Posterior reversible leukoencephalopathy syndrome (PRES) is a clinical condition whose actual incidence is still unknown. It was first described in 1996 and may be observed in acute patients, a majority of whom present such signs and symptoms as headache, altered level of consciousness, seizures, and/or bilateral loss of vision (cortical blindness). Neuroimaging studies usually reveal posterior leukoencephalopathy.1 This condition has been reported in the literature in association with various clinical entities, including hypertension, eclampsia, systemic neoplasms, kidney disease, sepsis, transplants, and immunosuppressive therapy.2–4
We present the case of a patient with postpartum eclampsia who developed acute psychotic symptoms and attempted suicide in the hospital. Imaging findings were characteristic of PRES, which disappeared after the clinical improvement which followed effective treatment for postpartum eclampsia.
Our patient was an 18-year-old pregnant woman (39.3 weeks) from Cali, Colombia, with no relevant history and no alterations detected in the prenatal checkups. She consulted the emergency department due to premature rupture of membranes of 2hours’ progression. An obstetric examination revealed that the foetus was in the breech position; 12hours after admission the patient was taken to the operating theatre for caesarian delivery, with no complications for the mother or baby.
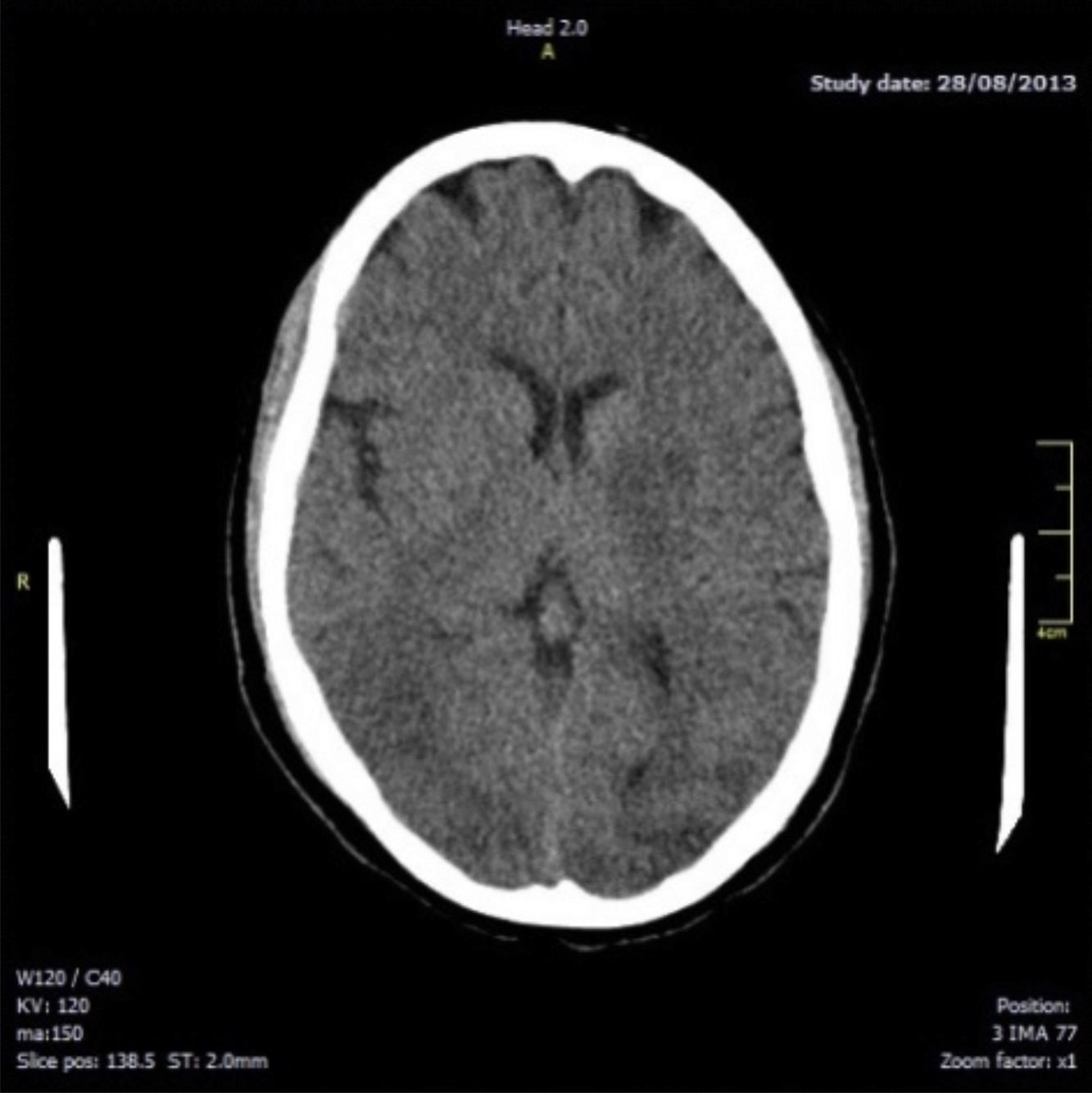
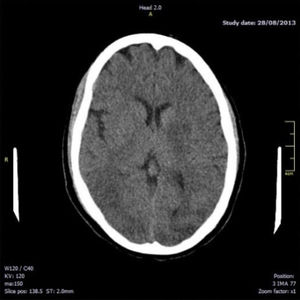
Fourteen hours after delivery, the patient reported intense holocranial headache with phosphenes; there were no signs of increased blood pressure. She subsequently presented a generalised tonic–clonic seizure lasting 1minute, associated with sphincter relaxation; this resolved with endovenous administration of benzodiazepines. An emergency brain computed tomography (CT) scan revealed hypodensities in the white matter of both hemispheres, involving the temporal-occipital region bilaterally and mainly the left-sided basal ganglion region (Fig. 1). These signs are highly indicative of symmetrical bilateral posterior leukoencephalopathy.
Complementary tests detected proteinuria and an increased level of lactate dehydrogenase (LDH) (Table 1), suggesting possible eclampsia with no initial presence of high blood pressure values. The patient started treatment with intravenous magnesium sulphate and remained under systemic and neurological monitoring at the intensive care unit.
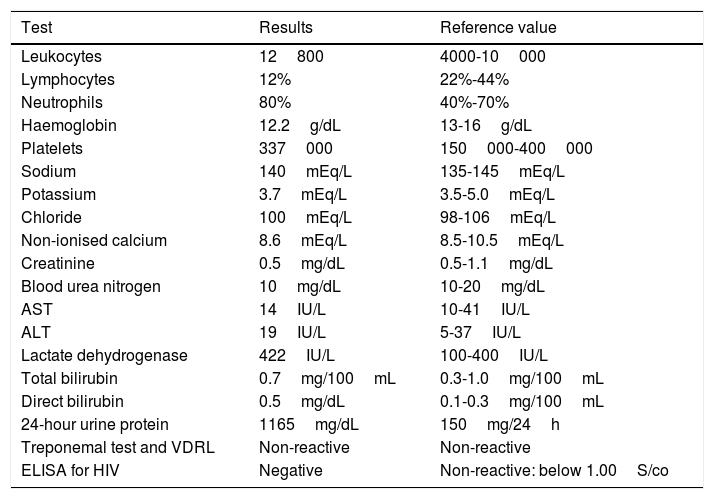
Findings from laboratory testing during hospitalisation.
| Test | Results | Reference value |
|---|---|---|
| Leukocytes | 12800 | 4000-10000 |
| Lymphocytes | 12% | 22%-44% |
| Neutrophils | 80% | 40%-70% |
| Haemoglobin | 12.2g/dL | 13-16g/dL |
| Platelets | 337000 | 150000-400000 |
| Sodium | 140mEq/L | 135-145mEq/L |
| Potassium | 3.7mEq/L | 3.5-5.0mEq/L |
| Chloride | 100mEq/L | 98-106mEq/L |
| Non-ionised calcium | 8.6mEq/L | 8.5-10.5mEq/L |
| Creatinine | 0.5mg/dL | 0.5-1.1mg/dL |
| Blood urea nitrogen | 10mg/dL | 10-20mg/dL |
| AST | 14IU/L | 10-41IU/L |
| ALT | 19IU/L | 5-37IU/L |
| Lactate dehydrogenase | 422IU/L | 100-400IU/L |
| Total bilirubin | 0.7mg/100mL | 0.3-1.0mg/100mL |
| Direct bilirubin | 0.5mg/dL | 0.1-0.3mg/100mL |
| 24-hour urine protein | 1165mg/dL | 150mg/24h |
| Treponemal test and VDRL | Non-reactive | Non-reactive |
| ELISA for HIV | Negative | Non-reactive: below 1.00S/co |
ALT: alanine aminotransferase; AST: aspartate transaminase; ELISA: enzyme-linked immunosorbent assay; LDH: lactate dehydrogenase; VDRL: Venereal Disease Research Laboratory test; HIV: human immunodeficiency virus.
Thirty-six hours after the onset of neurological symptoms, we observed increased mean arterial pressure (MAP) values (120-130mmHg); hypertension was controlled with intravenous labetalol complemented with oral antihypertensive drugs. The patient was also anxious, with hallucinations and soliloquies, and subsequently presented psychomotor agitation and hetero-aggression; she attempted to jump from the fifth floor of the hospital. At 24hours, she presented 2 further generalised tonic–clonic seizures lasting 30 and 60seconds, respectively, which were controlled with endovenous administration of benzodiazepines (diazepam), magnesium sulphate, and phenytoin. A new brain CT showed similar findings to those obtained in the first study.
During follow-up of the patient after the psychotic symptoms and seizures, we observed a significant improvement of neurological symptoms. She continued presenting somnolence, and blood pressure values normalised (AT 130/79, HR 108, and RR 17). Level of consciousness considerably improved, with normal orientation in time, space, and person, preserved judgement, logical thinking, coherent responses, and normal cognitive competence; no signs of focal neurological or functional deficit were observed. She was treated with oral antihypertensive drugs and intravenous magnesium sulphate, which satisfactorily controlled arterial pressure; the neurological and psychiatric manifestations fully resolved. The patient was systematically assessed with metabolic, infectious, immunological, and rheumatoid studies, which all yielded normal results. Together with the psychiatry department, we diagnosed the patient with PRES and established that the psychotic symptoms had resolved with the medical treatment of eclampsia. The patient remained asymptomatic until day 9 of hospitalisation. A follow-up brain CT showed no abnormalities, and the patient was discharged.
This young patient, with no relevant history and normal blood pressure throughout pregnancy, presented neurological signs and symptoms of acute progression including intense headache, phosphenes, seizures, and psychosis with behavioural alterations which led her to attempt suicide during her hospital stay; her final diagnosis was PRES. In this type of patients, we should perform differential diagnosis including, for example, delirium, which is frequent in hospitalised patients. However, during the first hours of follow-up, she did not present alterations in orientation or level of consciousness, which enabled us to rule out delirium.5,6 Although headache, seizures, altered level of consciousness, focal neurological signs, and such visual alterations as cortical blindness are typical findings of PRES1,4; concomitant psychosis is rarely reported in the literature.6 PRES therefore may cause a wide spectrum of both clinical and radiological manifestations. Axial CT images may be normal in these patients.4 However, some cases, such as the one described, initially show radiological findings typical of PRES, such as white matter hypodensities in the parieto-occipital region bilaterally, which is often indistinguishable from an acute ischaemic cerebrovascular event. In this case, magnetic resonance imaging should be the diagnostic technique of choice, showing hypointense and isointense lesions on T1- and diffusion-weighted sequences and hyperintensities on T2-weighted sequences.4 These lesions are caused by vasogenic oedema secondary to a functional alteration in cerebrovascular autoregulation.7
Our patient was a pregnant woman presenting headache, seizures, proteinuria, and increased blood pressure values, who was initially diagnosed with postpartum eclampsia; proper management with antihypertensive drugs and magnesium phosphate achieved effective control of arterial hypertension, and consequently a rapid resolution of neuropsychiatric symptoms and the changes characteristic of PRES identified at onset of neurological symptoms.5,8
In pregnant women with preeclampsia and eclampsia, PRES may contribute to the clinical manifestation of severe neurological complications. Early identification of these patients and timely treatment of the underlying systemic condition (in this case, eclampsia) and consequent neurological manifestations are important for the effective resolution of the neurological symptoms and radiological changes typical of this syndrome. This enables prevention of the irreversible neurological complications and disability typical of these patients.
Please cite this article as: Díaz-Ramírez GS, Salgado-Cifuentes CA, Zúñiga-Escobar G, Morales-Plaza CD. Síndrome de leucoencefalopatía posterior reversible asociado a psicosis: una presentación inusual. Neurología. 2019;34:549–551.