The aim of this study was to evaluate the effects of deep brain stimulation of the subthalamic nucleus (DBS-SN) on cognitive function in patients with Parkinson's disease (PD) 5 years after surgery.
Material and methodsWe conducted a prospective study including 50 patients with PD who underwent DBS-SN (62.5% were men; mean age of 62.2±8.2 years; mean progression time of 14.1±6.3 years). All patients were assessed before the procedure and at one year after surgery; 40 patients were further followed up until the 5-year mark. Follow-up assessments included the following neuropsychological tests: Mini-Mental State Examination (MMSE), Mattis Dementia Rating Scale (MDRS), letter-number sequencing of the WAIS-III (WAIS-III-LN), clock-drawing test, Rey auditory verbal learning test (RAVLT), Benton Visual Retention Test (BVRT), Judgment of Line Orientation (JLO) test, FAS Phonemic Verbal Fluency Test, Stroop test, and the Montgomery–Asberg Depression Rating Scale (MADRS).
ResultsPatients were found to score lower on the MMSE (−0.89%), clock-drawing test (−2.61%), MDRS (−1.72%), and especially phonemic (−13.28%) and sematic verbal fluency tests (−12.40%) at one year after surgery. Delayed recall on the RAVLT worsened one year after the procedure (−10.12%). At 5 years, impairment affected mainly verbal fluency; scores decreased an additional 16.10% and 16.60% in semantic and phonemic verbal fluency, respectively. Moderate decreases were observed in immediate recall (−16.87%), WAIS-III-LN (−16.67%), and JLO test (−11.56%).
DiscussionIn our sample, DBS-SN did not result in global cognitive impairment 5 years after surgery. Verbal function was found to be significantly impaired one year after the procedure. Impaired learning and visuospatial function may be attributed to degeneration associated with PD.
El objetivo es evaluar los efectos de la estimulación cerebral profunda del núcleo subtalámico bilateral (STN-DBS) sobre el estado cognitivo de los pacientes con enfermedad de Parkinson 5 años después de la cirugía.
Materiales y métodosEn este estudio prospectivo se incluyeron 50 pacientes con enfermedad de Parkinson (62,5% hombres, edad media 62,2±8,2 años y duración de la enfermedad 14,1±6,3 años) sometidos a STN-DBS. Todos los pacientes fueron evaluados preoperatoriamente y un año después de la cirugía, y 40 pacientes fueron seguidos hasta 5 años. En cada visita se realizaron las siguientes evaluaciones neuropsicológicas: Mini-Mental State Examination, Mattis Dementia Rating Scale (MDRS), test de secuencias números-letras de WAIS III-LN, Prueba de dibujo de reloj, Prueba de aprendizaje verbal auditivo Rey, la Prueba de retención visual de Benton, la Prueba de juicio de orientación de línea de Benton, la fluidez verbal fonética y semántica, la Prueba Stroop y la Escala de clasificación de depresión de Montgomery-Asberg.
ResultadosAnualmente se observaron reducciones en la puntación de Mini-Mental State Examination (−0,89%), Prueba del dibujo de reloj (−2,61%) y MDRS (−1,72%), fueron más marcados tanto para la fluidez verbal fonética (−13,28%) como semántica (−12,40%). Para la Prueba de aprendizaje verbal auditivo Rey observamos un deterioro en la capacidad de recuerdo diferido (−10,12%) un año después de la cirugía. A los 5 años la mayor parte del deterioro se produjo en la fluidez verbal, con reducciones adicionales de 16,10% y 16,60% para la fluidez verbal semántica y fonética, respectivamente. Se observó un empeoramiento más moderado del recuerdo inmediato (−16,87%), WAIS III-LN (−16,67%) y de la prueba de orientación lineal de Benton (−11,56%).
DiscusiónLa STN-DBS no condujo a deterioro cognitivo global a los 5 años de la cirugía. Hubo un deterioro significativo en la función verbal desde el primer año de la cirugía. El deterioro de la capacidad de aprendizaje y de las funciones visuoespaciales podría atribuirse al propio proceso degenerativo de la enfermedad.
Deep brain stimulation (DBS) is reported to be effective for short-term treatment of motor symptoms of Parkinson's disease (PD). It has also been observed to reduce dopaminergic drug use, resulting in fewer motor complications.1,2 No significant cognitive deficits have been observed in the first years after DBS, with the exception of reduced verbal fluency, probably caused by the surgery to the subthalamic nucleus (STN): cognitive alterations are less frequent when surgery is performed on the internal globus pallidus (iGP).3–5 Cognitive function may change when pulse generators are turned on,6,7 with alterations in the ability to inhibit interference (evaluated with the interference version of the Stroop Color and Word Test [SCWT]).6 As mentioned previously, outcomes may vary according to the target of surgery. According to several studies, patients receiving DBS in the STN (STN-DBS) perform more poorly in conditional associative learning tasks than those receiving iGP stimulation.6 However, STN-DBS improves cognitive flexibility, according to neuropsychological test results.6,8 Motor and cognitive improvements after the intervention result in greater patient quality of life.9–11
Little is known about the long-term course of motor symptoms in patients treated with DBS. According to the first 5-year follow-up studies of the effects of DBS, the benefits of treatment are partially lost during the first years after surgery.12–15 Few studies have analysed the impact of DBS on cognition at 5 years. One study, with a small patient sample, reported a decline in verbal fluency and abstract reasoning 5 years after the intervention.16 Other studies focusing on the clinical course of motor function report cognitive impairment at 5 years, which was attributed to the clinical course of the disease.14,15
The purpose of this study was to evaluate the effects of DBS on cognitive function at 5 years after surgery, and to compare these effects against the degeneration inherent to PD.
Material and methodsWe gathered a sample of 50 patients diagnosed with PD according to the United Kingdom Parkinson's Disease Society Brain Bank criteria (except for family history of PD) and the recommendations of the Core Assessment Program for Surgical Interventional Therapies in Parkinson's disease. All patients underwent tetrapolar electrode implantation (3389 DBS, Medtronic; Minneapolis, USA) in both STN. The intervention was performed at the movement disorders unit of Hospital Universitario de Cruces in Baracaldo (Spain). Mean age (standard deviation [SD]) at the time of surgery was 62.2 (8.2) years, with a mean disease duration of 14.1 (6.3) years. The sample included 32 men (64%) and 18 women (36%). Exclusion criteria were as follows: diagnosis of dementia, clinical manifestations suggestive of atypical parkinsonism, concomitant diseases contraindicating surgery, and severe psychiatric disorders. Patients undergoing unilateral implantation, those undergoing surgery to the iGP, and patients treated at other centres were excluded from our sample. We also excluded 19 patients who had been evaluated according to a different neuropsychological protocol to that used in our study, whose design is based on published evidence and the consensus of our surgery team.
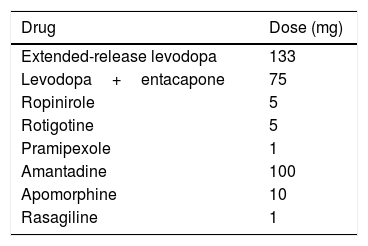
We recorded birth date, date of PD diagnosis and onset of dopaminergic therapy, education level, medical and psychiatric history, and levodopa equivalent dose. We calculated the levodopa equivalent daily dose (LEDD)17 for all dopaminergic drugs (Table 1). All patients were evaluated with the Unified Parkinson's Disease Rating Scale (UPDRS I-IV). Quality of life was evaluated with the Parkinson's Disease Questionnaire (PDQ-39) during “drug-on” periods at baseline (before surgery) and during “drug-on/DBS-on” periods at follow-up (pulse generators turned on). Patients used diaries to record the duration of “off” periods.
Levodopa equivalent daily dose17 (equivalent to 100mg levodopa) for different dopaminergic drugs.
| Drug | Dose (mg) |
|---|---|
| Extended-release levodopa | 133 |
| Levodopa+entacapone | 75 |
| Ropinirole | 5 |
| Rotigotine | 5 |
| Pramipexole | 1 |
| Amantadine | 100 |
| Apomorphine | 10 |
| Rasagiline | 1 |
Surgery was performed according to the protocol previously described in the literature.9,13
Neuropsychological studyBaseline neuropsychological assessments were performed 2 months before surgery. Patients were prospectively evaluated 12 and 60 months after neurosurgery. All patients were evaluated during “drug-on” periods before surgery and during “drug-on/DBS-on” periods at postoperative follow-up consultations. All assessments were performed by the same neuropsychologist. The neuropsychological assessment is structured in 2 parts: a semi-structured interview with the patient and an informant, and a neuropsychological test battery designed to evaluate multiple cognitive domains which are frequently impaired in patients with PD. Patients completed cognitive tests including the Mini-Mental State Examination (MMSE) and the Mattis Dementia Rating Scale 2 (MDRS-2), which includes subscales testing attention, memory, initiation/perseveration, construction, and conceptualisation. Memory was evaluated with 2 additional tests: the Rey Auditory Verbal Learning Test (RAVLT), which evaluates short-term auditory-verbal memory (immediate and delayed recall trials), and the Benton Visual Retention Test (BVRT), for visual memory. The SCWT was used to measure processing speed, selective attention, and cognitive flexibility. The letter-number sequencing subtest of the Wechsler Adult Intelligence Scale (WAIS-III) was administered to evaluate attention and working memory. Visuospatial ability was evaluated with the Benton Judgment Of Line Orientation (JLO) test and the clock-drawing test. To assess phonemic and semantic verbal fluency, patients were asked to produce as many words beginning with the letter “p” and to name as many animals as possible, with one minute for each category. In all tests, higher scores reflect better performance. The Montgomery–Asberg Depression Rating Scale (MADRS) was administered at each consultation to evaluate depression.
Given the inappropriateness on ethical grounds of including a control group in studies evaluating surgery outcomes, we retrospectively selected a group of 47 patients with PD (mean age [SD] of 66.1 years [6.1], with a mean disease duration of 8.8 years [4.4]), who were followed up for 5 years and were eligible for DBS according to the neurological criteria used, but who did not undergo the procedure for various reasons (patient not willing to undergo surgery, concomitant diseases, etc.). Despite its methodological limitations, this design gives an idea of the incidence of dementia over the same period of time in a group of patients with similar characteristics to those of our patients receiving DBS. We analysed the incidence of dementia based on the criteria established in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), performance in assessment scales (mainly the UPDRS I and the MMSE), and interviews with family members.
Informed consentBefore undergoing DBS, patients read and signed informed consent forms approved by the local research ethics committee, with family members acting as witnesses.
Statistical analysisQuantitative data are expressed as means (SD) and qualitative variables as percentages. Given the small size of our sample, non-parametric tests were used to compare means (Friedman and Wilcoxon tests). To analyse the predictive capacity of MDRS-2 scores for detecting dementia at 5 years (cut-off point <123), we used bivariate correlations (Pearson correlation coefficient), stepwise linear regression, and ROC curves to establish scaled scores with higher sensitivity for dementia screening. Statistical analysis was performed using the SPSS statistics software, version 20.0 (IBM; Armonk, NY, USA).
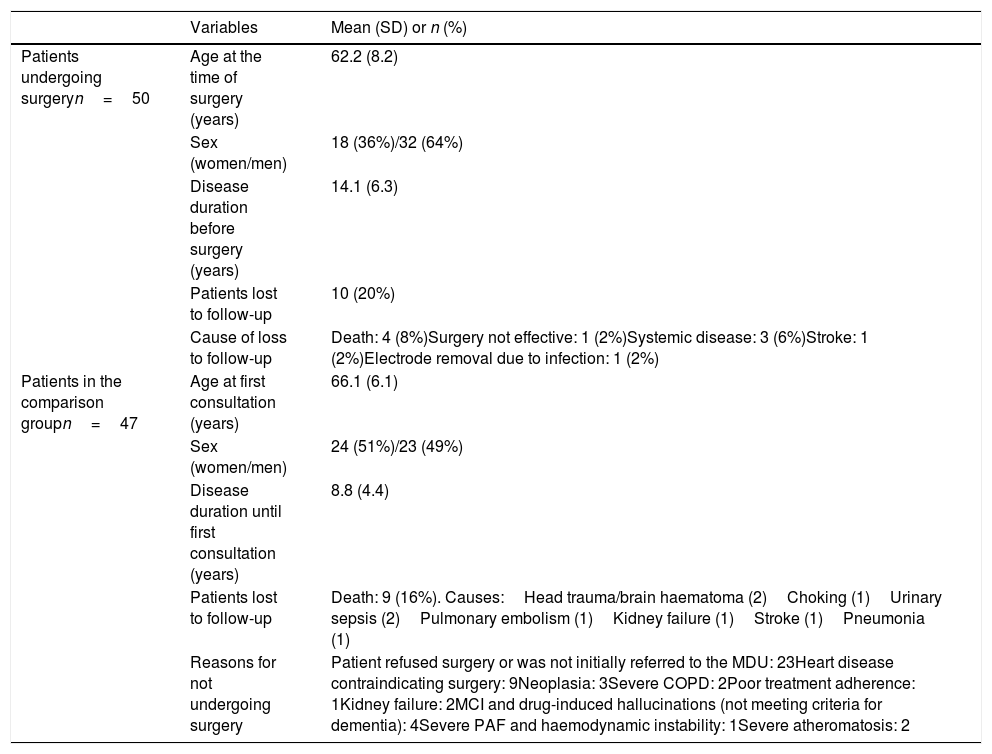
ResultsEighty percent of patients undergoing DBS were evaluated 5 years after the procedure; the remaining 20% were lost to follow-up: 4 died, 5 presented concomitant diseases preventing assessment, and the remaining patient was not assessed due to ineffectiveness of surgery (the pulse generator and electrodes were removed) (Table 2). Forty-eight patients were evaluated at one year, and 40 patients were assessed 5 years after the procedure.
Baseline characteristics of the 50 patients undergoing surgery (including causes of loss to follow-up during the first 5 years) and the 47 patients of the comparison group (including cause of loss to follow-up and reason for not undergoing surgery).
| Variables | Mean (SD) or n (%) | |
|---|---|---|
| Patients undergoing surgeryn=50 | Age at the time of surgery (years) | 62.2 (8.2) |
| Sex (women/men) | 18 (36%)/32 (64%) | |
| Disease duration before surgery (years) | 14.1 (6.3) | |
| Patients lost to follow-up | 10 (20%) | |
| Cause of loss to follow-up | Death: 4 (8%)Surgery not effective: 1 (2%)Systemic disease: 3 (6%)Stroke: 1 (2%)Electrode removal due to infection: 1 (2%) | |
| Patients in the comparison groupn=47 | Age at first consultation (years) | 66.1 (6.1) |
| Sex (women/men) | 24 (51%)/23 (49%) | |
| Disease duration until first consultation (years) | 8.8 (4.4) | |
| Patients lost to follow-up | Death: 9 (16%). Causes:Head trauma/brain haematoma (2)Choking (1)Urinary sepsis (2)Pulmonary embolism (1)Kidney failure (1)Stroke (1)Pneumonia (1) | |
| Reasons for not undergoing surgery | Patient refused surgery or was not initially referred to the MDU: 23Heart disease contraindicating surgery: 9Neoplasia: 3Severe COPD: 2Poor treatment adherence: 1Kidney failure: 2MCI and drug-induced hallucinations (not meeting criteria for dementia): 4Severe PAF and haemodynamic instability: 1Severe atheromatosis: 2 |
COPD: chronic obstructive pulmonary disease; MCI: mild cognitive impairment; MDU: movement disorders unit; PAF: pure autonomic failure.
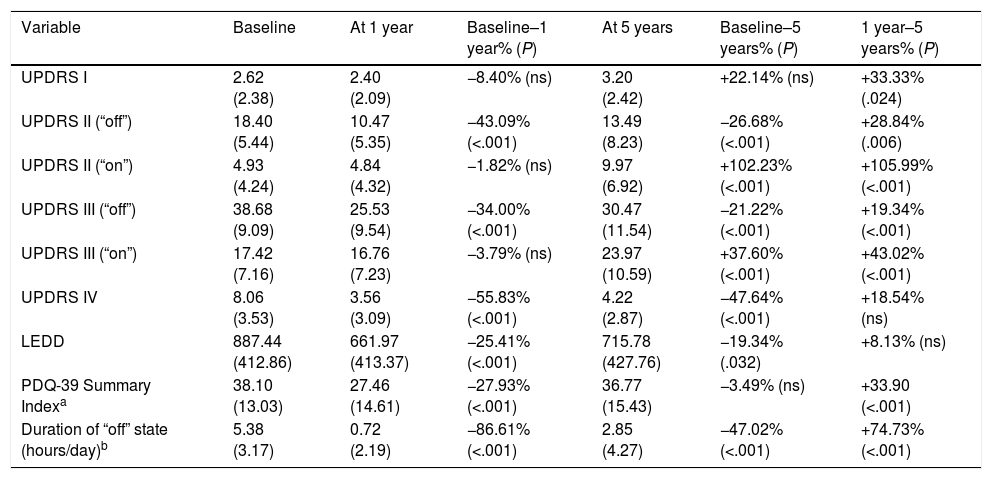
UPDRS I-IV scores are shown in Table 3. Patients were relatively young, with a mean age (SD) of 62.2 years (8.2) at the time of surgery, although with long disease progression times (14.1 years [6.3]). At baseline, a supramaximal dose of levodopa achieved a maximum improvement of 56% in UPDRS III scores. We observed significant differences between pre- and postoperative UPDRS III ratings, with scores showing improvements of 34% at year one and 22% 5 years after surgery in “drug-off/DBS-on” periods (Table 3). However, during “drug-on/DBS-on” periods, UPDRS III scores worsened by 37.60% 5 years after surgery compared to baseline and 1-year scores, which were similar (Table 3). We observed a significant decrease in the duration of “off” periods one year after the intervention; this improvement was still partially visible at 5 years (Table 3).
Scores for the different parts of the Unified Parkinson's Disease Rating Scale.
| Variable | Baseline | At 1 year | Baseline–1 year% (P) | At 5 years | Baseline–5 years% (P) | 1 year–5 years% (P) |
|---|---|---|---|---|---|---|
| UPDRS I | 2.62 (2.38) | 2.40 (2.09) | −8.40% (ns) | 3.20 (2.42) | +22.14% (ns) | +33.33% (.024) |
| UPDRS II (“off”) | 18.40 (5.44) | 10.47 (5.35) | −43.09% (<.001) | 13.49 (8.23) | −26.68% (<.001) | +28.84% (.006) |
| UPDRS II (“on”) | 4.93 (4.24) | 4.84 (4.32) | −1.82% (ns) | 9.97 (6.92) | +102.23% (<.001) | +105.99% (<.001) |
| UPDRS III (“off”) | 38.68 (9.09) | 25.53 (9.54) | −34.00% (<.001) | 30.47 (11.54) | −21.22% (<.001) | +19.34% (<.001) |
| UPDRS III (“on”) | 17.42 (7.16) | 16.76 (7.23) | −3.79% (ns) | 23.97 (10.59) | +37.60% (<.001) | +43.02% (<.001) |
| UPDRS IV | 8.06 (3.53) | 3.56 (3.09) | −55.83% (<.001) | 4.22 (2.87) | −47.64% (<.001) | +18.54% (ns) |
| LEDD | 887.44 (412.86) | 661.97 (413.37) | −25.41% (<.001) | 715.78 (427.76) | −19.34% (.032) | +8.13% (ns) |
| PDQ-39 Summary Indexa | 38.10 (13.03) | 27.46 (14.61) | −27.93% (<.001) | 36.77 (15.43) | −3.49% (ns) | +33.90 (<.001) |
| Duration of “off” state (hours/day)b | 5.38 (3.17) | 0.72 (2.19) | −86.61% (<.001) | 2.85 (4.27) | −47.02% (<.001) | +74.73% (<.001) |
LEDD decreased significantly at one year (−25.41%) and 5 years (−19.34%) after surgery compared to baseline. Only one patient received advanced PD treatment during the 5-year follow-up period (apomorphine infusion). The associated complications (UPDRS IV) also showed marked, sustained improvements (−55.83% at one year and −47.64% at 5 years). These changes had a significant positive impact on performance in activities of daily living (UPDRS II), which was more marked one year after surgery (−43.09%) than at 5 years (−26.28%). However, these improvements are poorly reflected in quality of life (PDQ-39) at 5 years after surgery, with nearly imperceptible changes compared to baseline (−3.49%); quality of life worsened between the first and the fifth year of follow-up (+33.90%) (Table 3).
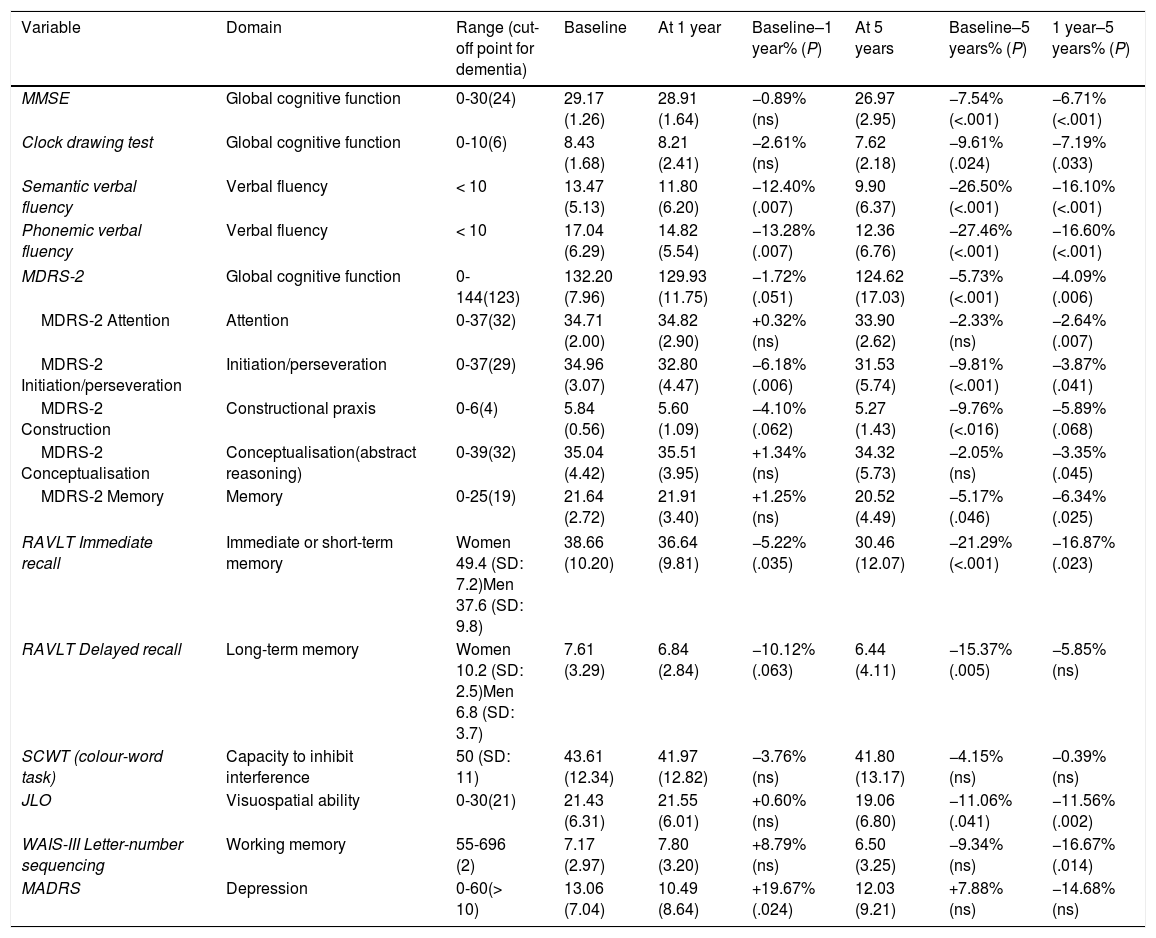
Neuropsychological test results at one year of follow-upAssessment scores for global cognitive function showed non-significant reductions one year after surgery (MMSE: −0.89%; clock-drawing test: −2.61%; MDRS-2: −1.72%) (Table 4). Visuospatial ability (JLO test) remained virtually unchanged one year after surgery. Patients showed impaired learning during the SCWT: 4 patients were unable to complete the test at baseline, compared to 11 patients at one year and 11 patients at 5 years. The remaining patients showed no significant neuropsychological impairment (Table 4). Both phonemic (−13.28%) and semantic verbal fluency (−12.40%) worsened significantly in the surgery group. The RAVLT revealed poorer delayed recall at one year (−10.12%); immediate recall worsened to a lesser extent (−5.22%). Working memory (letter-number sequencing subtest of the WAIS-III) showed non-significant improvements. Mood also improved: MADRS scores improved by 19.67%, reflecting less severe depressive symptoms (Table 4).
Scores on scales measuring cognitive function and evaluating depressive symptoms at baseline, at one year, and at 5 years of surgery.
| Variable | Domain | Range (cut-off point for dementia) | Baseline | At 1 year | Baseline–1 year% (P) | At 5 years | Baseline–5 years% (P) | 1 year–5 years% (P) |
|---|---|---|---|---|---|---|---|---|
| MMSE | Global cognitive function | 0-30(24) | 29.17 (1.26) | 28.91 (1.64) | −0.89% (ns) | 26.97 (2.95) | −7.54% (<.001) | −6.71% (<.001) |
| Clock drawing test | Global cognitive function | 0-10(6) | 8.43 (1.68) | 8.21 (2.41) | −2.61% (ns) | 7.62 (2.18) | −9.61% (.024) | −7.19% (.033) |
| Semantic verbal fluency | Verbal fluency | < 10 | 13.47 (5.13) | 11.80 (6.20) | −12.40% (.007) | 9.90 (6.37) | −26.50% (<.001) | −16.10% (<.001) |
| Phonemic verbal fluency | Verbal fluency | < 10 | 17.04 (6.29) | 14.82 (5.54) | −13.28% (.007) | 12.36 (6.76) | −27.46% (<.001) | −16.60% (<.001) |
| MDRS-2 | Global cognitive function | 0-144(123) | 132.20 (7.96) | 129.93 (11.75) | −1.72% (.051) | 124.62 (17.03) | −5.73% (<.001) | −4.09% (.006) |
| MDRS-2 Attention | Attention | 0-37(32) | 34.71 (2.00) | 34.82 (2.90) | +0.32% (ns) | 33.90 (2.62) | −2.33% (ns) | −2.64% (.007) |
| MDRS-2 Initiation/perseveration | Initiation/perseveration | 0-37(29) | 34.96 (3.07) | 32.80 (4.47) | −6.18% (.006) | 31.53 (5.74) | −9.81% (<.001) | −3.87% (.041) |
| MDRS-2 Construction | Constructional praxis | 0-6(4) | 5.84 (0.56) | 5.60 (1.09) | −4.10% (.062) | 5.27 (1.43) | −9.76% (<.016) | −5.89% (.068) |
| MDRS-2 Conceptualisation | Conceptualisation(abstract reasoning) | 0-39(32) | 35.04 (4.42) | 35.51 (3.95) | +1.34% (ns) | 34.32 (5.73) | −2.05% (ns) | −3.35% (.045) |
| MDRS-2 Memory | Memory | 0-25(19) | 21.64 (2.72) | 21.91 (3.40) | +1.25% (ns) | 20.52 (4.49) | −5.17% (.046) | −6.34% (.025) |
| RAVLT Immediate recall | Immediate or short-term memory | Women 49.4 (SD: 7.2)Men 37.6 (SD: 9.8) | 38.66 (10.20) | 36.64 (9.81) | −5.22% (.035) | 30.46 (12.07) | −21.29% (<.001) | −16.87% (.023) |
| RAVLT Delayed recall | Long-term memory | Women 10.2 (SD: 2.5)Men 6.8 (SD: 3.7) | 7.61 (3.29) | 6.84 (2.84) | −10.12% (.063) | 6.44 (4.11) | −15.37% (.005) | −5.85% (ns) |
| SCWT (colour-word task) | Capacity to inhibit interference | 50 (SD: 11) | 43.61 (12.34) | 41.97 (12.82) | −3.76% (ns) | 41.80 (13.17) | −4.15% (ns) | −0.39% (ns) |
| JLO | Visuospatial ability | 0-30(21) | 21.43 (6.31) | 21.55 (6.01) | +0.60% (ns) | 19.06 (6.80) | −11.06% (.041) | −11.56% (.002) |
| WAIS-III Letter-number sequencing | Working memory | 55-696 (2) | 7.17 (2.97) | 7.80 (3.20) | +8.79% (ns) | 6.50 (3.25) | −9.34% (ns) | −16.67% (.014) |
| MADRS | Depression | 0-60(> 10) | 13.06 (7.04) | 10.49 (8.64) | +19.67% (.024) | 12.03 (9.21) | +7.88% (ns) | −14.68% (ns) |
JLO: Benton Judgment of Line Orientation; MADRS: Montgomery–Asberg Depression Rating Scale; MDRS: Mattis Dementia Rating Scale; MMSE: Mini-Mental State Examination; ns: not significant; RAVLT: Rey Auditory Verbal Learning Test; SCWT: Stroop Color and Word Test; SD: standard deviation.
Global cognitive function was poorer at 5 years after surgery (MMSE: −7.54%; clock-drawing test: −9.61%; MDRS-2: −5.73%). Decreases were most marked in verbal fluency. From year one to year 5, semantic and phonemic fluency decreased an additional 16.10% and 16.6%, respectively. Cognitive function was considerably worse at 5 years (−26.50% in semantic fluency, −27.46% in phonemic verbal fluency). Changes were most marked in learning ability (RAVLT, immediate recall: −16.87%) and working memory (WAIS-III, letter-number sequencing subtest: 16.67%). Visuospatial ability was also considerably impaired compared to follow-up year one (−11.56%).
A year after surgery, 2 patients (4%) scored ≤ 26 on the MMSE, compared to 9 patients (22%) at 5 years. None of the patients scored below 20 points at 5 years after surgery. On MDRS, 10 and 14 patients (21% and 35%) scored below the cut-off score (130 points) at one and 5 years, respectively. The lowest MDRS-2 score of any patient in our sample was 81 points. Depressive symptoms (MADRS) improved during the first year following surgery but returned to baseline values at 5 years (Table 4).
Predictors of cognitive impairmentWe observed a strong correlation between baseline and 5-year follow-up MDRS-2 scores (correlation coefficient 0.78; P<.001). Stepwise linear regression, with MDRS-2 score at 5 years as the dependent variable and all baseline quantitative variables (age; progression time; scores on the UPDRS-I-IV, LEDD, MDRS-2, MMSE, clock-drawing test, WAIS-III, phonemic verbal fluency test, SCWT) as independent variables, identified baseline MDRS-2 score as the only predictive variable. ROC curves for the event “MDRS-2 score < 123 (cut-off point) at 5 years” show that baseline MDRS-2 scores above 130 had 23% sensitivity and baseline MDRS-2 scores above 137 had 0% sensitivity for predicting dementia at 5 years (area under the ROC curve: 0.15; P<.001).
Results from the comparison groupNine of the 47 patients included in the comparison group (19%) died within 5 years of follow-up; they had a mean age (SD) of 73.4 years (4.5) and a mean disease progression time of 13.1 years (4.8). Eighteen patients (38.3%) met DSM-IV criteria for dementia at 5 years of follow-up; this group had a mean age of 71.3 years (4.6). Table 2 shows the reasons that DBS was not administered to these patients.
DiscussionThe results of our long-term follow-up study reveal a slight decrease in MDRS-2, MMSE, and clock-drawing test scores in 5-year survivors; these decreases are not attributable to STN-DBS since worsening mostly occurred between years one and 5 of follow-up. During that period, a significant increase was observed in the number of patients scoring below 26 on the MMSE. The percentage of patients with cognitive impairment increased when more sensitive scales, such as the MDRS-2, were used. Thus, 35% (n=14) scored below 130 on the MDRS-2 scale at 5 years of follow-up. Interestingly, 21% of patients already scored below 130 at follow-up year one, due to poor performance in the initiation/perseveration subscale. Other studies using the MDRS-2 have also reported a similar decrease at 5 years after surgery.14,15 In a recent study with a shorter follow-up period (3 years), 14% of patients scored below 130 on the MDRS-2.18 In another study, however, 28% of patients developed dementia (DSM-IV criteria) during the 3-year follow-up period.19 Fasano et al.20 studied 32 consecutive patients undergoing STN-DBS, observing a slight decline in cognitive function 8 years after surgery, mainly in abstract reasoning and executive function. Verbal fluency was the most severely affected cognitive domain. However, only 20 patients were evaluated at 8 years; furthermore, the patients included were relatively young, with a mean age (SD) of 56.9 years (7.2).
In our study, 38.3% of patients from the comparison group met the DSM-IV diagnostic criteria for dementia at 5 years of follow-up. Dementia was defined differently in the DBS and comparison groups since the study was based on daily clinical practice; however, we may hypothesise that the incidence of dementia (DSM-IV criteria) in the study group at 5 years was similar to that of the comparison group. Other studies report variations in the estimated incidence of dementia in patients with PD, with similar ranges to those reported in studies including patients receiving medical treatment.21 Little is known about the impact of mild cognitive impairment on DBS outcomes in patients with PD. In a recent study, presence of mild cognitive impairment was not found to affect overall DBS outcomes. However, impairment of certain cognitive domains had a more deleterious effect; visuospatial impairment, for example, was predictive of poorer surgical outcomes.22 In our study, none of the patients scoring over 137 on the MDRS-2 at baseline scored below 123 at 5 years. Only 23% of those scoring over 130 showed cognitive impairment 5 years after surgery. According to the MDRS-2, construction and initiation/perseveration are more severely impaired than other cognitive domains. Impairment of posterior cortical functions including constructional praxis, visuospatial function, and semantic verbal fluency has been found to predict dementia in patients with PD.23 Our study revealed significantly impaired semantic and phonemic verbal fluency 5 years after surgery. However, as impairment was more severe one year after the procedure, it cannot be explained by the surgery or DBS alone.3,16,24
Learning ability was impaired at one year after surgery, as the RAVLT results show (immediate recall)3; delayed recall was less severely affected. SCWT results were not evaluable since 22% and 27% of patients were unable to complete the test at one and 5 years after surgery, respectively. The patients who did complete the test showed no significant impairment. Other studies have shown short-term impairment in patients’ capacity to inhibit interference.8
Depressive symptoms may worsen following STN-DBS; this effect depends on electrode location,25 whether dopaminergic treatment is reduced,13,26 and patients’ expectations of surgery. However, most studies report overall improvements in depressive symptoms.27 Our study showed a 20% improvement in MADRS scores one year after surgery; improvements were no longer observed at 5 years, however.
As reported by other studies, motor symptoms improved in patients undergoing STN-DBS; the benefits of DBS persisted at 5 years.13–15 Dopaminergic drug dose was significantly reduced and motor complications were significantly less frequent both at one and at 5 years. However, motor function worsened during “drug-on/DBS-on” periods at 5 years,13,15 especially in the case of axial symptoms.13,15,28,29 Our sample is unusual in that patients were younger (mean age [SD] of 62.2 years [8.2]) and had better baseline motor function (mean UPDRS-III score in “off” periods: 38.68 [9.09]) than those of other studies.14,15 Motor function improvements result in better performance in activities of daily living (UPDRS-III) at one (43.09%; P<.001) and 5 years (26.48%; P<.001). However, no improvements in quality of life (PDQ-39) were observed 5 years after surgery13; this may partially be explained by worsening of axial and cognitive symptoms, which have a great impact on quality of life.30,31 The mortality rate observed in our series (8%) is as expected for surgical patients in our sample's age group. Mortality was higher in the comparison group (19%); this may be explained by the fact that some patients were not eligible for surgery due to the presence of severe concomitant diseases (heart disease, kidney disease, etc.).
Our study has several limitations, including the lack of a control group. This may have masked the impairment caused by the natural course of PD. We included a group of retrospectively selected patients to analyse the number of patients with PD developing dementia after 5 years. Other limitations include the small sample size and the fact that 20% of patients were lost to follow-up, mainly due to death or systemic diseases. However, the few 5-year follow-up studies in the literature that analyse cognitive function, as well as the homogeneity of our sample and the fact that all patients were evaluated by the same neuropsychologist, confer consistency and validity to our results.
In conclusion, significant impairment of verbal function can be observed one year after surgery and increases over the 5 years following the procedure, although this cannot be explained by surgery exclusively. Degeneration caused by PD itself may explain visuospatial and learning impairment. Improvements in motor function are less pronounced at 5 years. Performance in activities of daily living improves, lowering the required doses of antiparkinson drugs, which in turn reduces the frequency of motor complications.
FundingThis study was partially funded by research grant INT-BC-2016-1 from Biocruces Bizkaia Health Research Institute.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Acera M, Molano A, Tijero B, Bilbao G, Lambarri I, Villoria R, et al. Impacto de la estimulación subtalámica a largo plazo sobre la situación cognitiva de los pacientes con enfermedad de Parkinson avanzada. Neurología. 2019;34:573–581.