Numerous polymorphisms in candidate genes coding for haemostatic system proteins have been proposed as risk factors for thrombosis.
MethodsWe performed a case-control study of consecutive ischaemic stroke survivors aged ≤45 years, treated at our neurology department from 2006 to 2014. Polymerase chain reaction–restriction fragment length polymorphism identified the following polymorphisms: Thr325Ile and Ala147Thr in TAFI, 4G/5G in PAI-1, PLA1/A2 in platelet glycoprotein IIb/IIIa, Glu298Asp in eNOS, and C677T in 5,10-MTHFR. A multivariate logistic regression analysis was performed to evaluate the independent risk of stroke.
Results204 cases and 204 age- and sex-matched controls were included in the study. Clinical and genetic variables associated with ischaemic stroke were hypertension (P=.03), tobacco use (P=.02), and the polymorphisms Glu298Asp (genotype: P=.001, allele frequency: P=.001) and C677T (genotype: P=.01); the Ala147Thr, Thr325IIe, 4G/5G, and PLA1/A2 mutations were not associated with ischaemic stroke. The 298Asp (P=.03) and T (P=.01) alleles, hypertension (P=.03), tobacco use (P=.01) and family history of stroke (P=.04) were identified as independent risk factors.
ConclusionThe polymorphisms Glu298Asp and C677T, affecting the eNOS and 5,10-MTHFR enzymes, respectively, and smoking, hypertension, and family history of stroke were associated with ischaemic stroke in young Mexican patients; this was not the case for the Thr325Ile, Ala147Thr, 4G/5G, and PLA1/A2 polymorphisms of the genes coding for fibrinolytic proteins and platelet receptors.
Diversos polimorfismos en genes candidatos que codifican proteínas del sistema hemostático se han propuesto como factores de riesgo para el desarrollo de trombosis.
MétodosCasos y controles, sobrevivientes de enfermedad vascular cerebral (EVC) isquémico idiopático ≤ 45 años de edad del servicio de Neurología incluidos de manera consecutiva del 2006al 2014. Por PCR-RFLP se identificaron los polimorfismos: Thr325Ile y Ala147Thr del gen de TAFI, 4G/5G del gen de PAI-1, PLA1/A2 del gen de la glicoproteína plaquetaria IIb/IIIa, Glu298Asp del gen de eNOS, y C677T del gen de la 5,10 MTHFR. Se realizó un análisis multivariado de regresión logística para calcular el riesgo independiente de EVC.
Resultados204 casos y 204 controles pareados por edad y sexo. Se asoció al polimorfismo Glu298Asp (genotipo p=0.001 y frecuencia alélica p=0.001), C677T (genotipo p=0.01), hipertensión (p=0.03) y tabaquismo (p=0.02) con la presencia de EVC isquémico, no así para los polimorfismos Ala147Thr, Thr325IIe, 4G/5G, y PLA1/A2. Se identificó como factor de riesgo independiente al alelo 298Asp (p=0.03), T (p=0.01), hipertensión (p=0.03), tabaquismo (p=0.01) y AHFEAT (p=0.04).
ConclusionesLos polimorfismos Glu298Asp y C677T de los genes que codifican a la enzima eNOS y 5,10 MTHFR, tabaquismo, hipertensión y AHFEAT, se asociaron a la presencia de EVC isquémico en jóvenes mexicanos, no así el Thr325Ile, Ala147Thr, 4G/5G, PLA1/A2 en genes que codifican proteínas del sistema de fibrinólisis y receptores plaquetarios.
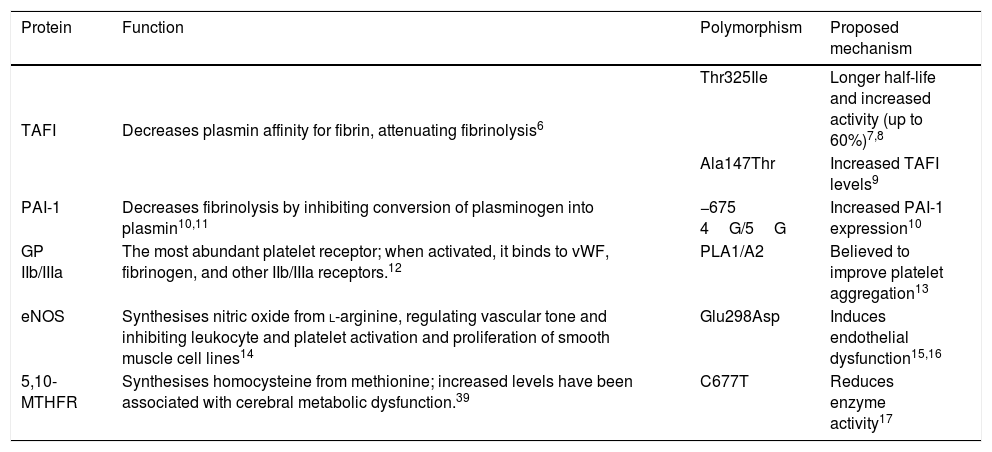
Cardiovascular diseases have become the leading cause of death in the world, with thrombosis being the most frequent aetiological mechanism.1 Approximately 67% of cases of cerebrovascular disease (CVD) are ischaemic; a considerable reduction has been observed in CVD incidence, probably due to early identification and good control of traditional risk factors.1,2 Prevalence of CVD in patients aged <45 years ranges from 3% to 5%; aetiology is not identified in over half of cases.3 Due to the reduced time of exposure to environmental cardiovascular risk factors, genetic risk factors are thought to be crucial in the development of thrombosis in young patients.4 Several polymorphisms in candidate genes coding for proteins of the haemostatic system have been proposed as risk factors for the development of thrombosis.5 Thrombin activatable fibrinolysis inhibitor (TAFI) is a procarboxypeptidase that attenuates fibrinolysis by removing C-terminal lysine residues of fibrin from the clot surface, decreasing the affinity of plasmin for its substrate.6 The Thr325Ile polymorphism in the gene coding for TAFI, located on chromosome 13q14.11, results in the substitution of threonine (Thr) by isoleucine (Ile) at position 325, lengthening the protein’s half-life and increasing its activity by up to 60%.7,8 The Ala147Thr polymorphism has been associated with increased TAFI expression, which explains up to 61.6% of variance in plasma levels.9 Tissue plasminogen activator inhibitor-1 (PAI-1) decreases fibrinolysis by inhibiting the conversion of plasminogen into plasmin and the migration and proliferation of smooth muscle cells between the lipid core of the atheromatous plaque and the arterial lumen, thus predisposing to plaque rupture by decreasing collagen synthesis. Insertion of a guanine (5G) at position 675 upstream of the transcription start site of the gene coding for PAI-1, located on chromosome 7q22.1, is associated with increased expression.10 The most abundant platelet receptor, platelet glycoprotein (GP) IIb/IIIa, is a calcium-dependent heterodimer that, when activated, undergoes a structural change that enables it to bind to ligands containing the amino acid sequence arginine-glycine-aspartic acid-serine, such as the von Willebrand factor, fibrinogen, and other IIb/IIIa receptors.12 Substitution of thymide (T) by cytosine (C) at position 1565 of exon 2 of the gene encoding the IIIa subunit, located on the long arm of chromosome 17, induces the replacement of leucine by proline at position 33, which is believed to improve platelet aggregation.13 The endothelial nitric oxide synthase (eNOS) enzyme catalyses the synthesis of nitric oxide from L-arginine; nitric oxide regulates vascular tone and prevents atherothrombotic disease by inhibiting leukocyte and platelet activation and smooth muscle cell activation.14 The substitution of G by T at position 894 of exon 7 of the gene encoding the eNOS enzyme, located on chromosome 7q35-36, translates into the substitution of glutamine (Glu) by aspartic acid (Asp) at position 298, inducing endothelial dysfunction.15,16 The C677T polymorphism in the gene encoding the 5,10-methylenetetrahydrofolate reductase (5,10-MTHFR) enzyme, located on chromosome 1p36.3, causes the substitution of valine (Val) by alanine (Ala) and is responsible for reduced enzyme activity (Table 1).17
Genotypes proposed as risk factors for idiopathic ischaemic cerebrovascular disease.
| Protein | Function | Polymorphism | Proposed mechanism |
|---|---|---|---|
| TAFI | Decreases plasmin affinity for fibrin, attenuating fibrinolysis6 | Thr325Ile | Longer half-life and increased activity (up to 60%)7,8 |
| Ala147Thr | Increased TAFI levels9 | ||
| PAI-1 | Decreases fibrinolysis by inhibiting conversion of plasminogen into plasmin10,11 | −675 4G/5G | Increased PAI-1 expression10 |
| GP IIb/IIIa | The most abundant platelet receptor; when activated, it binds to vWF, fibrinogen, and other IIb/IIIa receptors.12 | PLA1/A2 | Believed to improve platelet aggregation13 |
| eNOS | Synthesises nitric oxide from l-arginine, regulating vascular tone and inhibiting leukocyte and platelet activation and proliferation of smooth muscle cell lines14 | Glu298Asp | Induces endothelial dysfunction15,16 |
| 5,10-MTHFR | Synthesises homocysteine from methionine; increased levels have been associated with cerebral metabolic dysfunction.39 | C677T | Reduces enzyme activity17 |
5,10-MTHFR: 5,10-methylenetetrahydrofolate reductase; eNOS: endothelial nitric oxide synthase enzyme; GP IIb/IIIa: platelet glycoprotein IIb/IIIa; PAI-1: tissue plasminogen activator inhibitor-1; TAFI: thrombin activatable fibrinolysis inhibitor; vWF: Von Willenbrand factor.
The aim of the present study is to identify the association and interaction between the polymorphisms Thr325Ile and Ala147Thr of the gene encoding TAFI; 4G/5G of the gene encoding PAI-1; PLA1/A2 of the gene encoding the GP IIIa subunit; Glu298Asp of the gene encoding eNOS; and C677T of the gene coding for 5,10-MTHFR; and ischaemic CVD in young Mexican patients.
Patients and methodsWe performed a case-control study of consecutive patients diagnosed with idiopathic ischaemic CVD aged 45 or younger, admitted from 2006 to 2014. All patients signed informed consent forms and were referred to the Thrombosis, Haemostasis and Atherogenesis Medical Research Unit at Hospital General Regional no. 1, which belongs to the Mexican Institute of Health (IMSS, for its Spanish initials). We recorded data on the following variables: age, sex, history of type 2 diabetes mellitus (DM2), systemic arterial hypertension (SAHT), dyslipidaemia, smoking, and hereditary family history of atherothrombotic disease (HFHAD). We tested all participants for Thr325Ile, Ala147Thr, 4G/5G, PLA1/A2, Glu298Asp, and C677T polymorphisms. This project was reviewed and approved by the ethics committee of the IMS, in accordance with the principles of the Declaration of Helsinki of the World Medical Association and the World Health Organization, revised in Tokyo (Japan).
All patients with CT-confirmed diagnosis of ischaemic CVD previously underwent the following studies: carotid and vertebral Doppler ultrasound, transthoracic or transoesophageal echocardiogram, angiography, heart MRI scan, 12-lead electrocardiogram, and automated heart rate monitoring of at least 24h’ duration. These studies were performed to rule out those patients with risk factors for cardioembolic CVD, such as atrial fibrillation, intraventricular thrombus, acute myocardial infarction, prosthetic valves, cardiomyopathy, endocarditis, atrial myxoma, persistent foramen ovale, atrial septal aneurysm, carotid artery stenosis ≥ 50%, vertebrobasilar artery dissection, and arteritis. We determined the concentrations of antithrombin, protein C and protein S (Diagnostica Stago), lupus anticoagulant (DVV test, DVV confirm®; American Diagnostic, USA), modified activated protein C resistance (normal range >2.0, Coatest+APC Resistance V-S; Chromogenix, Sweden), and anticardiolipin antibodies (normal range <10 U; Sanofi Diagnostics Pasteur, France) in all participants in the 6 months following stroke.
Ischaemic CVD was diagnosed according to the Trial of ORG 10172 in Acute Stroke Treatment criteria.18 The control group included apparently healthy volunteers who consecutively attended the blood bank as potential donors, with no relevant history (DM, SAHT, CVD, or thrombotic disease) and biochemical parameters within normal ranges. Controls consented to participate in the study and were age- and sex-matched with the patient group.
Identification of polymorphismsThe Thr325Ile and Thr147Ala polymorphisms of the TAFI gene were amplified under the following thermal conditions: denaturation for 30s at 94°C, annealing at 60°C for 30s, and 30 cycles of extension at 72°C for 60s. Using the SpeI restriction enzyme, we obtained a band of 245 bp for the Thr allele and 364 bp for the Ile allele of the Thr325Ile polymorphism. Using the BbvI enzyme, we obtained bands of 124 and 304 bp for the Ala allele and 428 bp for the Thr allele of the Thr147Ala polymorphism.
For the 4G/5G polymorphism of the PAI-1 gene, we applied an initial denaturation at 94°C for 3min, followed by 30 cycles of denaturation at 94°C for 3s, annealing at 60°C for 30s, and extension at 72°C for 30s, and a final extension at 72°C for 60s. Using the Bsl I enzyme, we obtained a band of 99 bp for the 5G allele and 98 bp for the 4G allele.
For the PLA1/A2 polymorphism of the GP IIIa subunit, we applied an initial denaturation at 94°C for 5min, followed by 35 cycles of denaturation at 94°C for one minute, annealing at 57°C for 45s, and extension at 72°C for 60s, and a final extension at 72°C for 15min. Using the MspI enzyme, we obtained a band of 221 bp for the PLA1 allele and 177 bp for the PLA2 allele.
The Glu298Asp polymorphism of the eNOS gene required denaturation at 94°C for 30s, annealing at 60°C for 30s, and 30 cycles of extension at 72°C for 30s. With the Mbo I enzyme, we obtained 2 fragments of 119 and 87 bp for the Asp298 allele and 206 bp for the Glu298 allele.
The C677T polymorphism of the 5,10-MTHFR gene required denaturation at 94°C for 20s, annealing at 62°C for 20s, and 30 cycles of extension at 72°C for 20s. Using the Hinf I enzyme, we obtained a band of 175 bp for the T allele and 198 bp for the C allele.
Statistical analysisContinuous variables are expressed as means (standard deviation). Categorical variables are shown as number of patients (n) and percentages (%). We compared continuous variables in the patient and control groups using the t test. Categorical variables were compared using the chi-square test. The Shapiro–Wilk test was used to test for normality of all the variables and to determine the data distribution. The allele frequency of each polymorphism was assessed in every group using the chi-square test in accordance with the Hardy–Weinberg equilibrium. We performed a multivariate logistic regression analysis to calculate the independent risk of presenting ischaemic CVD, expressed as the odds ratio. The independent variables in the model were the clinical variables and the polymorphisms showing statistical significance in the univariate analysis. Values of P<.05 were considered statistically significant. Statistical analysis was performed using the SPSS statistics software, version 20 (SPSS Inc., Chicago, IL, USA).
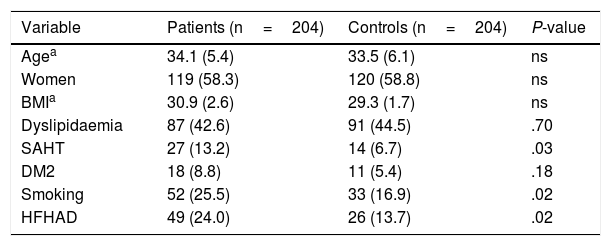
ResultsWe included 251 patients aged ≤45 years diagnosed with ischaemic CVD over a period of 8 years, from 2006 to 2014. We excluded 33 patients due to protein S deficiency (n=7), protein C deficiency (n=1), anticardiolipin antibodies (n=15), lupus anticoagulant (n=6), acquired activated protein C resistance (n=4), antithrombin deficiency (n=1), and 14 patients due to cerebral haemorrhage. Our sample finally included 204 young patients with idiopathic ischaemic CVD origin and 204 age- and sex-matched controls. Table 2 shows the clinical and demographic characteristics of both groups. In the patient group, mean age was 34.1 (5.4) years; 58.3% of patients (n=119) were women, and mean BMI was 30.9 (2.6) kg/m2. Listed in order of frequency, the classical cardiovascular risk factors observed in the sample were: dyslipidaemia in 42.6% (n=87), smoking in 25.5% (n=52), HFHAD in 24% (n=49), SAHT in 13.2% (n=27), and DM2 in 8.8% (n=18). Compared to controls, patients showed a higher frequency of SAHT (13.2% vs. 6.7%, P=.03), smoking (25.5% vs 16.9%, P=.02), and HFHAD (24% vs 13.7%, P=.02). There were no significant differences between groups for the variables age, sex, BMI, dyslipidaemia, or DM2.
Clinical and demographic characteristics of 204 patients aged ≤45 years with idiopathic ischaemic CVD and 204 controls.
| Variable | Patients (n=204) | Controls (n=204) | P-value |
|---|---|---|---|
| Agea | 34.1 (5.4) | 33.5 (6.1) | ns |
| Women | 119 (58.3) | 120 (58.8) | ns |
| BMIa | 30.9 (2.6) | 29.3 (1.7) | ns |
| Dyslipidaemia | 87 (42.6) | 91 (44.5) | .70 |
| SAHT | 27 (13.2) | 14 (6.7) | .03 |
| DM2 | 18 (8.8) | 11 (5.4) | .18 |
| Smoking | 52 (25.5) | 33 (16.9) | .02 |
| HFHAD | 49 (24.0) | 26 (13.7) | .02 |
Data are expressed as frequency (%), except where otherwise stated.
BMI: body mass index; DM2: type 2 diabetes mellitus; HFHAD: heredity and family history of atherothrombotic disease; ns: not significant; SAHT: systemic arterial hypertension.
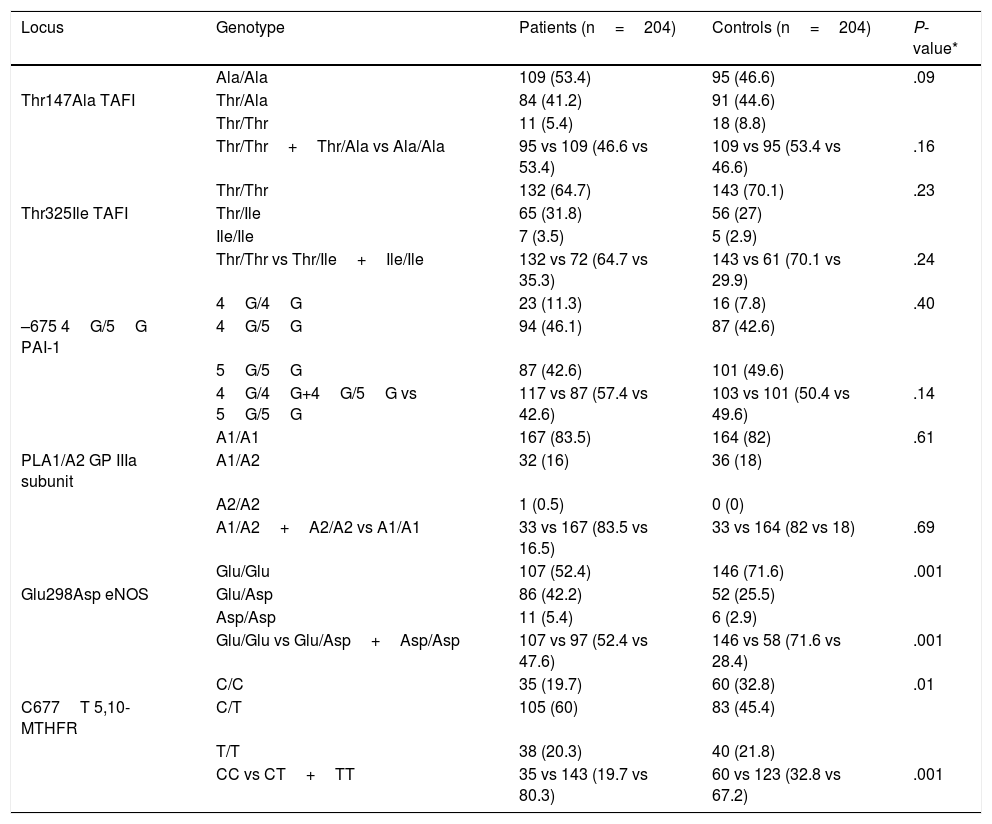
Table 3 shows the genotypic distribution of the Thr325Ile, Ala147Thr, 4G/5G, PLA1/A2, Glu298Asp, and C677T polymorphisms in the patient and control groups.
Genotypic distribution of the Ala147Thr, Thr325Ile, 4G/5G, PLA1/A2, Glu298Asp, and C677T polymorphisms in 204 patients aged ≤45 years with idiopathic ischaemic CVD and 204 controls.
| Locus | Genotype | Patients (n=204) | Controls (n=204) | P-value* |
|---|---|---|---|---|
| Ala/Ala | 109 (53.4) | 95 (46.6) | .09 | |
| Thr147Ala TAFI | Thr/Ala | 84 (41.2) | 91 (44.6) | |
| Thr/Thr | 11 (5.4) | 18 (8.8) | ||
| Thr/Thr+Thr/Ala vs Ala/Ala | 95 vs 109 (46.6 vs 53.4) | 109 vs 95 (53.4 vs 46.6) | .16 | |
| Thr/Thr | 132 (64.7) | 143 (70.1) | .23 | |
| Thr325Ile TAFI | Thr/Ile | 65 (31.8) | 56 (27) | |
| Ile/Ile | 7 (3.5) | 5 (2.9) | ||
| Thr/Thr vs Thr/Ile+Ile/Ile | 132 vs 72 (64.7 vs 35.3) | 143 vs 61 (70.1 vs 29.9) | .24 | |
| 4G/4G | 23 (11.3) | 16 (7.8) | .40 | |
| –675 4G/5G PAI-1 | 4G/5G | 94 (46.1) | 87 (42.6) | |
| 5G/5G | 87 (42.6) | 101 (49.6) | ||
| 4G/4G+4G/5G vs 5G/5G | 117 vs 87 (57.4 vs 42.6) | 103 vs 101 (50.4 vs 49.6) | .14 | |
| A1/A1 | 167 (83.5) | 164 (82) | .61 | |
| PLA1/A2 GP IIIa subunit | A1/A2 | 32 (16) | 36 (18) | |
| A2/A2 | 1 (0.5) | 0 (0) | ||
| A1/A2+A2/A2 vs A1/A1 | 33 vs 167 (83.5 vs 16.5) | 33 vs 164 (82 vs 18) | .69 | |
| Glu/Glu | 107 (52.4) | 146 (71.6) | .001 | |
| Glu298Asp eNOS | Glu/Asp | 86 (42.2) | 52 (25.5) | |
| Asp/Asp | 11 (5.4) | 6 (2.9) | ||
| Glu/Glu vs Glu/Asp+Asp/Asp | 107 vs 97 (52.4 vs 47.6) | 146 vs 58 (71.6 vs 28.4) | .001 | |
| C/C | 35 (19.7) | 60 (32.8) | .01 | |
| C677T 5,10-MTHFR | C/T | 105 (60) | 83 (45.4) | |
| T/T | 38 (20.3) | 40 (21.8) | ||
| CC vs CT+TT | 35 vs 143 (19.7 vs 80.3) | 60 vs 123 (32.8 vs 67.2) | .001 |
eNOS: endothelial nitric oxide synthase enzyme; GP IIb/IIIa: platelet glycoprotein IIb/IIIa; 5,10-MTHFR: 5,10-methylenetetrahydrofolate reductase; PAI-1: tissue plasminogen activator inhibitor-1; TAFI: thrombin activatable fibrinolysis inhibitor.
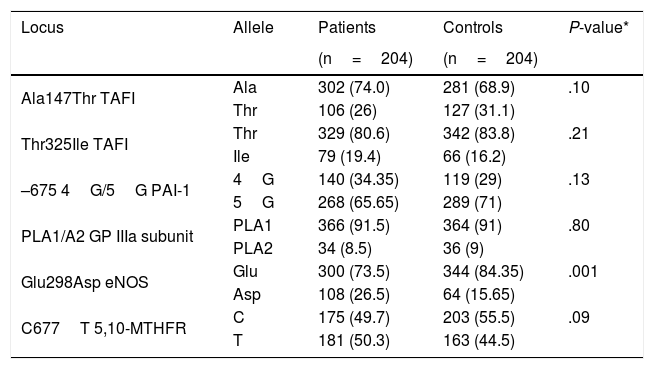
Table 4 shows the allele frequency of the Thr325Ile, Ala147Thr, 4G/5G, PLA1/A2, Glu298Asp, and C677T polymorphisms in the patient and control groups.
Allele frequency of the Ala147Thr, Thr325Ile, 4G/5G, PLA1/A2, Glu298Asp, and C677T polymorphisms in 204 patients aged ≤45 years with idiopathic ischaemic CVD and 204 controls.
| Locus | Allele | Patients | Controls | P-value* |
|---|---|---|---|---|
| (n=204) | (n=204) | |||
| Ala147Thr TAFI | Ala | 302 (74.0) | 281 (68.9) | .10 |
| Thr | 106 (26) | 127 (31.1) | ||
| Thr325Ile TAFI | Thr | 329 (80.6) | 342 (83.8) | .21 |
| Ile | 79 (19.4) | 66 (16.2) | ||
| –675 4G/5G PAI-1 | 4G | 140 (34.35) | 119 (29) | .13 |
| 5G | 268 (65.65) | 289 (71) | ||
| PLA1/A2 GP IIIa subunit | PLA1 | 366 (91.5) | 364 (91) | .80 |
| PLA2 | 34 (8.5) | 36 (9) | ||
| Glu298Asp eNOS | Glu | 300 (73.5) | 344 (84.35) | .001 |
| Asp | 108 (26.5) | 64 (15.65) | ||
| C677T 5,10-MTHFR | C | 175 (49.7) | 203 (55.5) | .09 |
| T | 181 (50.3) | 163 (44.5) |
eNOS: endothelial nitric oxide synthase enzyme; GP IIb/IIIa: platelet glycoprotein IIb/IIIa; 5,10-MTHFR: 5,10-methylenetetrahydrofolate reductase; PAI-1: tissue plasminogen activator inhibitor-1; TAFI: thrombin activatable fibrinolysis inhibitor.
*Chi-square test.
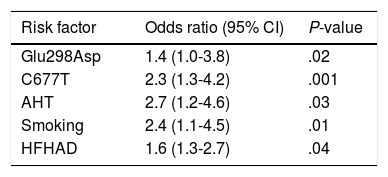
We identified the Glu298Asp allele (P=.02), the C677T polymorphism (P=.001), hypertension (P=.03), smoking (P=.01), and HFHAD (P=.04) as independent risk factors (Table 5).
Multivariate logistic regression analysis with ischaemic CVD as the dependent variable.
| Risk factor | Odds ratio (95% CI) | P-value |
|---|---|---|
| Glu298Asp | 1.4 (1.0-3.8) | .02 |
| C677T | 2.3 (1.3-4.2) | .001 |
| AHT | 2.7 (1.2-4.6) | .03 |
| Smoking | 2.4 (1.1-4.5) | .01 |
| HFHAD | 1.6 (1.3-2.7) | .04 |
HFHAD: heredity and family history of atherothrombotic disease; SAHT: systemic arterial hypertension.
In this study, we analysed 6 different polymorphisms located in genes coding for proteins of the fibrinolytic system and associated with endothelial dysfunction and platelet hyperaggregability.
The increase in plasma TAFI concentration has been associated with a higher risk and severity of ischaemic CVD.19–21 Although in at least 25% of cases, plasma TAFI levels are determined by several polymorphisms located on gene CPB2,22 the effect of the Thr325Ile and Ala147Thr polymorphisms on increased cardiovascular risk is not conclusive.23 According to our findings, the Ala147Thr and Thr325IIe polymorphisms of the TAFI gene are not associated with occurrence of ischaemic CVD (genotype, P=.09 and allele frequency, P=.10; and genotype, P=.23 and allele frequency, P=.21, respectively) in patients aged ≤45 years. Ladenvall et al.24 also report negative results in patients with ischaemic CVD aged under 70 years, and Akatsu et al.25 report negative results in 253 patients with a mean age of 80 years. In contrast, Kozian et al.26 showed an association between the Ile homozygous genotype of the TAFI gene with higher incidence of early ischaemic CVD in a cohort of over 3300 patients; the Ala147Thr polymorphism did not show such an association. Ischaemic CVD manifests with a highly heterogeneous clinical and aetiological spectrum; the prevalence of traditional and environmental cardiovascular risk factors varies in different populations, as does the genetic substrate between different ethnic groups. Occurrence and age of onset of ischaemic CVD are probably determined by specific combinations of genetic and environmental risk factors at different stages of life,27 which would partly explain this inconsistency in the results reported.
The role of PAI-1 in the pathophysiology of ischaemic CVD is more complex than in other thrombotic diseases, such as myocardial infarction. Despite the fact that PAI-1 promotes atherosclerosis and is considered the main inhibitor of fibrinolysis, its expression by astrocytes of the brain parenchyma is a protective factor against tPA-induced lesions to the blood-brain barrier, cerebral oedema, and haemorrhagic transformation of the infarcted area.28,29 Recent studies suggest that the 4G allele of the PAI-1 gene has a neuroprotective effect against ischaemic CVD but increases risk of myocardial infarction.30 However, the genotypic distribution of the 4G/5G polymorphism varies greatly in different regions of the world, both in healthy individuals and patients with ischaemic CVD (ranging from 28% to 90% for the heterozygous 4G/5G genotype).31 In our sample, neither the genotype (P=.40) nor the allele frequency (P=.13) of the 4G risk allele was associated with ischaemic CVD in young Mexican patients aged ≤45 years. In contrast, Ranellou et al.31 reported a higher frequency of the 4G/5G heterozygous genotype in Greek patients with CVD aged <50 years versus controls (P=.02). In a meta-analysis including 8336 cases and 14 403 controls, Hu et al.32 identified the 4G/4G homozygous genotype as a risk factor for ischaemic CVD. Furthermore, our research group has identified increased serum PAI-1 levels in young patients with ischaemic CVD. Higher levels of PAI-1 have been observed in subjects with acute ischaemic CVD than in convalescent patients and patients in the chronic phase.33 In mice with transient middle cerebral artery occlusion, inhibition of TAFI and PAI-1 by monoclonal antibodies significantly decreased fibrin formation and stroke size by 50%.34 There is evidence that PAI-1 has a dual effect on ischaemic CVD pathophysiology, both participating in the development and progression of atherosclerotic plaque, and acting as neuroprotective factor, limiting lesion severity.
In our sample, the Leu33Pro polymorphism in the gene encoding the GP IIIa subunit was not associated with the presence of ischaemic CVD (genotype, P=.61 and allele frequency, P=.80) in individuals aged ≤45 years. Similar results were reported by Van Goor et al.35 who found no association between the PLA2 allele and presence of cryptogenic CVD in 80 patients aged ≤45 years. However, an interaction has been reported between the PLA1/A2 genotype and history of smoking in a subgroup of patients with lacunar CVD aged between 55 and 69 years (P=.024).36 In our study, history of smoking was more frequent in patients than in controls (P=.02). In in vitro studies, patients with ischaemic CVD displaying the PLA1/A2 heterozygous genotype have shown increased platelet aggregation, GP IIb/IIIa receptor expression, and fibrinogen binding capacity, in comparison with individuals with the PLA1/A1 genotype.37 This variability in the results of different studies may be due to interaction between several environmental and genetic factors. There is a need for further studies assessing the interaction between smoking, serum fibrinogen concentration, and polymorphisms in genes coding for platelet receptors.
In the present study, the Glu298Asp polymorphism of the eNOS gene was associated with presence of ischaemic CVD in a Mexican sample of individuals aged ≤45 years (genotype, P=.001 and allele frequency, P=.001, respectively). In a case-control study, Kumar et al.38 identified the Glu298Asp polymorphism as a risk factor for ischaemic CVD in a population from North India (P=.028). A meta-analysis including a total of 6733 patients and 7305 controls observed a significant association (P<.001) between the Glu298Asp polymorphism and presence of ischaemic CVD.39
Homocysteine is an amino acid formed during methionine metabolism; increased plasma concentrations have been associated with cerebral metabolic dysfunction, and are considered an independent risk factor for ischaemic CVD.40 In our study, the TT homozygous genotype and CT heterozygous genotype of the C677T polymorphism in the 5,10-MTHFR gene were found to be associated with presence of ischaemic CVD in a Mexican sample of individuals aged ≤45 years (P=.01). Jiang et al.41 identified 251 cases of ischaemic CVD in a period of 6.2 years in a cohort of 39 165 individuals; subjects with SAHT and the TT genotype presented a higher risk of developing CVD, with an odds ratio of 10.6. In contrast, Ranellou et al.31 observed no significant differences in the allele frequency of the C677T polymorphism between Greek patients with ischaemic CVD aged <50 years versus controls (P=.78).31 A previous study conducted by our research group identified a significant increase (P<.001) in serum homocysteine levels after a postoral methionine load in a group of young patients with ischaemic CVD versus controls.42
The discrepancy in the results reported in different studies suggests that patients with ischaemic CVD present a complex cerebrovascular genotype; there is a need for a careful aetiological classification of patients, epidemiological studies in larger populations, a detailed functional assessment of genetic variants, and validation of findings in different ethnic groups, to determine the precise role of these genes in the development of ischaemic CVD.43
Regarding modifiable risk factors, hypertension, smoking, and family history of cerebral artery disease were identified as independent risk factors for ischaemic CVD in this group of patients.
Some strengths of our study are: 1) study groups were age-and sex-matched, 2) age was limited to ≤45 years to minimise the effect of exposure to traditional risk factors; and 3) we analysed 6 polymorphisms in the 5 candidate genes of the haemostatic system in each individual. Limitations of the study include the lack of determination of serum TAFI and nitric oxide levels and aggregometry testing.
In accordance with the results obtained, we propose the Glu298Asp and C677T polymorphisms of the genes coding for the eNOS enzyme and 5,10-MTHFR, respectively, as possible risk factors for ischaemic CVD. Our findings suggest endothelial dysfunction as the pathophysiological mechanism involved. Further studies are needed to study the thrombotic mechanisms present in patients with idiopathic CVD, and to check whether these results are consistent in other populations.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThis project was funded with the support of the CONACyT Sectoral Fund for Research in Health and Social Security (No. 261887) and grants from the Health Research Fund from the IMSS and IMSS Foundation (FIS/IMSS/PROT/PRIO/13/023, FIS/IMSS/PROT/G13/1195, FIS/IMSS/PROT/G16/1616).
Please cite this article as: Jiménez-González MC, et al. Identificación de factores de riesgo genéticos asociados a la enfermedad vascular cerebral de tipo isquémico en jóvenes mexicanos. Neurología. 2021;36:337–345.