External lumbar drainage is a promising measure for the prevention of delayed aneurysmal subarachnoid haemorrhage-related ischaemic complications.
MethodsControlled studies evaluating the effects of external lumbar drainage in patients with aneurysmal subarachnoid haemorrhage were included. Primary outcomes were: new cerebral infarctions and severe disability. Secondary outcomes were: clinical deterioration due to delayed cerebral ischaemia, mortality, and the need of definitive ventricular shunting. Results were presented as pooled relative risks, with their 95% confidence intervals (95% CI).
ResultsA total of 6 controlled studies were included. Pooled relative risks were: new cerebral infarctions, 0.48 (95% CI: 0.32-0.72); severe disability, 0.5 (95% CI: 0.29-0.85); delayed cerebral ischaemia-related clinical deterioration, 0.46 (95% CI: 0.34-0.63); mortality, 0.71 (95% CI: 0.24-2.06), and need of definitive ventricular shunting, 0.80 (95% CI: 0.51-1.24). Assessment of heterogeneity only revealed statistically significant indexes for the analysis of severe disability (I2=70% and P=.01).
ConclusionExternal lumbar drainage was associated with a statistically significant decrease in the risk of delayed cerebral ischaemia-related complications (cerebral infarctions and clinical deterioration), as well as the risk of severe disability; however, it was not translated in a lower mortality. Nevertheless, it is not prudent to provide definitive recommendations at this time because of the qualitative and quantitative heterogeneity among included studies. More randomised controlled trials with more homogeneous outcomes and definitions are needed to clarify its impact in patients with aneurysmal subarachnoid haemorrhage.
El drenaje lumbar externo de líquido cefalorraquídeo es una medida promisoria para la prevención de las complicaciones de la isquemia cerebral tardía asociada a la hemorragia subaracnoidea espontánea de origen aneurismático.
MétodosSe incluyeron los estudios controlados que evaluaran los efectos del drenaje lumbar externo en pacientes con hemorragia subaracnoidea aneurismática. Los desenlaces primarios fueron: nuevos infartos cerebrales y discapacidad grave. Los desenlaces secundarios fueron: deterioro clínico causado por isquemia cerebral tardía, mortalidad y necesidad de derivación ventricular definitiva. Los resultados se presentaron en riesgos relativos combinados, con un intervalo de confianza del 95% (IC 95%).
ResultadosFueron incluidos un total de 6 estudios controlados. Los riesgos relativos combinados fueron: nuevos infartos cerebrales, 0,48 (IC 95%: 0,32-0,72); discapacidad grave, 0,5 (IC 95%: 0,29-0,85); deterioro clínico causado por isquemia cerebral tardía, 0,46 (IC 95%: 0,34-0,63); mortalidad, 0,71 (IC 95%: 0,24-2,06) y necesidad de derivación ventricular definitiva, 0,80 (IC 95%: 0,51-1,24). La evaluación de la heterogeneidad demostró índices estadísticamente significativos únicamente en el análisis de discapacidad grave (I2=70% y p=0,01).
ConclusiónEl drenaje lumbar externo se asoció con una reducción estadísticamente significativa del riesgo de complicaciones causadas por la isquemia cerebral tardía (infartos cerebrales y deterioro clínico), así como del riesgo de discapacidad grave; sin embargo, esto no se tradujo en una menor mortalidad. No obstante, no es prudente emitir recomendaciones definitivas debido a la heterogeneidad cualitativa y cuantitativa entre los estudios. Son necesarios más ensayos clínicos con definiciones homogéneas de sus desenlaces para aclarar sus efectos en los pacientes con hemorragia subaracnoidea aneurismática.
Cerebral vasospasm and delayed cerebral ischaemia (DCI) are the most frequent and severe complications of aneurysmal subarachnoid haemorrhage (ASH), occurring in between 20% and 50% of all cases.1–3 Vasospasm is characterised by a progressive reduction in arterial diameter in the circle of Willis, and it is usually observed between days 4 and 14 after symptom onset.4–6 DCI manifests clinically as neurological impairment which cannot be explained by such other conditions as hyponatraemia, hypoxaemia, infections, pulmonary oedema, drug intoxication, hydrocephalus, or rebleeding.7 Lesions secondary to DCI may cause cerebral infarctions irrespective of the development of cerebral vasospasm.8,9
A great deal of the diagnostic and therapeutic resources required for managing patients with ASH are aimed at identifying, preventing, or treating vasospasms and DCI. Several studies have explored the use of glucocorticoids, endothelin-1 antagonists, statins, magnesium sulfate, acetylsalicylic acid, hypothermia, transdermal nitroglycerin, ebselen, and thrombolytics. However, none of these treatments has been shown to be effective in clinical practice.1,10–12 In contrast, such other alternatives as oral nimodipine, haemodynamic therapy, intraarterial vasodilator therapy, and endovascular angioplasty have been proved useful for the prevention and treatment of vasospasm-related ischaemic neurological deficits. Their effectiveness, however, is limited.13
Although the pathophysiological mechanisms of vasospasm are yet to be fully understood, we currently know that breakdown products of haemoglobin play a major role in this process.14,15 Incidence of vasospasm and DCI shows a close correlation with volume, density, and persistence of clots in the subarachnoid space and ventricular cavities.4,14 In line with this idea, it has been suggested that early surgical removal of these clots may reduce the frequency and severity of vasospasms.16 Komotar et al.17 conducted a systematic review to assess the usefulness of cisternal drainage with microsurgical fenestration of the lamina terminalis, but concluded that this technique had no benefits.17
An external ventricular drain (EVD) also favours drainage of CSF containing breakdown products of haemoglobin; however, its clinical effectiveness has not been proved. In addition, this technique has been associated with an increased risk of developing posthaemorrhagic hydrocephalus.18–21
Lumbar drainage (LD) is another alternative for extracting CSF; it has lower rates of haemorrhagic, obstructive, and infectious complications than ventricular drainage.22 Its use in patients with ASH is based on evidence provided by Macdonald22 and Hänggi et al.23 These authors found that CSF drainage may reduce the incidence and potential consequences of vasospasms.
Some studies have revealed that the concentration of blood components in CSF in patients with ASH is greater when CSF is collected using an LD than with an EVD. This proves that blood products accumulate mainly in the lumbar and basal cisterns.24,25 These findings indicate that draining CSF from the lumbar cistern allows for more efficient clearance of breakdown products of haemoglobin from CSF and may therefore have detectable clinical benefits.
Some preliminary studies exploring the usefulness of LD in patients with ASH suggest that this technique reduces the risk of morbidity, mortality, and vasospasm-related ischaemic complications26; however, no meta-analyses have been conducted to evaluate LD.
MethodsWe conducted a systematic literature review following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.27
Literature searchThe online search took place in July 2013. We searched the following databases: MEDLINE/PubMed, EMBASE, Google Scholar, Cochrane Library, CINAHL, EBSCO, HINARI, and SciELO. We did not limit our search by language or dates of publication.
The search employed the following keywords: ‘subarachnoid hemorrhage’, ‘hydrocephalus’, ‘lumbar drain’, ‘external ventricular drain’, ‘cerebral infarction’, ‘stroke’, ‘vasospasm’, and ‘delayed ischaemic neurologic deficit’.
Selection criteriaInclusion criteriaType of studies:
- 1.
Observational studies or controlled clinical trials.
- 2.
Studies with a prospective or retrospective design.
Type of participants:
- 1.
Adults only (18 and older) with ASH.
- 2.
Patients who underwent aneurysm occlusion with microsurgery or endovascular treatment.
Type of intervention:
- 1.
LD placement during the acute phase of aneurysm rupture (first 7 days of bleeding).
- 2.
LD for at least 3 consecutive days.
Type of outcome assessment. We included studies that analysed any of the following parameters:
- 1.
New cerebral infarctions linked to DCI.
- 2.
Disability, assessed with either the modified Rankin Scale (mRS) or the Glasgow Outcome Scale (GOS).28–30
- 1.
Concomitant use of thrombolytics or other intrathecal drugs and LD.
- 2.
Study population of fewer than 10 patients.
- 3.
No control group.
- 4.
Subarachnoid haemorrhage caused by anything other than intracranial aneurysm rupture (trauma, surgery, vasculitis, arteriovenous malformations, cavernous malformations, venous angiomas, capillary telangiectasias, etc.).
- 5.
Aneurysms caused by trauma or infections.
- 1.
New cerebral infarctions linked to DCI.
- 2.
Severe disability.
- 1.
Clinical deterioration caused by DCI.
- 2.
Mortality.
- 3.
Need for a permanent shunt.
- -
New cerebral infarctions linked to DCI: cerebral infarctions identified in CT or MRI scans performed during the first 6 weeks after ASH, or in pathology studies in autopsies. These infarctions may not be related to surgical or endovascular procedures (microsurgical or endovacular repair and/or ventricular drain placement).
- -
Severe disability: scores of 0, 1, or 2 on the mRS, or of 4 or 5 on the GOS.
- -
Clinical deterioration caused by DCI: focal neurological signs (including hemiparesis, aphasia, apraxia, hemianopsia, or hemispatial neglect) or a decrease of at least 2 points on the GOS, after ruling out other causes of clinical deterioration (metabolic or electrolyte changes, hydrocephalus, rebleeding, complications during aneurysm repair, etc.).
- -
Mortality: death during follow-up period (regardless of the cause).
- -
Need for a permanent shunt: permanent implantation of a ventricular shunt is required to treat posthaemorrhagic hydrocephalus.
Article titles were evaluated by 2 independent reviewers who identified potentially relevant articles based on the abstracts. We subsequently gathered all the potentially relevant articles in full-text format and applied the inclusion and exclusion criteria mentioned above. The same reviewers collected data and assessed methodological quality. Discrepancies between reviewers were solved by consensus.
Data extractionWe gathered the following data: study design, sample size, method for repairing the aneurysm causing ASH, time of LD placement, duration of LD, CSF drainage speed with LD, definitions of the primary and secondary outcomes, and time elapsed from symptom onset to outcome assessment.
To assess disability, outcomes were dichotomised; we recorded the number of individuals with disability in the group treated with LD and in the control group. We used the equivalence between the mRS and the GOS established by Wong et al.31; according to these authors, favourable outcomes correspond to scores of 0, 1, or 2 on the mRS and to scores of 4 or 5 on the GOS. This equivalence is routinely used in meta-analyses evaluating the effectiveness of pharmacological interventions in patients with ASH and shows good concordance between scales.7,31–33
When the data necessary for analysis and quality assessment were not published in the full-text article, we requested this information from the main authors via e-mail. This approach to obtaining the data necessary for our analysis had to be employed in the case of only one study.34
Assessment of methodological qualityObservational studies were assessed with the Newcastle–Ottawa Scale, which is recommended by the Cochrane Non-Randomised Studies Methods Group35; randomised studies were assessed on the Jadad scale, also known as the Oxford quality scoring system.36,37
Synthesis, data analysis, and assessment of heterogeneityTherapeutic effects were analysed by estimating the relative risk (RR) using a random-effects model and 95% confidence intervals (95% CI) for all dichotomous analyses.9,33
Statistical heterogeneity was assessed using the I2 statistic and the chi-square test. For purposes of data interpretation, I2 values>50% and P<.1 in the chi-square test were considered statistically significant.38
Statistical analysis was performed using Review Manager statistical software, version 5 (The Cochrane Collaboration, Oxford, UK).
We assessed potential publication bias using funnel plots.
In addition, we conducted a subgroup analysis to determine the magnitude of the therapeutic effect according to the methodology of the studies (controlled observational studies and clinical trials).
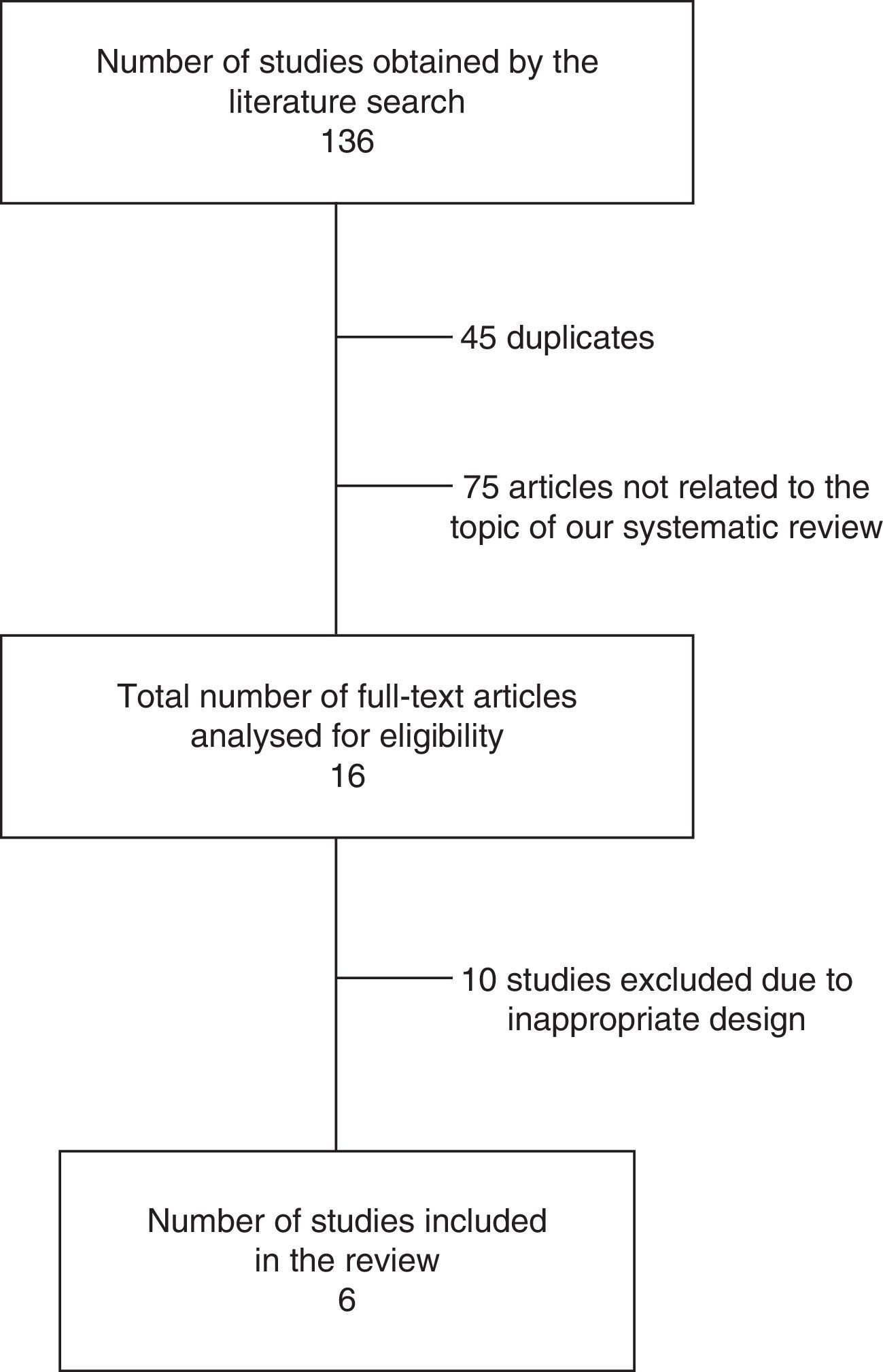
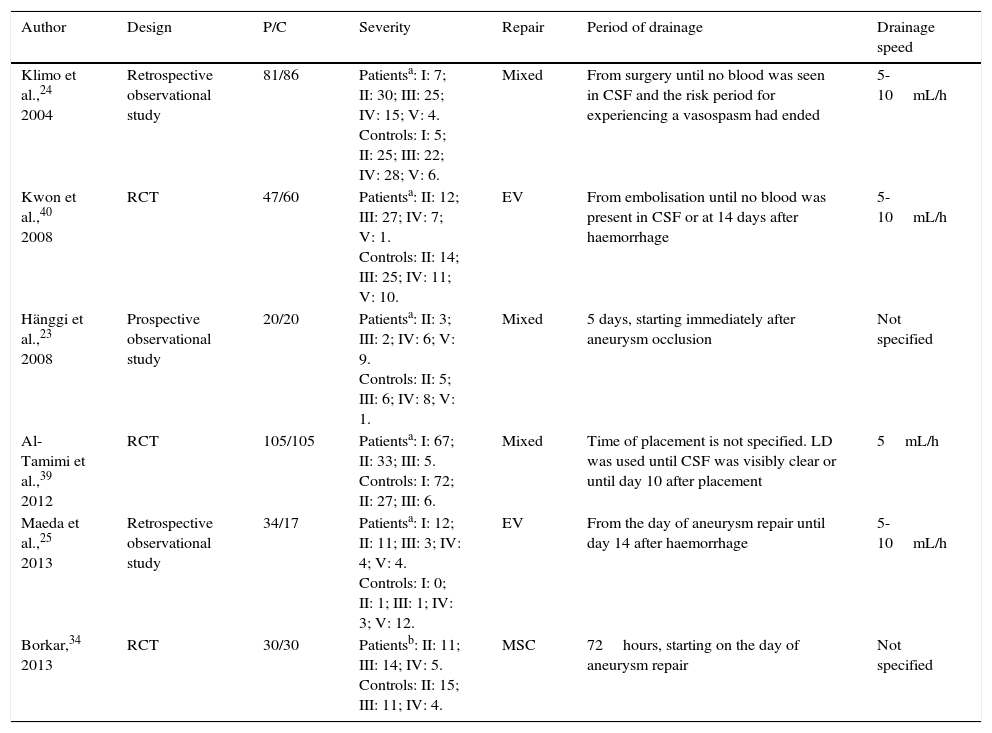
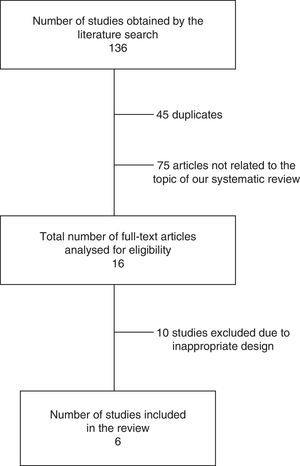
ResultsSearch resultsOur literature search identified 136 articles. After evaluating the abstract, reading the full-text article, and applying the inclusion and exclusion criteria, we selected a total of 6 controlled studies (635 individuals) evaluating at least one of the primary or secondary outcomes of our meta-analysis (Table 1). Three of these studies randomly assigned participants to treatment or control groups (randomised clinical trials).34,39,40
Characteristics of the included studies.
| Author | Design | P/C | Severity | Repair | Period of drainage | Drainage speed |
|---|---|---|---|---|---|---|
| Klimo et al.,24 2004 | Retrospective observational study | 81/86 | Patientsa: I: 7; II: 30; III: 25; IV: 15; V: 4. Controls: I: 5; II: 25; III: 22; IV: 28; V: 6. | Mixed | From surgery until no blood was seen in CSF and the risk period for experiencing a vasospasm had ended | 5-10mL/h |
| Kwon et al.,40 2008 | RCT | 47/60 | Patientsa: II: 12; III: 27; IV: 7; V: 1. Controls: II: 14; III: 25; IV: 11; V: 10. | EV | From embolisation until no blood was present in CSF or at 14 days after haemorrhage | 5-10mL/h |
| Hänggi et al.,23 2008 | Prospective observational study | 20/20 | Patientsa: II: 3; III: 2; IV: 6; V: 9. Controls: II: 5; III: 6; IV: 8; V: 1. | Mixed | 5 days, starting immediately after aneurysm occlusion | Not specified |
| Al-Tamimi et al.,39 2012 | RCT | 105/105 | Patientsa: I: 67; II: 33; III: 5. Controls: I: 72; II: 27; III: 6. | Mixed | Time of placement is not specified. LD was used until CSF was visibly clear or until day 10 after placement | 5mL/h |
| Maeda et al.,25 2013 | Retrospective observational study | 34/17 | Patientsa: I: 12; II: 11; III: 3; IV: 4; V: 4. Controls: I: 0; II: 1; III: 1; IV: 3; V: 12. | EV | From the day of aneurysm repair until day 14 after haemorrhage | 5-10mL/h |
| Borkar,34 2013 | RCT | 30/30 | Patientsb: II: 11; III: 14; IV: 5. Controls: II: 15; III: 11; IV: 4. | MSC | 72hours, starting on the day of aneurysm repair | Not specified |
C: controls; MSC: microsurgical clipping; LD: lumbar drain; P: patients; RCT: randomised controlled trial; EV: endovascular; CSF: cerebrospinal fluid.
The results of our literature review are shown in Fig. 1, and the main characteristics of the studies are summarised in Table 1.
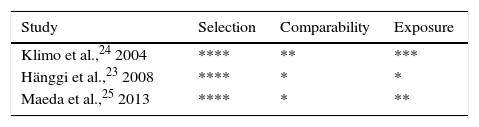
Quality of the studiesObservational studiesThe Newcastle–Ottawa Scale demonstrated that all the observational studies included appropriate definitions of patients and outcomes. Likewise, all studies included a representative sample from the population potentially able to benefit from this treatment (patients with ASH). Control groups in each of the studies consisted of series of patients who were not selected because they had a condition other than ASH. Results were assessed using blind interviews and by reviewing medical histories to ensure that data had an acceptable level of reliability. The results of each study are summarised in Table 2.
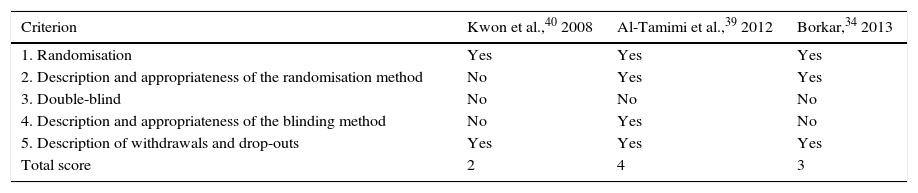
Randomised controlled trialsWe included 3 controlled studies in which patients were randomised to the study groups.34,39,40 In one of them, however, the randomisation method is not explicitly described.40 Given that LD is an intervention, patients will know whether or not they have received this treatment. As a result, none of the studies was double-blind. The study by Al-Tamimi et al.39 was the only one in which outcomes were assessed by blinded interviewers. Finally, after applying the Jadad scale, only the studies by Borkar and Al-Tamimi et al. were considered to show high methodological quality.34,39 A detailed assessment of each of these studies is shown in Table 3.
Quality assessment of the randomised controlled clinical trials included in our study, using the Jadad scale.
| Criterion | Kwon et al.,40 2008 | Al-Tamimi et al.,39 2012 | Borkar,34 2013 |
|---|---|---|---|
| 1. Randomisation | Yes | Yes | Yes |
| 2. Description and appropriateness of the randomisation method | No | Yes | Yes |
| 3. Double-blind | No | No | No |
| 4. Description and appropriateness of the blinding method | No | Yes | No |
| 5. Description of withdrawals and drop-outs | Yes | Yes | Yes |
| Total score | 2 | 4 | 3 |
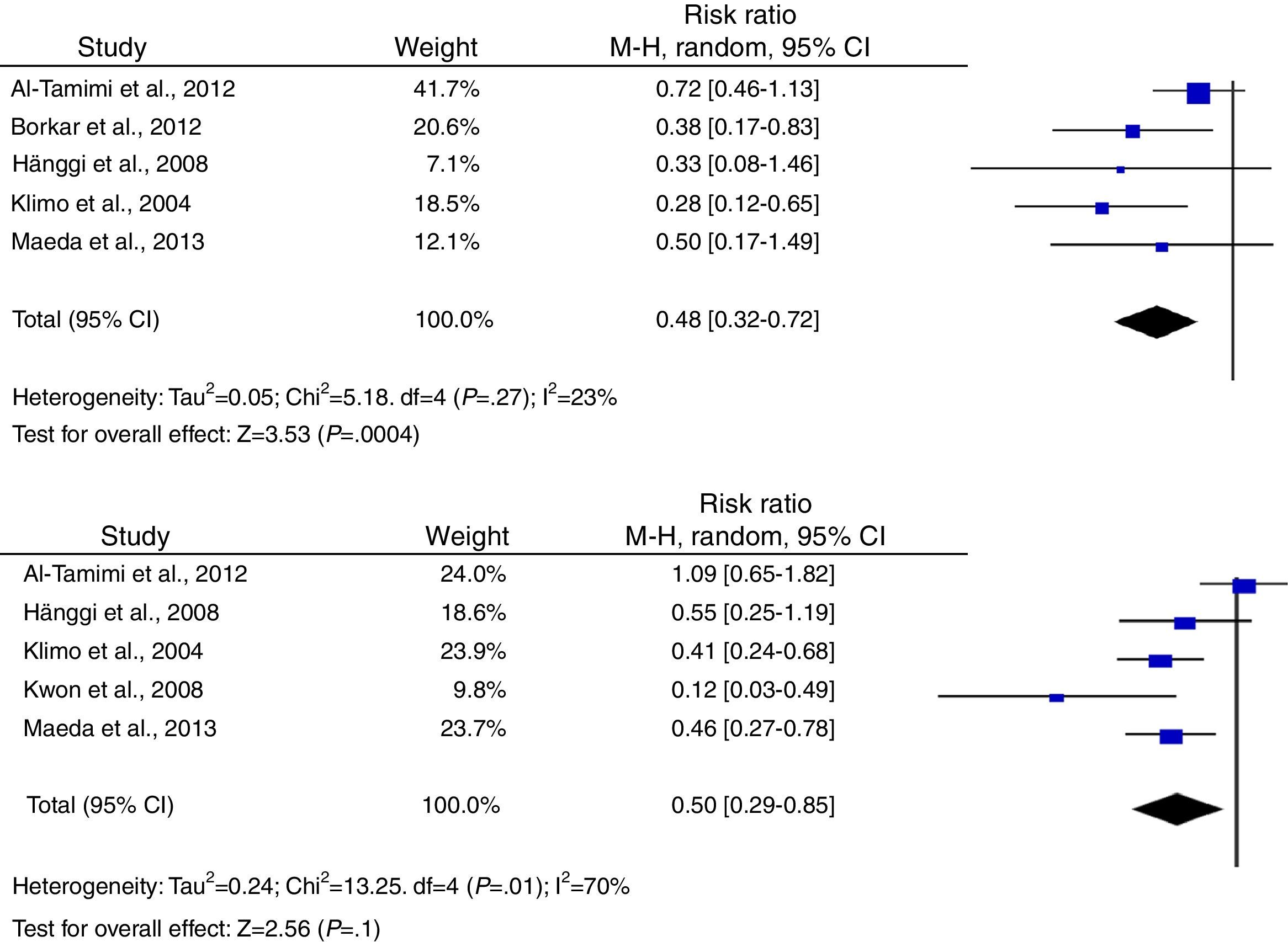
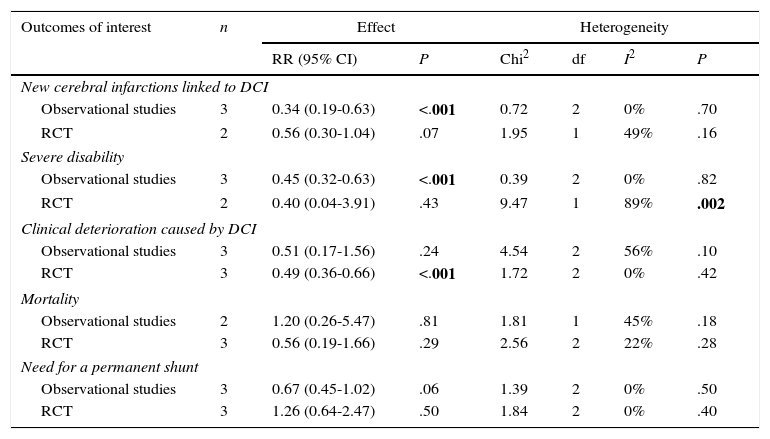
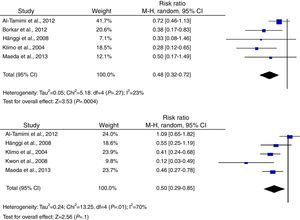
According to the analysis of primary outcomes, LD was associated with a lower risk of new cerebral infarctions visible by CT or MRI. The combined RR of the 5 studies evaluating this outcome was 0.48 (95% CI, 0.32-0.72) and met criteria for statistical significance (P<.001). Analyses revealed no statistically significant heterogeneity (I2=23%, P=.27).23–25,34,39 Five studies were included in the analysis of severe disability; combined results showed that LD is associated with a lower risk of this outcome (RR, 0.50; 95% CI, 0.29-0.85; P=.01). However, results were statistically heterogeneous (I2=70%; P=.01) (Fig. 2).23–25,34,39,40
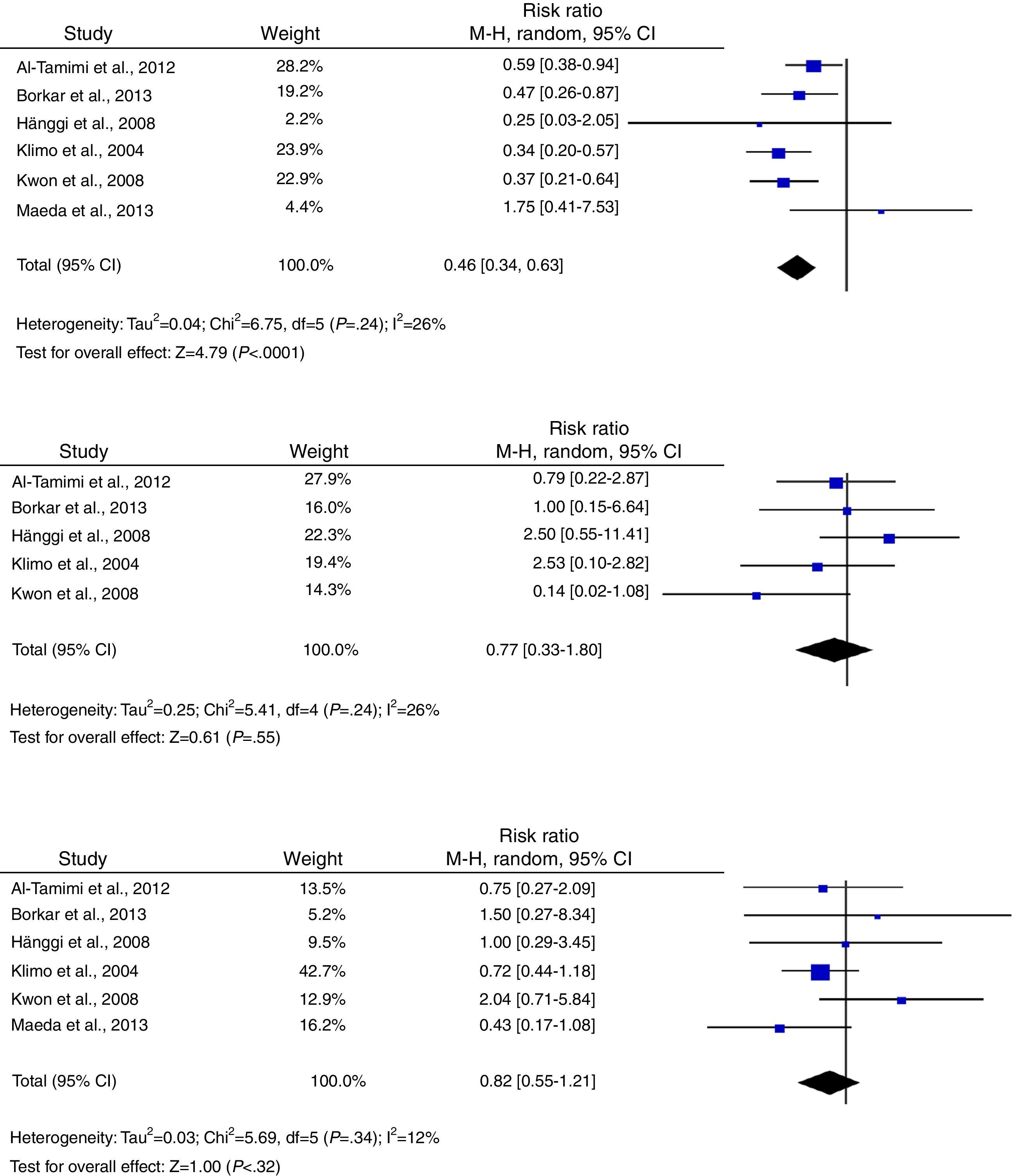
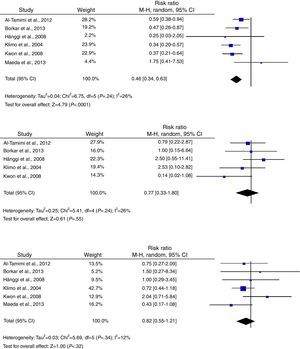
Regarding secondary outcomes, results were more homogeneous in the analyses corresponding to clinical deterioration caused by DCI, mortality, and need for a permanent shunt. The risk of clinical deterioration caused by DCI was lower in the patients treated with LD than in controls (RR, 0.46; 95% CI, 0.34-0.63; P<.001). Results were consistent among studies included in the analysis (I2=26%; P=.24).23–25,34,39,40 Five studies evaluated the effects of LD on the risk of mortality. The analysis yielded a combined RR of 0.77 (95% CI, 0.33-1.80), which demonstrates the lack of either positive or negative effects (I2=44%; P=.25).23,24,39,40 Similarly, the results of the 6 studies examining the influence of LD on the need for surgery for posthaemorrhagic hydrocephalus demonstrated that this treatment has no effect on the need for a permanent shunt (RR, 0.82; 95% CI, 0.55-1.21; P=.31) (Fig. 3).23–25,34,39,40
Analysis by subgroupsStudies were divided according to their methodology: observational studies and clinical trials were analysed separately. Estimations of the effects of this treatment and heterogeneity are shown in Table 4.
Subgroup analysis by study design.
| Outcomes of interest | n | Effect | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P | Chi2 | df | I2 | P | ||
| New cerebral infarctions linked to DCI | |||||||
| Observational studies | 3 | 0.34 (0.19-0.63) | <.001 | 0.72 | 2 | 0% | .70 |
| RCT | 2 | 0.56 (0.30-1.04) | .07 | 1.95 | 1 | 49% | .16 |
| Severe disability | |||||||
| Observational studies | 3 | 0.45 (0.32-0.63) | <.001 | 0.39 | 2 | 0% | .82 |
| RCT | 2 | 0.40 (0.04-3.91) | .43 | 9.47 | 1 | 89% | .002 |
| Clinical deterioration caused by DCI | |||||||
| Observational studies | 3 | 0.51 (0.17-1.56) | .24 | 4.54 | 2 | 56% | .10 |
| RCT | 3 | 0.49 (0.36-0.66) | <.001 | 1.72 | 2 | 0% | .42 |
| Mortality | |||||||
| Observational studies | 2 | 1.20 (0.26-5.47) | .81 | 1.81 | 1 | 45% | .18 |
| RCT | 3 | 0.56 (0.19-1.66) | .29 | 2.56 | 2 | 22% | .28 |
| Need for a permanent shunt | |||||||
| Observational studies | 3 | 0.67 (0.45-1.02) | .06 | 1.39 | 2 | 0% | .50 |
| RCT | 3 | 1.26 (0.64-2.47) | .50 | 1.84 | 2 | 0% | .40 |
RCT: randomised controlled trial; DCI: delayed cerebral ischaemia; 95% CI: 95% confidence interval; RR: relative risk.
A random-effects model was used for all the analyses. Statistically significant values are shown in bold.
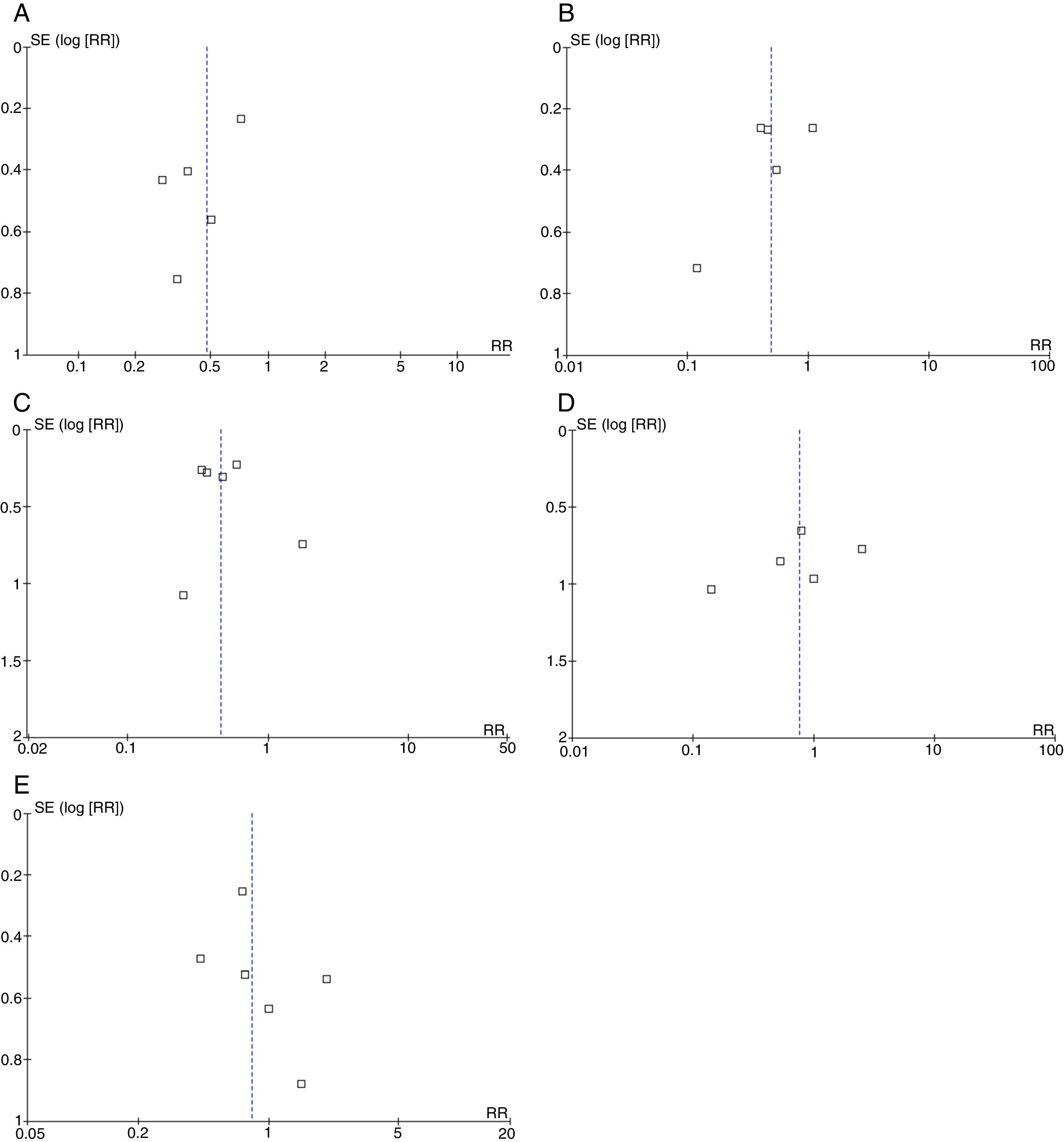
Broadly speaking, the included studies were considered to have a high risk of bias since full-text articles did not offer explanations of the methods used to assess final outcomes, especially with regard to evaluator blinding. Funnel plots showed no asymmetries potentially indicative of publication bias for any of the outcomes. However, the reliability of these analyses is low since fewer than 10 studies were available (Fig. 4) (Appendix A).35
DiscussionThe results of the present meta-analysis show that LD is useful for preventing cerebral infarctions linked to DCI; these results correspond to the combined analysis and the subgroup analysis for observational studies. However, the analysis of the 2 high-quality randomised trials showed no differences in RR, probably due to the statistical power of the data.34,39 These findings are especially relevant for patients with ASH: some studies have demonstrated a strong association between DCI and poor functional recovery in the medium and long term.41 In addition, we found a decrease in risk of severe disability associated with LD, which is another primary outcome recommended for evaluating interventions aimed at preventing vasospasm and DCI.8,9 Nevertheless, the heterogeneity of functional outcomes was statistically significant, which does not allow us to establish firm recommendations.
Despite the beneficial effects of draining CSF with an LD in patients with DCI, this technique had no statistically significant effect on the risk of mortality. Again, this finding points to a lack of association between incidence of complications linked to DCI and clinical outcomes in patients with SAH. Some authors have pointed out that this discrepancy may be due to the appearance of adverse events and complications that may counteract the benefits of certain treatments.13 This hypothesis was supported by a meta-analysis on the effectiveness of clazosentan, an endothelin receptor antagonist, which was found to be effective for preventing ischaemic neurological deficit and cerebral infarctions.13 However, mortality and disability rates in patients were no lower than in controls, since pulmonary, haemodynamic, and haematological complications associated with this drug negated its benefits.42–44
Although complications associated with LD may have had an influence on the similarities in mortality risk between groups, this hypothesis may be refuted based on several studies proving that LD has a good safety profile in patients with ASH, since it does not increase the risk of rebleeding or mortality.45–47 Brain herniation is another potential complication of LD in patients with ASH, and especially in those with intracranial hypertension. For this reason, the studies in the present meta-analysis excluded all patients with compression of the basal cisterns and those who were not good candidates for LD according to the neurosurgeon. Likewise, preliminary studies have proved the safety of LD, even in patients with intracranial hypertension, provided that an EVD is placed and an adequate pressure gradient is maintained.46 This evidence shows that complications associated with lumbar catheter placement are not sufficient to explain the lack of an effect on the risk of mortality; future research should explore other possible mechanisms.
Another potential benefit of LD is the decreased risk of posthaemorrhagic hydrocephalus: this procedure helps remove breakdown products of haemoglobin which accumulate in the subarachnoid space, blocking arachnoid villi.48,49 However, the combined analysis demonstrated that there were no differences in the risk of needing a permanent shunt for posthaemorrhagic hydrocephalus. This is probably due to the fact that a small amount of blood products may impede CSF reabsorption, and LD may be insufficient to remove these products.49
LimitationsOur study has a number of limitations inherent to its methodology and to the studies included in the meta-analysis. The most relevant limitation concerns the combined analysis of randomised and observational studies, which may result in differences between intervention groups (selection bias). Another issue is that some studies did not explicitly indicate that they had followed a strict research protocol (reporting bias). Although combined analyses of randomised and observational studies are frequently conducted in systematic reviews to increase accuracy of the results, they are not recommended by the Cochrane Non-Randomised Studies Methods Group as this may lead to bias.50 With this in mind, we conducted a subgroup analysis based on study methodology. The analysis of clinical trials showed benefits of LD limited to a reduced risk of clinical deterioration caused by DCI; this finding may indicate a bias in the results of the combined analysis.
We should also point out that the funnel plots drawn for the purpose of detecting any publication biases have a low reliability since only a small number of studies were analysed.35
In conclusion, the results of our meta-analysis show that LD may reduce the risk of complications caused by DCI (cerebral infarctions and clinical deterioration), and risk of severe disability, but it has no effect on the risk of mortality. However, due to the quantitative and qualitative heterogeneity of the included studies, these findings are not conclusive and should be updated using the results of forthcoming clinical trials with homogeneous definitions of outcomes. These additional findings will provide a better understanding of the effects of LD in patients with ASH.51,52
FundingThis study was funded by the Neuroscience and Neurological Science Research Group, Cartagena de Indias, Colombia.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Dr Sachin A. Borkar from the department of neurosurgery at the All India Institute of Medical Sciences (New Delhi, India), for providing us with additional data from his study.
We also wish to thank Dr Keith Suárez-Jaramillo from the department of internal medicine at the University of Texas Health Science Centre (San Antonio, Texas), for his invaluable assistance with the English version of the abstract.
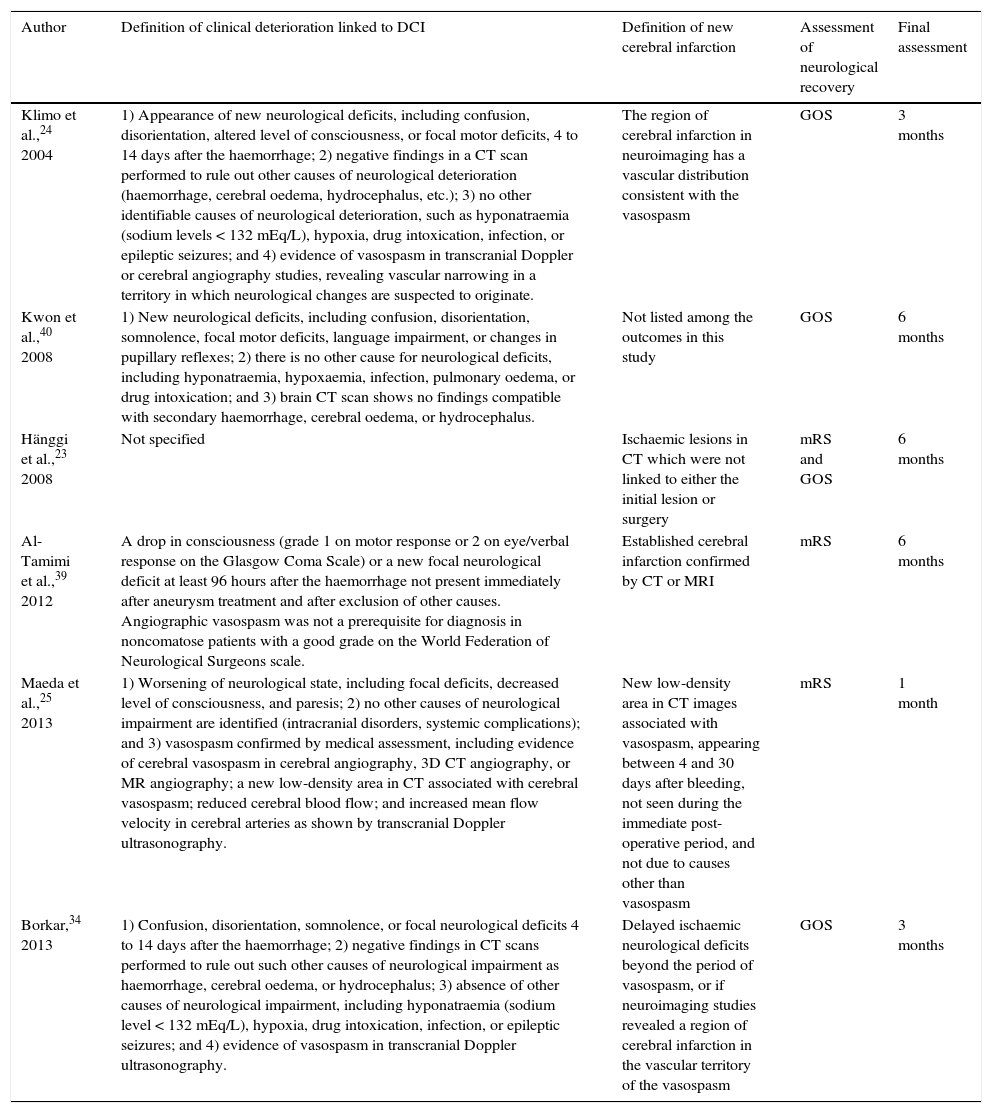
| Author | Definition of clinical deterioration linked to DCI | Definition of new cerebral infarction | Assessment of neurological recovery | Final assessment |
|---|---|---|---|---|
| Klimo et al.,24 2004 | 1) Appearance of new neurological deficits, including confusion, disorientation, altered level of consciousness, or focal motor deficits, 4 to 14 days after the haemorrhage; 2) negative findings in a CT scan performed to rule out other causes of neurological deterioration (haemorrhage, cerebral oedema, hydrocephalus, etc.); 3) no other identifiable causes of neurological deterioration, such as hyponatraemia (sodium levels < 132 mEq/L), hypoxia, drug intoxication, infection, or epileptic seizures; and 4) evidence of vasospasm in transcranial Doppler or cerebral angiography studies, revealing vascular narrowing in a territory in which neurological changes are suspected to originate. | The region of cerebral infarction in neuroimaging has a vascular distribution consistent with the vasospasm | GOS | 3 months |
| Kwon et al.,40 2008 | 1) New neurological deficits, including confusion, disorientation, somnolence, focal motor deficits, language impairment, or changes in pupillary reflexes; 2) there is no other cause for neurological deficits, including hyponatraemia, hypoxaemia, infection, pulmonary oedema, or drug intoxication; and 3) brain CT scan shows no findings compatible with secondary haemorrhage, cerebral oedema, or hydrocephalus. | Not listed among the outcomes in this study | GOS | 6 months |
| Hänggi et al.,23 2008 | Not specified | Ischaemic lesions in CT which were not linked to either the initial lesion or surgery | mRS and GOS | 6 months |
| Al-Tamimi et al.,39 2012 | A drop in consciousness (grade 1 on motor response or 2 on eye/verbal response on the Glasgow Coma Scale) or a new focal neurological deficit at least 96 hours after the haemorrhage not present immediately after aneurysm treatment and after exclusion of other causes. Angiographic vasospasm was not a prerequisite for diagnosis in noncomatose patients with a good grade on the World Federation of Neurological Surgeons scale. | Established cerebral infarction confirmed by CT or MRI | mRS | 6 months |
| Maeda et al.,25 2013 | 1) Worsening of neurological state, including focal deficits, decreased level of consciousness, and paresis; 2) no other causes of neurological impairment are identified (intracranial disorders, systemic complications); and 3) vasospasm confirmed by medical assessment, including evidence of cerebral vasospasm in cerebral angiography, 3D CT angiography, or MR angiography; a new low-density area in CT associated with cerebral vasospasm; reduced cerebral blood flow; and increased mean flow velocity in cerebral arteries as shown by transcranial Doppler ultrasonography. | New low-density area in CT images associated with vasospasm, appearing between 4 and 30 days after bleeding, not seen during the immediate post-operative period, and not due to causes other than vasospasm | mRS | 1 month |
| Borkar,34 2013 | 1) Confusion, disorientation, somnolence, or focal neurological deficits 4 to 14 days after the haemorrhage; 2) negative findings in CT scans performed to rule out such other causes of neurological impairment as haemorrhage, cerebral oedema, or hydrocephalus; 3) absence of other causes of neurological impairment, including hyponatraemia (sodium level < 132 mEq/L), hypoxia, drug intoxication, infection, or epileptic seizures; and 4) evidence of vasospasm in transcranial Doppler ultrasonography. | Delayed ischaemic neurological deficits beyond the period of vasospasm, or if neuroimaging studies revealed a region of cerebral infarction in the vascular territory of the vasospasm | GOS | 3 months |
GOS: Glasgow Outcome Scale; mRS: modified Rankin Scale.
Please cite this article as: Alcalá-Cerra G, Paternina-Caicedo Á, Díaz-Becerra C, Moscote-Salazar LR, Gutiérrez-Paternina JJ, Niño-Hernández LM, et al. Drenaje lumbar externo de líquido cefalorraquídeo en pacientes con hemorragia subaracnoidea aneurismática: revisión sistemática y metaanálisis de estudios controlados. Neurología. 2016;31:431–444.