented very severe delays in psychomotor development, and all were lost to follow-up during the first year of life due to the family's abandoning treatment; during that time, patients failed to consciously interact with their environment and were obviously unable to speak or sit up. Delays in reaching all psychomotor milestones were also significant in the three patients without schizencephaly. They began walking and talking very late; 2 demonstrate very basic walking and language ability, which was achieved through a regimen of speech therapy and physical therapy with intramuscular botulinum toxin injections to combat the patients’ spasticity. One is still unable to walk without assistance due to spasticity, which causes hip subluxation (case 4).
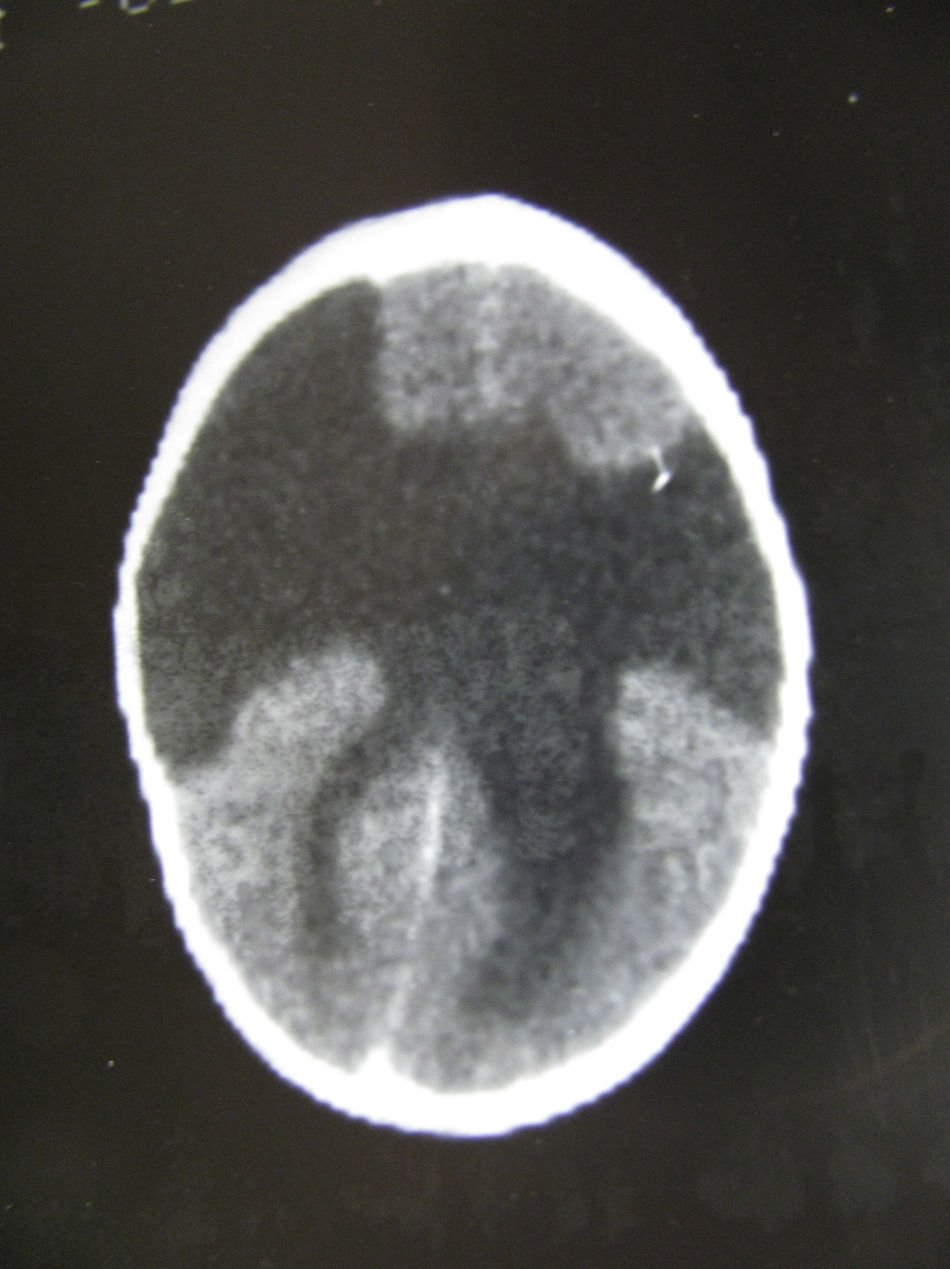
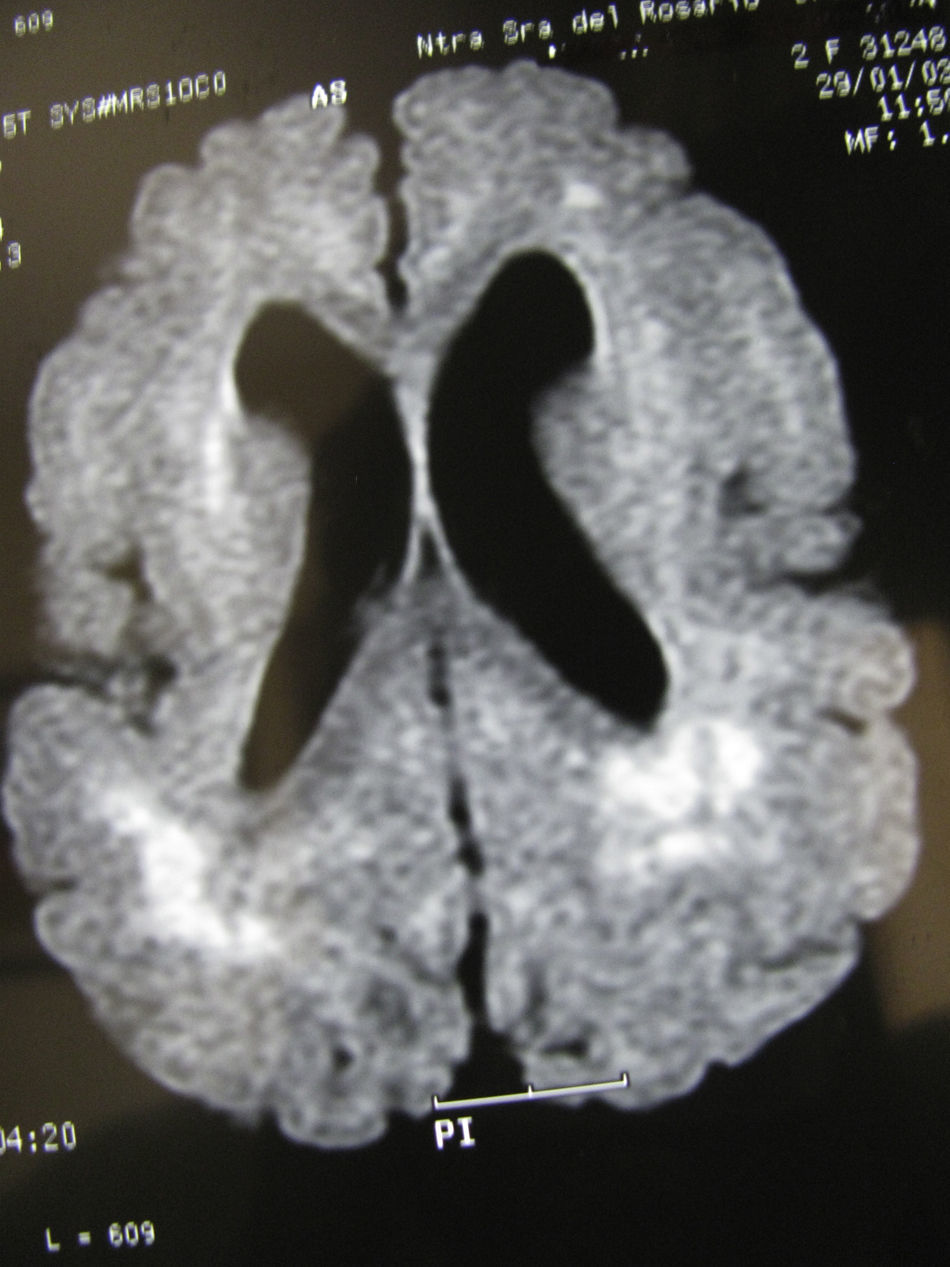
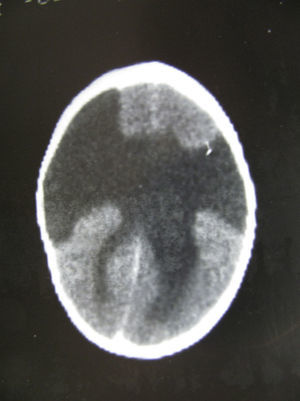
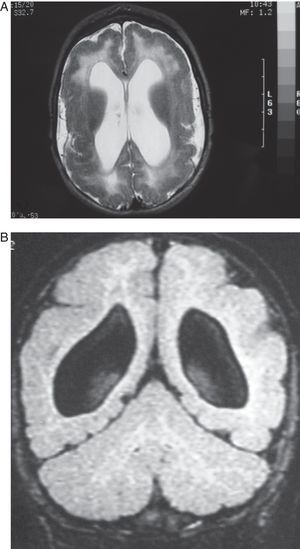
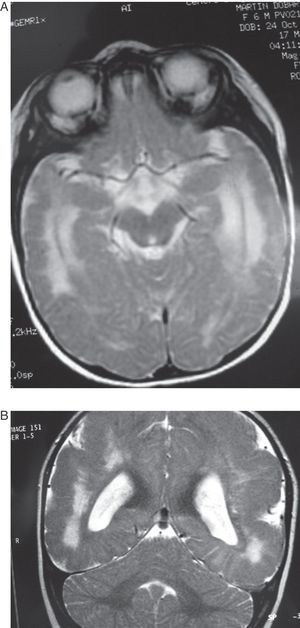
In this case (the only case in which a CT study was performed, Fig. 1), both the CT and complete MR studies showed significant alterations in both subcortical white matter and subcortical grey matter. Bilateral open-lipped schizencephaly (BOLS) was present in cases 1 and 2, with unilateral schizencephaly in case 3. Cases 4, 5, and 6 displayed polymicrogyria with areas of pachygyria in both hemispheres. These alterations were observed in all of the projections or slices (axial, coronal, and sagittal) (Fig. 2) in all sequences taken in cases 4, 5, and 6. There were no differences between the alterations recorded during the first year of life and those at 6 to 10 years. This stabilisation process for cortical lesions did not occur in white matter lesions. T2-weighted hyperintense MR images of white matter lesions evolved during the early years at the very least; in later years, an abnormality was still apparent and the signal was not completely normal (Figs. 3–5). These T2-weighted hyperintense areas became more isolated, but they did continue to appear for a few years. These areas were interpreted as a possible manifestation of delayed myelination. However, we cannot rule out a chronic inflammatory reaction due to attack by cytomegalovirus, since infection is followed by neurological sequelae that affect nearly all CNS functions.
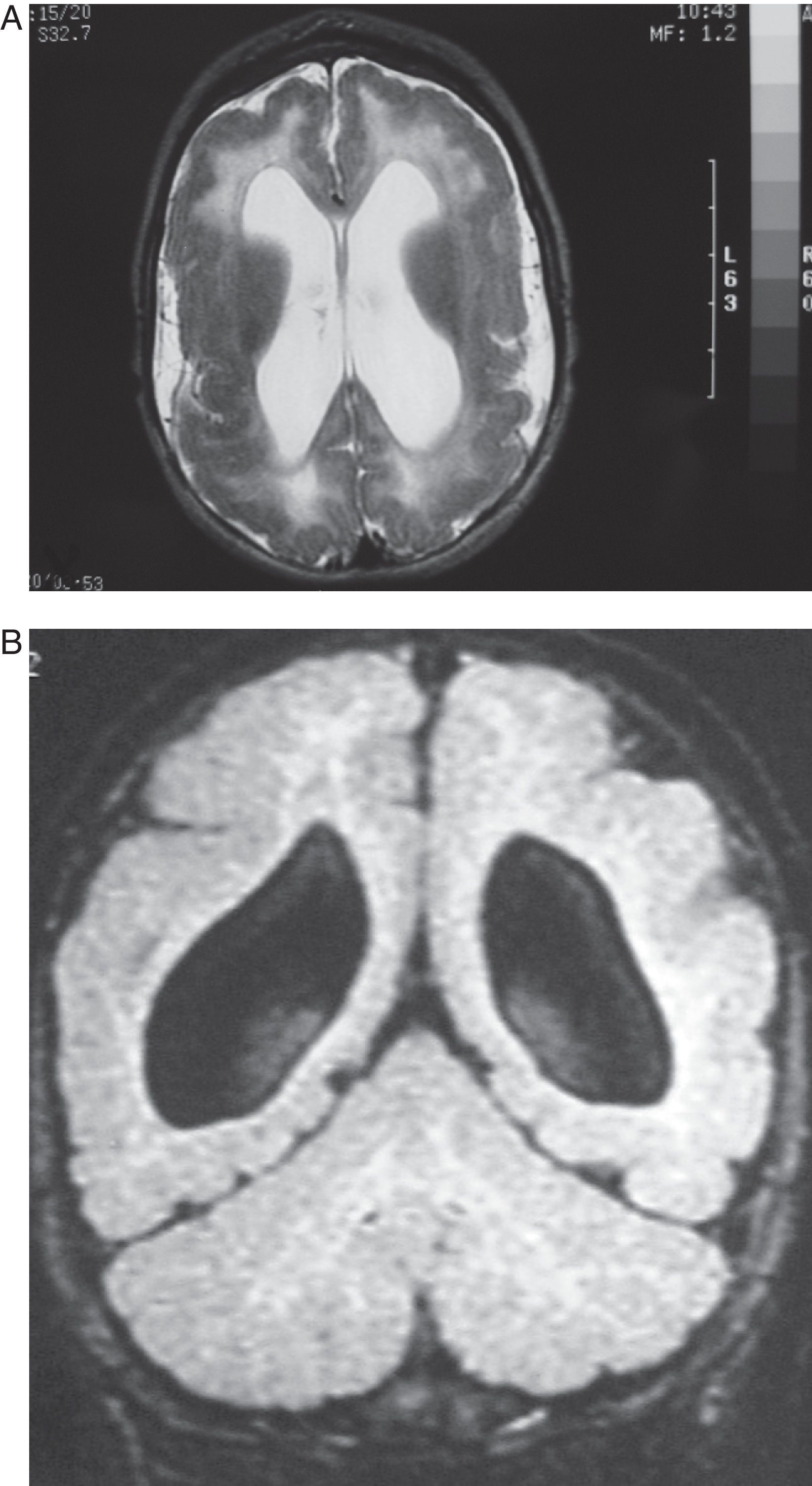
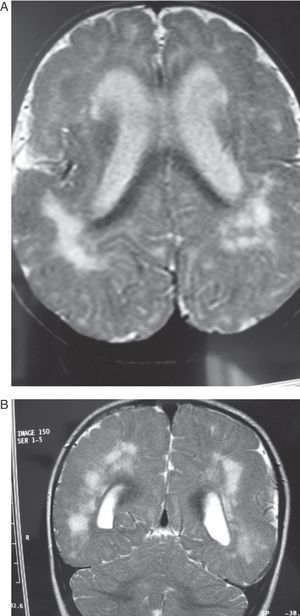
Case 4: Patient aged 5 months with intrauterine cytomegalovirus. MR study. (A) the T2-weighted axial slice shows pachygyria- and polymicrogyria-type cortical malformations, dilation of lateral ventricles, and diffuse hyperintense image in the white matter in both hemispheres. (B) T1-weighted coronal section of the same study, showing few gyri, the considerable width of the cortical grey matter (polymicrogyria) and substantial passive dilation and roundness of lateral ventricles.
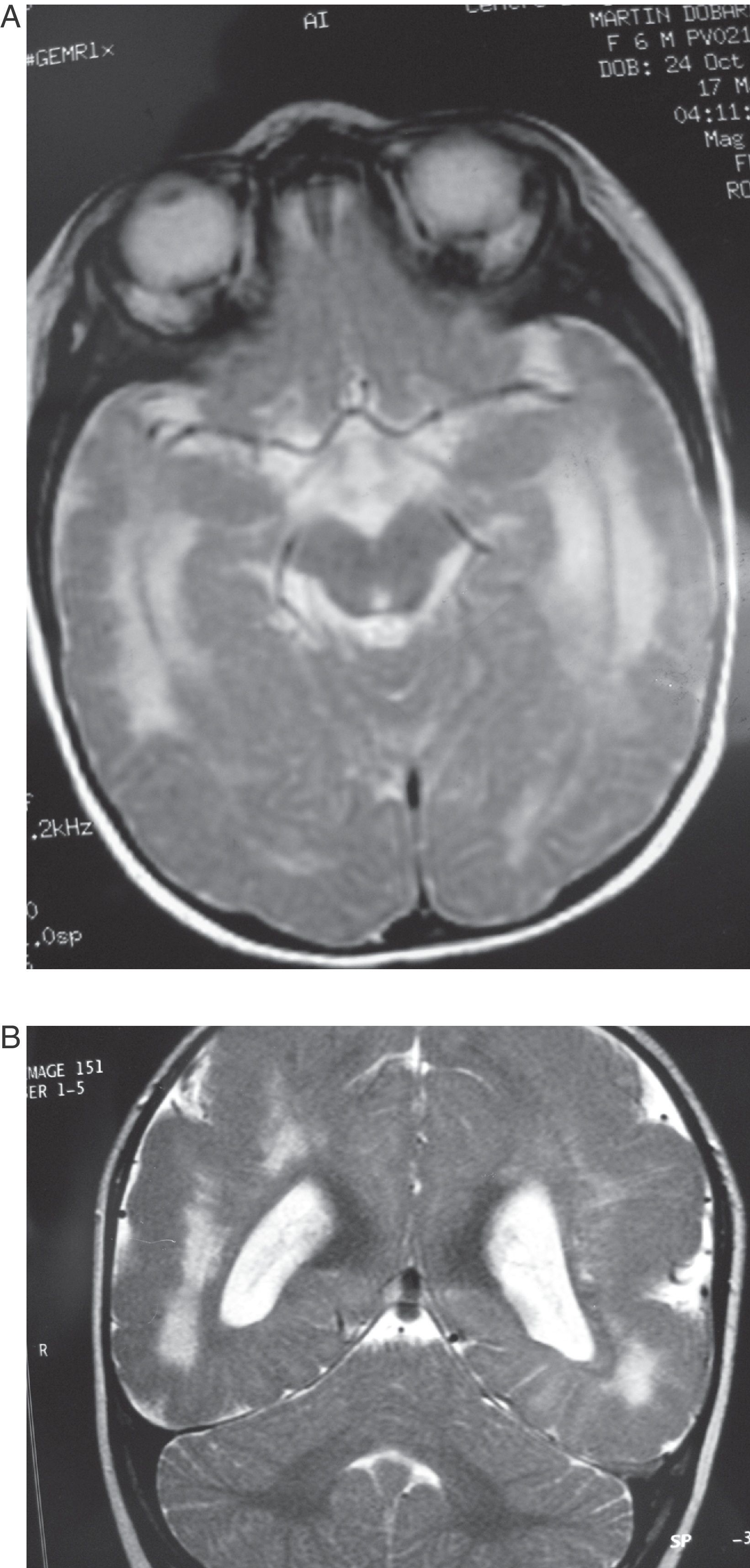
Case 5: Patient aged 6 months. (A) MR axial section at level of the base of the brain showing a wide hyperintense zone in the white matter on both hemispheres. (B) T2-weighted coronal section of the same study. Note the cortical abnormalities and diffuse hyperintensity in the white matter, predominantly in the posterior and anterior areas of the brain.
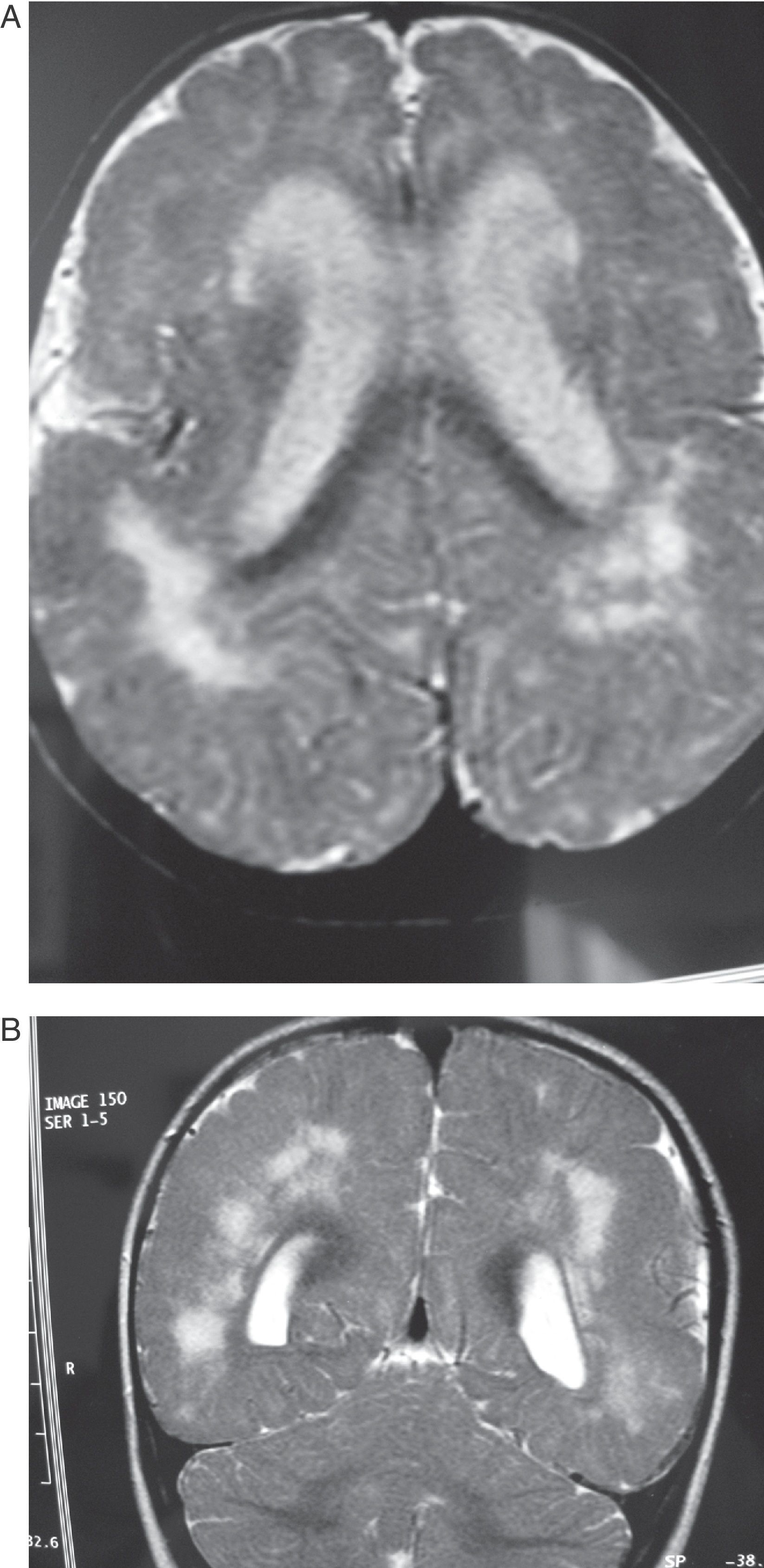
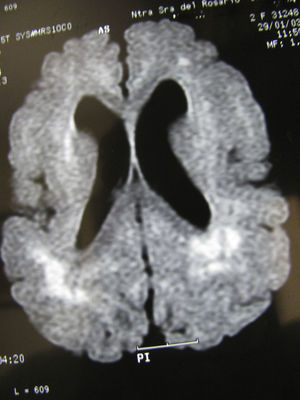
Same patient as in Fig. 3 at the age of one year. (A) The axial slice shows the same cortical and subcortical alterations that were visible at 6 months, but hyperintensity of the white matter lesions has decreased. (B) The coronal slice also shows alterations in the white matter similar to those seen at 6 months.
Same patient as in Figs. 3 and 4 at age two and a half. The axial section of the T1-weighted MR image shows cortical alterations similar to those observed in the patient at 6 months and 1 year; the hyperintense zones are more concentrated within a number of areas, especially in posterior areas.
Images of the ventricles in patients with schizencephaly were very different; they were extremely dilated, with hemispheric schizencephalic clefts in BOLS cases (1 and 2) or unilateral clefts (case 3). The septum pellucidum and the corpus callosum were absent in all 3 of these cases. The polymicrogyric edges of the schizencephalic clefts do not show up clearly in any of the images from these patients. One patient underwent study with CT only; in the others, MR imaging was not 3D, which is the only method for capturing a well-defined image of this anomaly. In the 3 patients with lissencephaly-pachygyria or polymicrogyria, the lateral ventricles appear dilated and rounded. This is probably due to white matter atrophy.
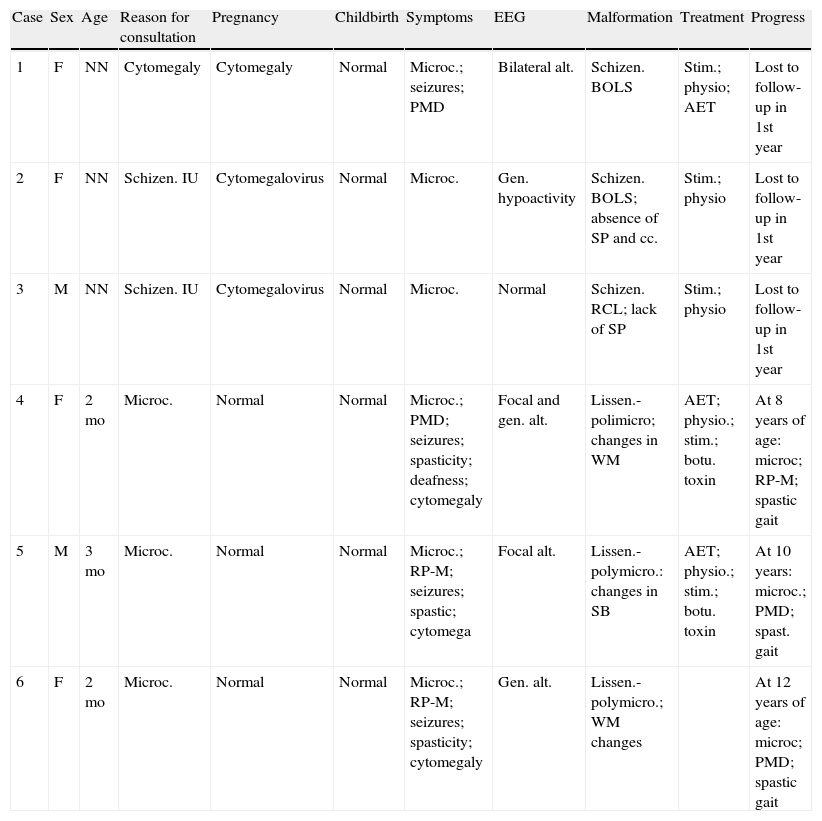
EEGs repeatedly showed some kind of focal alteration whose appearance varied depending on whether or not seizures were controlled at the time. Seizure control was achieved with pharmacological treatment in the 3 patients (cases 4–6) who were monitored over a number of years. The most relevant clinical, EEG, and imaging findings appear in Table 1.
Intrauterine cytomegalovirus sequelae.
| Case | Sex | Age | Reason for consultation | Pregnancy | Childbirth | Symptoms | EEG | Malformation | Treatment | Progress |
| 1 | F | NN | Cytomegaly | Cytomegaly | Normal | Microc.; seizures; PMD | Bilateral alt. | Schizen. BOLS | Stim.; physio; AET | Lost to follow-up in 1st year |
| 2 | F | NN | Schizen. IU | Cytomegalovirus | Normal | Microc. | Gen. hypoactivity | Schizen. BOLS; absence of SP and cc. | Stim.; physio | Lost to follow-up in 1st year |
| 3 | M | NN | Schizen. IU | Cytomegalovirus | Normal | Microc. | Normal | Schizen. RCL; lack of SP | Stim.; physio | Lost to follow-up in 1st year |
| 4 | F | 2mo | Microc. | Normal | Normal | Microc.; PMD; seizures; spasticity; deafness; cytomegaly | Focal and gen. alt. | Lissen.-polimicro; changes in WM | AET; physio.; stim.; botu. toxin | At 8 years of age: microc; RP-M; spastic gait |
| 5 | M | 3mo | Microc. | Normal | Normal | Microc.; RP-M; seizures; spastic; cytomega | Focal alt. | Lissen.-polymicro.: changes in SB | AET; physio.; stim.; botu. toxin | At 10 years: microc.; PMD; spast. gait |
| 6 | F | 2mo | Microc. | Normal | Normal | Microc.; RP-M; seizures; spasticity; cytomegaly | Gen. alt. | Lissen.-polymicro.; WM changes | At 12 years of age: microc; PMD; spastic gait |
AET, antiepileptic treatment; alt., alterations; botu toxin, spasticity treated with botulinum toxin; BOLS, bilateral open-lipped schizencephaly; cc., corpus callosum; F, female; gen., generalised; hypoact., hypoactivity; IU, intrauterine; lissen.-polymicro., lissencephaly-polymicrogyria; hip lux., hip luxation; m, months; M, male; microc.: microcephaly; NN, neonate; physio., physiotherapy; PMD, psychomotor delay; RCL, right-sided closed lip schizencephaly; schizen., schizencephaly; SP, septum pellucidum; spast., spastic; stim., stimulation; WM, white matter.
Intrauterine infection by cytomegalovirus is very likely the most common infection causing malformations in fetuses.11 Both the mothers and the children affected by the infection during gestation and the neonatal period showed the typical signs of the infection. These signs include hepatosplenomegaly, microcephaly, hearing disorders, uveitis, petechiae, delayed psychomotor development, and seizures.15–19 Until the advent of RM studies, which can be used to directly diagnose the main fetal brain abnormalities caused by cytomegalovirus, the imaging techniques used for diagnostic purposes were simple radiology and pneumoencephalography, which shows periventricular calcifications and microcephaly.
Unlike toxoplasmosis, another TORCH complex entity (in this case, parasitic) which creates immunity, cytomegalovirus is not eliminated after the first infection in most cases. Rather, it remains latent and may reactivate at a later time, especially in patients who are immunocompromised or pregnant. This is what occurred in 75% of the cases.20
Infection by cytomegalovirus occurs in 0.6%–0.7% of all neonates and it is the most common congenital neurological condition of infectious origin in both Spain21 and Sweden, where its prevalence is between 0.2% and 0.5%. Malformations of the cerebral cortex are its most severe sequelae.19,22
Children who show symptoms during the neonatal period are at high risk of presenting neurological sequelae at a later date. Many infants who appear to be normal at birth begin to display an array of mostly neurological abnormalities and others such as hearing disorders. These are often associated with white matter lesions, which may be extensive and appear early,23 as demonstrated by cases 4, 5, and 6 in our series.
One of the most reliable tests for detecting primary infection is the presence of IgG and IgM immunoglobulin. For a few years now, it has been possible to diagnose cytomegalovirus retrospectively by using polymerase chain reaction techniques on the DNA of blood stored on Guthrie test cards.23–25 Intrauterine detection is not difficult, but treatment provides only limited results, and the most promising current treatment consists of administering antibiotics and steroids to the mother.26 Absence of abdominal signs during gestation guarantees the survival of the affected infants. The presence of abdominal or brain signs is associated with poor prognosis, which brings up the possibility of administering intrauterine treatment to fetuses with cytomegalovirus infections.27
The presence of microcephaly and typical ocular lesions in a newborn, or delayed psychomotor development, hyperactivity, lack of motor coordination, hearing loss, and signs of cerebellar impairment in an older patient, indicate late-onset infection with intrauterine cytomegalovirus.28 MR studies of the brain lesions show, in addition to decreased brain mass and generalised ventricular dilation, changes in the grey and white matter. In the grey matter, we see changes in the shape of the sulci and gyri, with schizencephaly, lissencephaly, polymicrogyria, pachygyria, and cortical dysplasia.19,22,25,28–30 These types of cortical abnormalities depend on the moment in gestation in which the fetus was affected by cytomegalovirus.31 These abnormalities do not normally present alone, but are accompanied by similarly severe lesions in the cerebellum, such as global hypoplasia affecting the vermis and the cerebellar hemispheres.
The mechanism by which cytomegalovirus affects the cerebral parenchyma is quite controversial. Prevalent theories include affinity of the virus for germinal matrix cells and the vascular impairment which the virus causes in the fetus.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pascual-Castroviejo I, et al. Citomegalia congénita y malformaciones corticales y subcorticales. Neurología. 2012;27:336–42.