Pseudotumor cerebri (PC) in prepubertal patients displays certain characteristics that differentiate it from its presentation at the postpubertal stage. The aim of this study is to describe the characteristics of paediatric patients diagnosed with PC at our centre and to compare them according to their pubertal status.
Patients and methodsWe included patients aged between 1 and 18 years who were diagnosed with PC in a tertiary-level hospital between 2006 and 2019 and who met the updated diagnostic criteria for PC. They were classified according to body weight and pubertal status. Subsequently, we analysed results from lumbar punctures, neuroimaging studies, ophthalmological assessments, and treatments received during follow-up.
ResultsWe included 28 patients, of whom 22 were of prepubertal age and 6 were of postpubertal age. The mean age (standard deviation) was 9.04 (2.86) years. Among the postpubertal patients, 83.3% were boys, 66.7% of whom presented overweight/obesity. In the group of prepubertal patients, 27% were boys, 31.8% of whom were overweight. The most frequent symptoms were headache (89.9%) and blurred vision (42.9%). All patients presented papilloedema, and 21.4% manifested sixth nerve palsy. Possible triggers were identified in 28.6% of cases. Nineteen percent of patients presented clinical recurrence, all of whom were prepubertal patients. Complete clinical resolution was achieved in 55.6% of patients.
ConclusionPrepubertal patients with PC show lower prevalence of obesity, higher prevalence of secondary aetiologies, and higher recurrence rates than postpubertal patients.
El Síndrome de Pseudotumor Cerebri (SPTC) en pacientes prepuberales presenta características que lo diferencian respecto a su presentación en la etapa postpuberal. Nuestro objetivo es describir las características de los pacientes diagnosticados de SPTC pediátrico en nuestro centro y compararlas en función de su estado puberal.
Pacientes y métodosSe incluyeron a los pacientes diagnosticados de SPTC en un hospital de tercer nivel entre los años 2006 y 2019 con edades comprendidas entre 1 y 18 años que cumplieran los criterios diagnósticos actualizados del SPTC. Se clasificaron en función de su estado puberal y peso corporal. Posteriormente, se analizaron los datos de las punciones lumbares, estudios de neuroimagen, valoraciones oftalmológicas, así como el régimen terapéutico recibido a lo largo de su seguimiento.
ResultadoSe recogieron 28 pacientes, 22 prepuberales y 6 postpuberales, con edad media de 9,04 ± 2,86 años. El 83,3% de los pacientes postpuberales eran varones presentando sobrepeso/obesidad en el 66,7%. Eran varones el 27% de los pacientes prepuberales, de ellos asociaban sobrepeso el 31,8%. La sintomatología más frecuente fue cefalea (89,9%) y visión borrosa (42,9%). Todos los pacientes presentaron papiledema; un 21,4% de los casos presentaron parálisis del VI par. Se identificó un posible desencadenante en un 28,6%. El 19% presentaron recurrencia clínica, siendo todos ellos prepuberales. La resolución clínica completa se produjo en el 55,6% de los pacientes.
ConclusiónPacientes con SPTC presentan menor prevalencia de obesidad en la etapa prepuberal, junto con un mayor porcentaje de etiologías secundarias y tasa de recurrencia que los pacientes postpuberales.
Paediatric pseudotumour cerebri syndrome (PTCS) presents several distinctive features compared to the manifestation of the syndrome in adults.1
The syndrome is characterised by the appearance of signs (papilloedema, sixth cranial nerve palsy) and symptoms (headache, vision loss) of intracranial hypertension, in the absence of a structural lesion on neuroimaging or abnormal CSF findings. Depending on whether a trigger factor is identified, the syndrome is classified as primary (idiopathic intracranial hypertension) or secondary.2
PTCS is believed to be caused by an alteration in CSF circulation, due to either increased CSF production or decreased CSF absorption.3 Glymphatic system dysfunction has recently been linked to the development of the syndrome.4,5
Presentation of PTCS in adulthood is frequently associated with obesity, with a clear predominance in women6,7; in the paediatric population, however, this trend has only been observed in the postpubertal stage.8,9 Other distinctive features of paediatric PTCS include a higher proportion of cases of secondary pseudotumour cerebri and a higher frequency of asymptomatic idiopathic intracranial hypertension10; in these patients, the incidental finding of papilloedema constitutes the most frequent reason for study.
However, these distinctive features are not described consistently across series of paediatric PTCS. In a recent study by Mosquera et al.,11 secondary PTCS accounted for 41% of the sample, and none of the patients presented obesity. These findings stand in contrast with those presented by Bhalla et al.,12 who reported obesity in 20% of their cohort and paediatric secondary PTCS in only 16%.
These diverging results underscore the need for further research into paediatric PTCS. The purpose of this study is to analyse the epidemiological, clinical, and prognostic characteristics of paediatric patients with PTCS from our centre, and to compare them in terms of pubertal stage.
Patients and methodsWe conducted a descriptive, retrospective, longitudinal study of patients diagnosed with paediatric PTCS at the paediatric neurology unit of a tertiary-level hospital between February 2006 and November 2019.
The study included all patients aged 1–18 years at the time of diagnosis who met the revised diagnostic criteria for PTCS established by Friedman et al.2 A CSF opening pressure > 25 cm H2O (28 cm H2O in patients under sedation or presenting obesity) was required for a definite diagnosis, and > 20 cm H2O for a probable diagnosis. We excluded all patients for whom data on CSF opening pressure were not available and patients diagnosed with pseudopapilloedema but no other symptoms suggestive of intracranial hypertension.
The initial assessment included medical history taking, physical examination, and ophthalmological examination with ophthalmoscopy and visual acuity (VA) evaluation. After ruling out space-occupying lesions on head CT, we conducted a brain MRI study with and without contrast; most patients also underwent MRI angiography (venous phase). Lumbar puncture was performed with the patient sedated, in the lateral decubitus position, with knees in extension for opening pressure determination. Biochemistry, complete blood count, serology and autoimmunity tests, and hormone analysis were performed to rule out secondary causes. Humphrey visual field testing and Spectralis optical coherence tomography (OCT) were performed in cooperative patients.
Once diagnosis was established, treatment was started with acetazolamide dosed at 15−25 mg/kg; the dose was progressively increased based on patient response. During follow-up, patients were monitored for headache and underwent ophthalmoscopy, VA evaluation, OCT, and visual field testing, as well as visual evoked potentials and optic nerve ultrasound in some patients. Patients showing no response to treatment were administered such other drugs as furosemide, prednisone, spironolactone, or topiramate, or were treated surgically (ventriculoperitoneal shunt [VPS] or lumboperitoneal shunt [LPS]) in the event of severe visual impairment.
We studied the following variables: age, sex, clinical presentation, physical examination (VA, ophthalmoscopy, sixth cranial nerve palsy), comorbidities, laboratory test results, neuroimaging findings, CSF opening pressure, visual field testing, OCT, treatment and adverse effects, follow-up time, symptom resolution, and recurrence.
Patients were classified according to age-adjusted body mass index percentiles, as follows: normal weight (≥ 5th and < 85th percentiles), overweight (≥ 85th and < 95th percentiles), or obesity (≥ 95th percentile). The variable body weight was recoded into a dichotomous variable: normal weight or excessive weight (overweight and obesity).
Patients were also classified into 3 age groups: 1−5, 6−11, and 12−18 years. Based on previous studies, patients were classified as either prepubertal (< 12 years) or postpubertal (≥ 12 years).9,13 We conducted a descriptive analysis of qualitative data, which were expressed as absolute and relative frequencies, while quantitative variables were expressed as means and standard deviation (SD).
Hypothesis testing was performed bilaterally, using the Fisher exact test to compare percentages between subgroups; the threshold for statistical significance was set at P < .05. Data were collected, processed, and analysed with the SPSS statistics software, version 25 (IBM Corp.; Armonk, NY, USA).
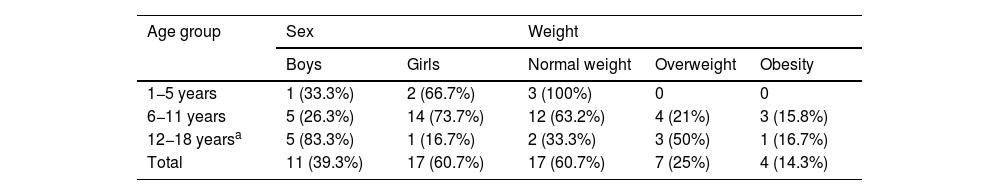
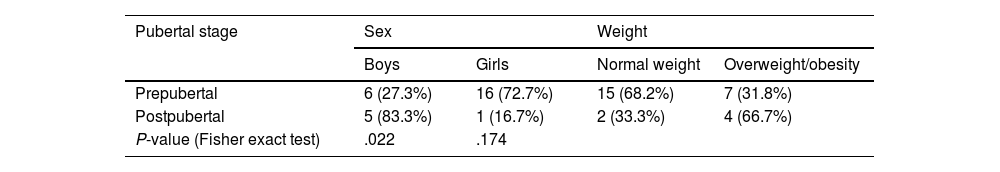
ResultsClinical characteristicsWe identified a total of 31 patients diagnosed with PTCS; 3 of these were excluded for not meeting the diagnostic criteria. Of the final sample (N = 28), 17 (60.3%) were girls and 11 (39.7%) were boys. Mean (SD) age was 9.04 (2.86) years. Table 1 presents the distribution of our sample by sex and weight classification. Table 2 presents the distribution of sex and weight classification by pubertal stage.
Sex and weight distribution by age group.
| Age group | Sex | Weight | |||
|---|---|---|---|---|---|
| Boys | Girls | Normal weight | Overweight | Obesity | |
| 1−5 years | 1 (33.3%) | 2 (66.7%) | 3 (100%) | 0 | 0 |
| 6−11 years | 5 (26.3%) | 14 (73.7%) | 12 (63.2%) | 4 (21%) | 3 (15.8%) |
| 12−18 yearsa | 5 (83.3%) | 1 (16.7%) | 2 (33.3%) | 3 (50%) | 1 (16.7%) |
| Total | 11 (39.3%) | 17 (60.7%) | 17 (60.7%) | 7 (25%) | 4 (14.3%) |
Sex and weight distribution by pubertal stage.
| Pubertal stage | Sex | Weight | ||
|---|---|---|---|---|
| Boys | Girls | Normal weight | Overweight/obesity | |
| Prepubertal | 6 (27.3%) | 16 (72.7%) | 15 (68.2%) | 7 (31.8%) |
| Postpubertal | 5 (83.3%) | 1 (16.7%) | 2 (33.3%) | 4 (66.7%) |
| P-value (Fisher exact test) | .022 | .174 | ||
Hypothesis contrast testing results were considered to be statistically significant if P < .05.
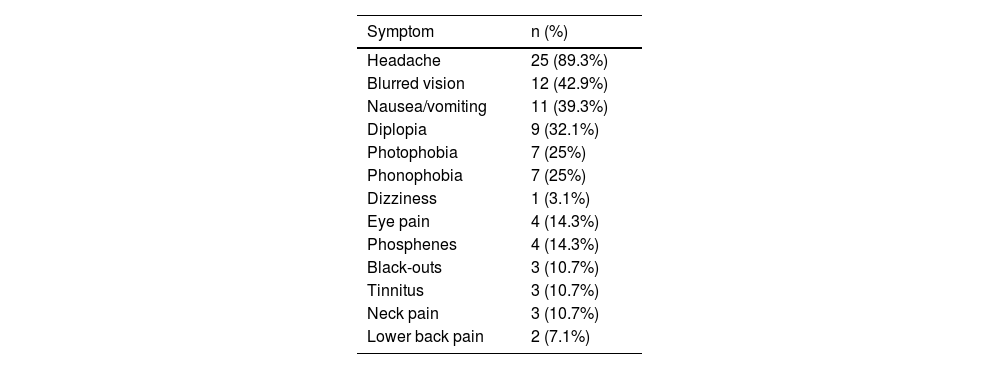
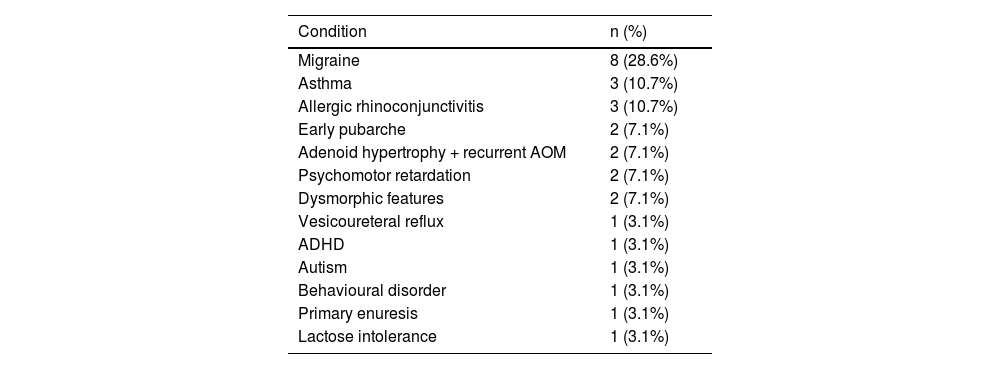
Table 3 summarises the initial manifestation in our sample; no patient was asymptomatic. Over half of patients (53.6%) presented at least one comorbidity; frequencies are presented in Table 4.
Frequency of symptoms of paediatric pseudotumour cerebri syndrome in our cohort.
| Symptom | n (%) |
|---|---|
| Headache | 25 (89.3%) |
| Blurred vision | 12 (42.9%) |
| Nausea/vomiting | 11 (39.3%) |
| Diplopia | 9 (32.1%) |
| Photophobia | 7 (25%) |
| Phonophobia | 7 (25%) |
| Dizziness | 1 (3.1%) |
| Eye pain | 4 (14.3%) |
| Phosphenes | 4 (14.3%) |
| Black-outs | 3 (10.7%) |
| Tinnitus | 3 (10.7%) |
| Neck pain | 3 (10.7%) |
| Lower back pain | 2 (7.1%) |
Medical history of our paediatric cohort of patients with pseudotumour cerebri syndrome.
| Condition | n (%) |
|---|---|
| Migraine | 8 (28.6%) |
| Asthma | 3 (10.7%) |
| Allergic rhinoconjunctivitis | 3 (10.7%) |
| Early pubarche | 2 (7.1%) |
| Adenoid hypertrophy + recurrent AOM | 2 (7.1%) |
| Psychomotor retardation | 2 (7.1%) |
| Dysmorphic features | 2 (7.1%) |
| Vesicoureteral reflux | 1 (3.1%) |
| ADHD | 1 (3.1%) |
| Autism | 1 (3.1%) |
| Behavioural disorder | 1 (3.1%) |
| Primary enuresis | 1 (3.1%) |
| Lactose intolerance | 1 (3.1%) |
ADHD: attention-deficit/hyperactivity disorder; AOM: acute otitis media.
The neurological examination detected sixth cranial nerve palsy in 6 patients (21.4%), which was bilateral in half of cases; 2 patients presented alternating exotropia. Eight patients (30.8%) presented VA below 1.0; of these, 3 presented VA below 0.5. A total of 23 patients (82.1%) presented bilateral papilloedema, one (3.6%) presented unilateral papilloedema, and in the remaining 4 (14.3%) differential diagnosis included pseudopapilloedema. Six patients (21.4%) presented splinter haemorrhages, which were unilateral in all cases.
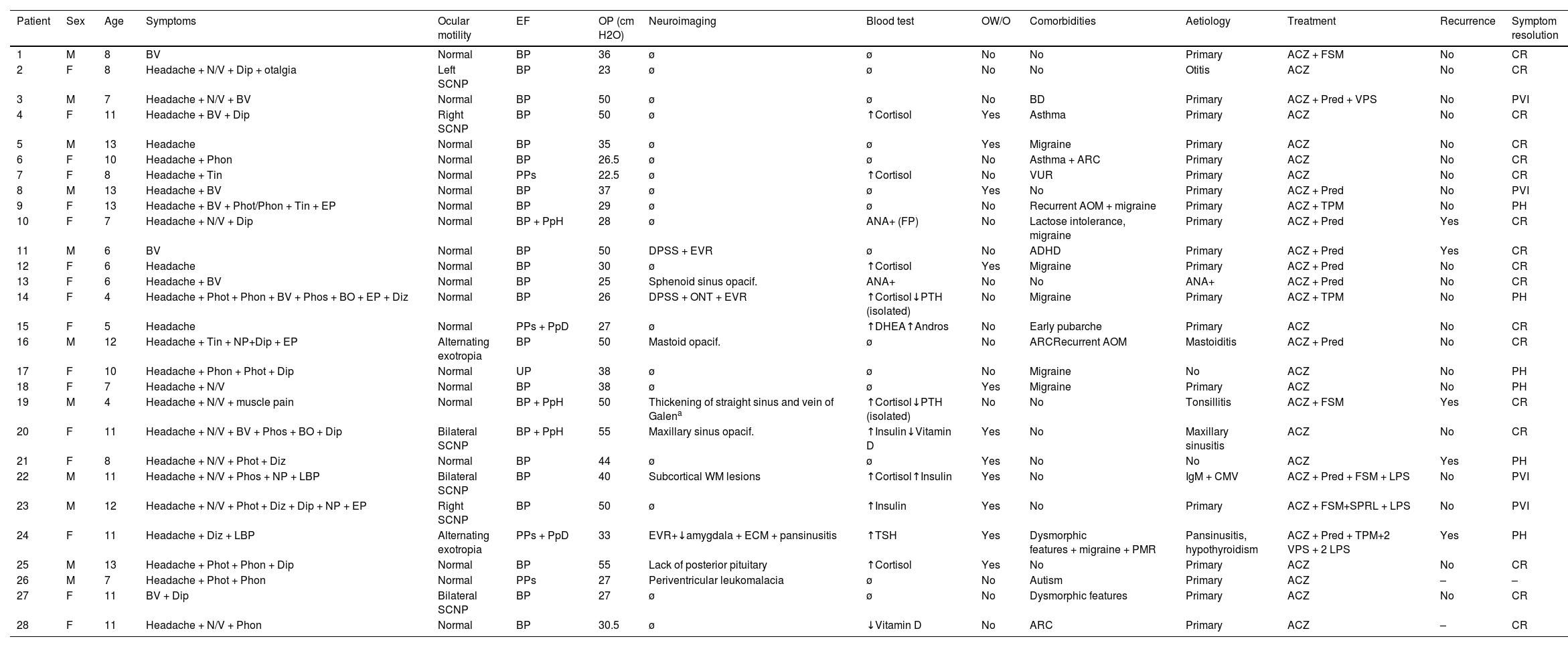
Complementary testsAll patients underwent brain MRI, and 88.5% also underwent venous MRI angiography. Four patients (14.3%) presented distension of the perioptic subarachnoid space (DPSS), with one also presenting optic nerve tortuosity. None of the patients presented globe flattening, transverse sinus stenosis, or empty sella syndrome. Other neuroimaging findings were sinus disease (4; 13.4%), enlarged Virchow-Robin spaces (3; 10.7%), enlarged cisterna magna (2; 7.1%), nonspecific subcortical signal intensity alterations (2; 7.1%), tonsillar descent (1; 3.6%), mastoid opacification (1; 3.6%), and absence of the posterior pituitary (1; 3.6%). Table 5 summarises the demographic and clinical characteristics of our sample.
Clinical and demographic characteristics of our cohort of paediatric patients with pseudotumour cerebri syndrome.
| Patient | Sex | Age | Symptoms | Ocular motility | EF | OP (cm H2O) | Neuroimaging | Blood test | OW/O | Comorbidities | Aetiology | Treatment | Recurrence | Symptom resolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 8 | BV | Normal | BP | 36 | ø | ø | No | No | Primary | ACZ + FSM | No | CR |
| 2 | F | 8 | Headache + N/V + Dip + otalgia | Left SCNP | BP | 23 | ø | ø | No | No | Otitis | ACZ | No | CR |
| 3 | M | 7 | Headache + N/V + BV | Normal | BP | 50 | ø | ø | No | BD | Primary | ACZ + Pred + VPS | No | PVI |
| 4 | F | 11 | Headache + BV + Dip | Right SCNP | BP | 50 | ø | ↑Cortisol | Yes | Asthma | Primary | ACZ | No | CR |
| 5 | M | 13 | Headache | Normal | BP | 35 | ø | ø | Yes | Migraine | Primary | ACZ | No | CR |
| 6 | F | 10 | Headache + Phon | Normal | BP | 26.5 | ø | ø | No | Asthma + ARC | Primary | ACZ | No | CR |
| 7 | F | 8 | Headache + Tin | Normal | PPs | 22.5 | ø | ↑Cortisol | No | VUR | Primary | ACZ | No | CR |
| 8 | M | 13 | Headache + BV | Normal | BP | 37 | ø | ø | Yes | No | Primary | ACZ + Pred | No | PVI |
| 9 | F | 13 | Headache + BV + Phot/Phon + Tin + EP | Normal | BP | 29 | ø | ø | No | Recurrent AOM + migraine | Primary | ACZ + TPM | No | PH |
| 10 | F | 7 | Headache + N/V + Dip | Normal | BP + PpH | 28 | ø | ANA+ (FP) | No | Lactose intolerance, migraine | Primary | ACZ + Pred | Yes | CR |
| 11 | M | 6 | BV | Normal | BP | 50 | DPSS + EVR | ø | No | ADHD | Primary | ACZ + Pred | Yes | CR |
| 12 | F | 6 | Headache | Normal | BP | 30 | ø | ↑Cortisol | Yes | Migraine | Primary | ACZ + Pred | No | CR |
| 13 | F | 6 | Headache + BV | Normal | BP | 25 | Sphenoid sinus opacif. | ANA+ | No | No | ANA+ | ACZ + Pred | No | CR |
| 14 | F | 4 | Headache + Phot + Phon + BV + Phos + BO + EP + Diz | Normal | BP | 26 | DPSS + ONT + EVR | ↑Cortisol↓PTH (isolated) | No | Migraine | Primary | ACZ + TPM | No | PH |
| 15 | F | 5 | Headache | Normal | PPs + PpD | 27 | ø | ↑DHEA↑Andros | No | Early pubarche | Primary | ACZ | No | CR |
| 16 | M | 12 | Headache + Tin + NP+Dip + EP | Alternating exotropia | BP | 50 | Mastoid opacif. | ø | No | ARCRecurrent AOM | Mastoiditis | ACZ + Pred | No | CR |
| 17 | F | 10 | Headache + Phon + Phot + Dip | Normal | UP | 38 | ø | ø | No | Migraine | No | ACZ | No | PH |
| 18 | F | 7 | Headache + N/V | Normal | BP | 38 | ø | ø | Yes | Migraine | Primary | ACZ | No | PH |
| 19 | M | 4 | Headache + N/V + muscle pain | Normal | BP + PpH | 50 | Thickening of straight sinus and vein of Galena | ↑Cortisol↓PTH (isolated) | No | No | Tonsillitis | ACZ + FSM | Yes | CR |
| 20 | F | 11 | Headache + N/V + BV + Phos + BO + Dip | Bilateral SCNP | BP + PpH | 55 | Maxillary sinus opacif. | ↑Insulin↓Vitamin D | Yes | No | Maxillary sinusitis | ACZ | No | CR |
| 21 | F | 8 | Headache + N/V + Phot + Diz | Normal | BP | 44 | ø | ø | Yes | No | No | ACZ | Yes | PH |
| 22 | M | 11 | Headache + N/V + Phos + NP + LBP | Bilateral SCNP | BP | 40 | Subcortical WM lesions | ↑Cortisol↑Insulin | Yes | No | IgM + CMV | ACZ + Pred + FSM + LPS | No | PVI |
| 23 | M | 12 | Headache + N/V + Phot + Diz + Dip + NP + EP | Right SCNP | BP | 50 | ø | ↑Insulin | Yes | No | Primary | ACZ + FSM+SPRL + LPS | No | PVI |
| 24 | F | 11 | Headache + Diz + LBP | Alternating exotropia | PPs + PpD | 33 | EVR+↓amygdala + ECM + pansinusitis | ↑TSH | Yes | Dysmorphic features + migraine + PMR | Pansinusitis, hypothyroidism | ACZ + Pred + TPM+2 VPS + 2 LPS | Yes | PH |
| 25 | M | 13 | Headache + Phot + Phon + Dip | Normal | BP | 55 | Lack of posterior pituitary | ↑Cortisol | Yes | No | Primary | ACZ | No | CR |
| 26 | M | 7 | Headache + Phot + Phon | Normal | PPs | 27 | Periventricular leukomalacia | ø | No | Autism | Primary | ACZ | – | – |
| 27 | F | 11 | BV + Dip | Bilateral SCNP | BP | 27 | ø | ø | No | Dysmorphic features | Primary | ACZ | No | CR |
| 28 | F | 11 | Headache + N/V + Phon | Normal | BP | 30.5 | ø | ↓Vitamin D | No | ARC | Primary | ACZ | – | CR |
ACZ: acetazolamide; ADHD: attention-deficit/hyperactivity disorder; ANA: antinuclear antibodies; Andros: androstenedione; AOM: acute otitis media; ARC: allergic rhinoconjunctivitis; BD: behavioural disorder; BO: black-outs; BP: bilateral papilloedema; BV: blurred vision; CMV: cytomegalovirus; CR: complete resolution; DHEA: dehydroepiandrosterone; Dip: diplopia; Diz: dizziness; DPSS: distension of the perioptic subarachnoid space; ECM: enlarged cisterna magna; EF: eye fundus; EP: eye pain; EVR: enlarged Virchow-Robin spaces; F: female; FP: false positive; FSM: furosemide; LBP: lower back pain; LPS: lumboperitoneal shunt; M: male; NP: neck pain; N/V: nausea/vomiting; ONT: optic nerve tortuosity; OP: opening pressure; opacif.: opacification; OW/O: overweight/obesity; PH: persistence of headache; Phon: phonophobia; Phos: phosphenes; Phot: photophobia; PMR: psychomotor retardation; PpD: peripapillary drusen; PpH: peripapillary haemorrhage; PPs: possible pseudopapilloedema; Pred: prednisone; PTH: parathyroid hormone; PVI: persistent visual impairment; SCNP: sixth cranial nerve palsy; SPRL: spironolactone; Tin: tinnitus; TPM: topiramate; UP: unilateral papilloedema; VPS: ventriculoperitoneal shunt; VUR: vesicoureteral reflux; WM: white matter; ø: normal; -: no data.
Mean (SD) CSF opening pressure was 35.05 cm H2O (11.52). Nine patients (32.1%) were diagnosed with probable PTCS, as they presented CSF opening pressure values of 20−28 cm H2O.
Laboratory tests detected elevated serum cortisol levels in 7 patients (25%), hyperinsulinaemia in 3 (10.7%), vitamin D deficiency in 2 (7.1%), elevated dehydroepiandrosterone and androstenedione levels in one (3.6%), CMV IgM positivity in one (3.6%), and ANA positivity in 2 (7.1%), one of the latter became negative for ANA during follow-up. Two patients (7.1%) presented low levels of parathyroid hormone, although this finding was isolated and had no impact on calcium or phosphorus metabolism.
A potential trigger for the symptoms was identified in 8 patients (28.6%): upper respiratory tract infection in 6 patients (otitis media in 1, mastoiditis in 1, tonsillitis in 1, sinusitis in 2, pansinusitis + hypothyroidism in 1), CMV IgM positivity associated with nonspecific MRI signal intensity alterations in one patient, and ANA positivity in the remaining patient. No significant differences in sex distribution or presence of excessive weight were observed between patients with primary or secondary PTCS. Prepubertal patients accounted for 75% of patients with primary PTCS and 87.7% of those with secondary PTCS. The Fisher exact test revealed no statistically significant differences in the percentage of prepubertal patients between aetiological subgroups (P = .640).
Twenty-one patients (75%) underwent OCT, which yielded normal results in 5 (23.8%) and revealed a generalised increase in peripapillary retinal nerve fibre layer thickness in 12 (57.1%) and a sectoral increase in peripapillary retinal nerve fibre layer thickness in 4 (19%). Visual field testing was performed in 26 patients (92.9%); results were considered uninformative in 10 patients (38.5%), normal in 4 (15.4%), and abnormal in 12 (46.15%). In the latter subgroup, alterations were mild in 9 patients (75%), moderate in one (8.3%), and severe in 2 (16.7%). The most frequent visual field defects were enlarged blind spot (4; 15.8%), partial arcuate defect (4; 15.8%), and visual field constriction (2; 7.7%). Visual evoked potentials were evaluated in 4 patients (14.3%), and were normal in all cases.
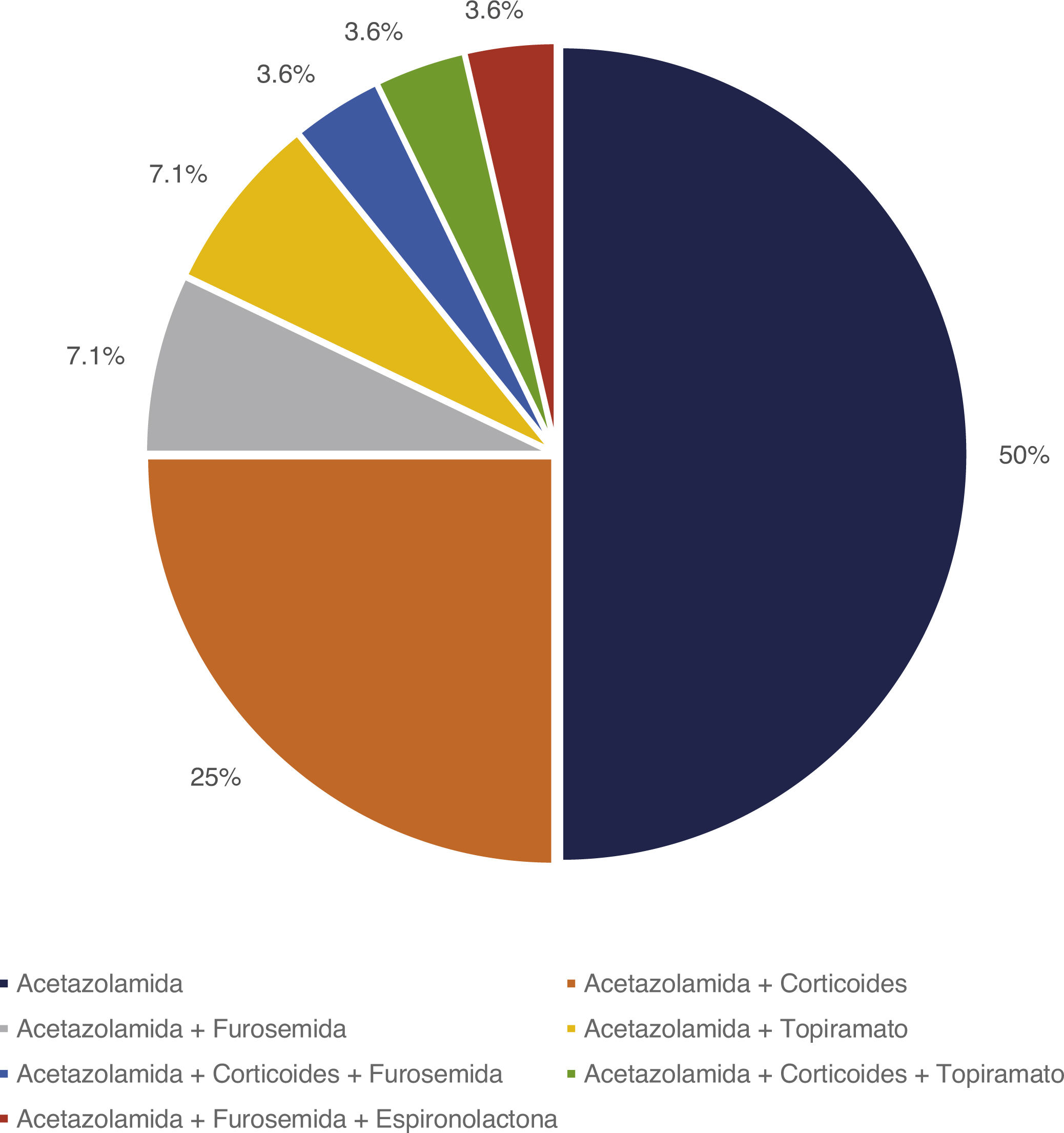
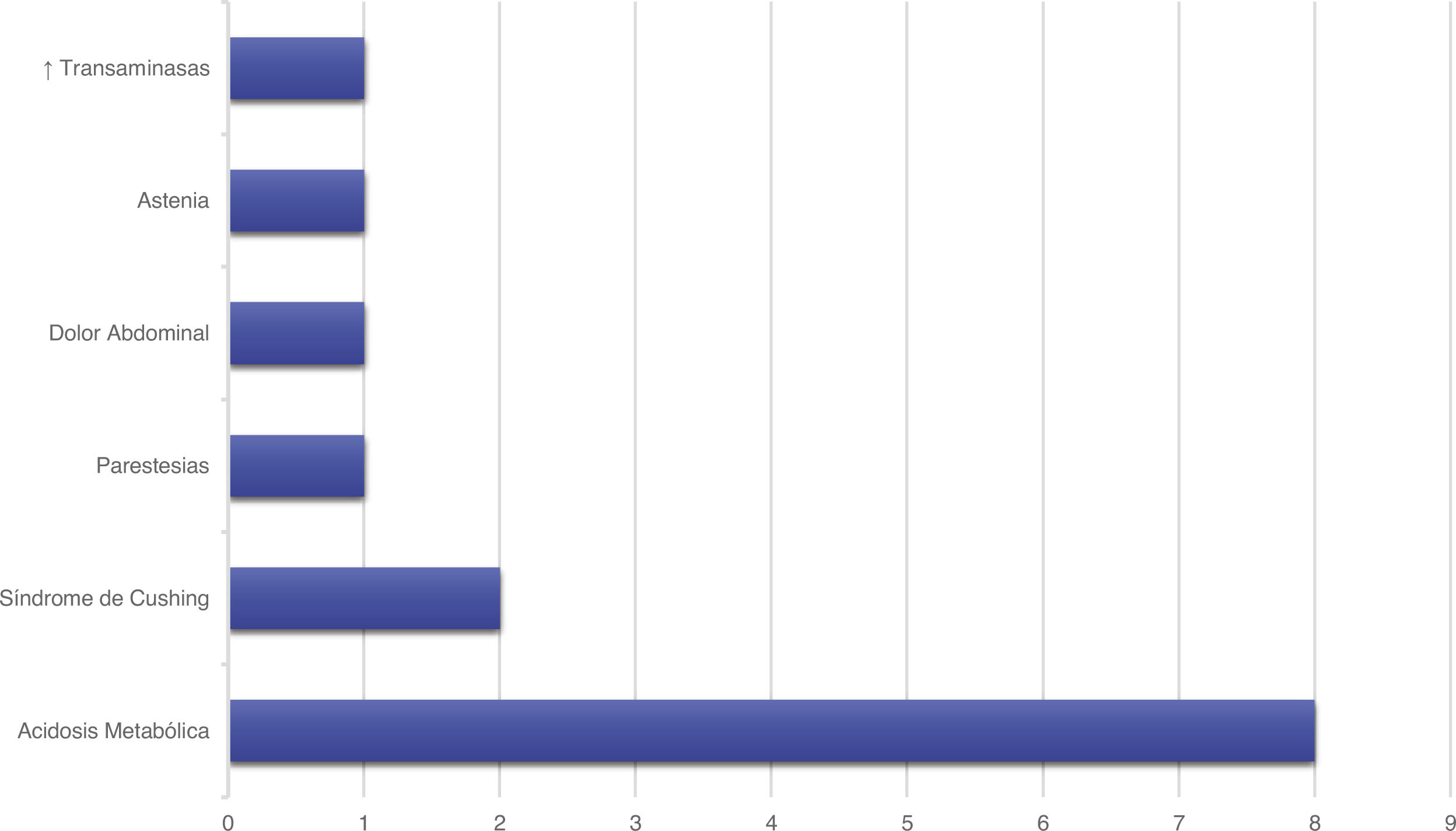
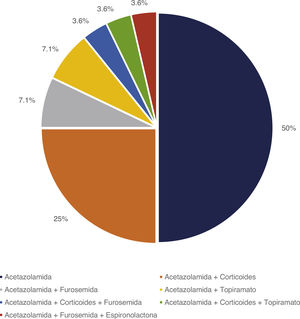
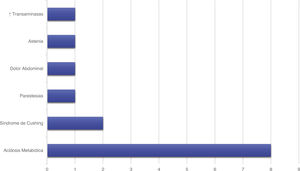
Treatment and progressionAll patients started treatment with acetazolamide, combined with other drugs according to the degree of treatment response. Fig. 1 summarises the pharmacological treatments administered. Twelve patients (42.9%) presented adverse drug reactions (Fig. 2), although these did not lead to treatment withdrawal in any case. Treatment was combined with dietary interventions in 10 patients (35.7%). Mean (SD) treatment duration was 39.51 (36.48) months.
Four patients (14.3%) required surgical treatment management due to lack of treatment response (LPS in 2 patients and VPS in one). One patient underwent C1 laminectomy (due to Chiari malformation) and LPS, and required 3 reinterventions (one LPS and 2 VPS due to shunt dysfunction secondary to infection/obstruction). After one of the VPS procedures, the patient presented haematoma in the caudothalamic area.
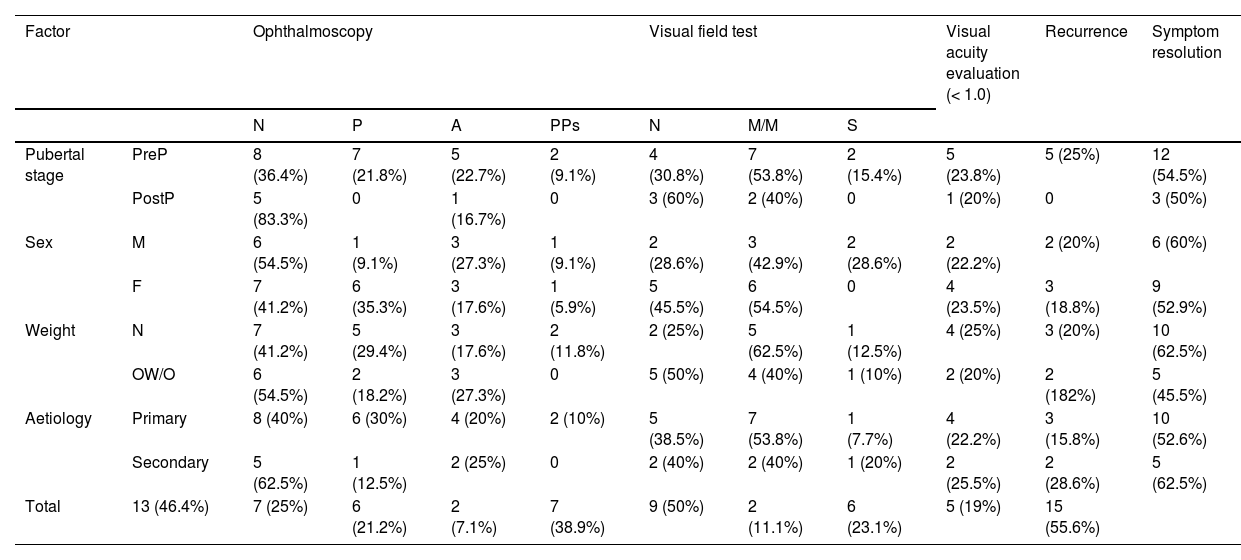
Mean (SD) follow-up time was 18.48 (19.31) months. Table 6 presents ophthalmoscopy, VA evaluation, and visual field testing results from the latest assessment, as well as the rates of recurrence and symptom resolution. Only one of the 22 patients for whom VA data were available presented VA below 0.8. Twenty-one patients underwent visual field testing, with results being uninformative in 3.
Prognosis of pseudotumour cerebri syndrome, by prepubertal stage, sex, weight classification, and aetiology.
| Factor | Ophthalmoscopy | Visual field test | Visual acuity evaluation (< 1.0) | Recurrence | Symptom resolution | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | A | PPs | N | M/M | S | |||||
| Pubertal stage | PreP | 8 (36.4%) | 7 (21.8%) | 5 (22.7%) | 2 (9.1%) | 4 (30.8%) | 7 (53.8%) | 2 (15.4%) | 5 (23.8%) | 5 (25%) | 12 (54.5%) |
| PostP | 5 (83.3%) | 0 | 1 (16.7%) | 0 | 3 (60%) | 2 (40%) | 0 | 1 (20%) | 0 | 3 (50%) | |
| Sex | M | 6 (54.5%) | 1 (9.1%) | 3 (27.3%) | 1 (9.1%) | 2 (28.6%) | 3 (42.9%) | 2 (28.6%) | 2 (22.2%) | 2 (20%) | 6 (60%) |
| F | 7 (41.2%) | 6 (35.3%) | 3 (17.6%) | 1 (5.9%) | 5 (45.5%) | 6 (54.5%) | 0 | 4 (23.5%) | 3 (18.8%) | 9 (52.9%) | |
| Weight | N | 7 (41.2%) | 5 (29.4%) | 3 (17.6%) | 2 (11.8%) | 2 (25%) | 5 (62.5%) | 1 (12.5%) | 4 (25%) | 3 (20%) | 10 (62.5%) |
| OW/O | 6 (54.5%) | 2 (18.2%) | 3 (27.3%) | 0 | 5 (50%) | 4 (40%) | 1 (10%) | 2 (20%) | 2 (182%) | 5 (45.5%) | |
| Aetiology | Primary | 8 (40%) | 6 (30%) | 4 (20%) | 2 (10%) | 5 (38.5%) | 7 (53.8%) | 1 (7.7%) | 4 (22.2%) | 3 (15.8%) | 10 (52.6%) |
| Secondary | 5 (62.5%) | 1 (12.5%) | 2 (25%) | 0 | 2 (40%) | 2 (40%) | 1 (20%) | 2 (25.5%) | 2 (28.6%) | 5 (62.5%) | |
| Total | 13 (46.4%) | 7 (25%) | 6 (21.2%) | 2 (7.1%) | 7 (38.9%) | 9 (50%) | 2 (11.1%) | 6 (23.1%) | 5 (19%) | 15 (55.6%) | |
Results from the latest ophthalmoscopy, visual field, and visual acuity tests. A: optic disc atrophy/pallor; F: female; M: male; M/M: mild/moderate visual field defect; N: normal; OW/O: overweight/obesity; P: papilloedema; PostP: postpubertal; PPs: probable pseudopapilloedema due to presence of peripapillary drusen; PreP: prepubertal; S: severe visual field defect.
Regarding remission of initial symptoms, 15 patients (55.6%) reported complete symptom resolution; 4 (14.8%) reported persistence of visual alterations, with partial improvements in 2 of them; and 8 patients (29.6%) reported persistent headache, with 6 of them being diagnosed with migraine.
Recurrence was defined as the reappearance of signs and/or symptoms of intracranial hypertension after resolution of the initial symptoms. Five patients (19%) presented recurrence. Mean (SD) age was 7.20 (2.58) years, and mean time from symptom resolution to recurrence was 14.20 (16.58) months. Treatment with acetazolamide was restarted in one patient, and 3 patients receiving acetazolamide in monotherapy required increased doses, with prednisone and furosemide being added in 2 cases, respectively. In one patient receiving acetazolamide and prednisone, the dose of acetazolamide was increased, although the drug was discontinued due to ineffectiveness, and topiramate was added. Three patients presented symptom resolution, with VA of 1.0; one patient continued to present sporadic headache with preserved VA; and another patient presented refractory headache and VA 0.7/1.0, requiring several neurosurgical interventions due to treatment resistance and complications.
All patients presenting recurrence were prepubertal, compared to 71.4% of patients presenting no recurrence. The Fisher exact test found no statistically significant differences in the percentage of prepubertal patients between the groups presenting and not presenting recurrence (P = .298).
DiscussionOur series of 28 patients presented a predominance of girls in the prepubertal group and boys in the postpubertal group. This finding stands in contrast with the results of previous studies, which report a similar sex distribution in the prepubertal stage, with female predominance after the age of 11–12 years.14 This difference is probably explained by the small number of patients in the postpubertal group (n = 6) and the small age difference between groups (maximum age of 13 years), which hinders differentiation between subgroups.
We observed a progressive increase in the percentage of patients with overweight or obesity in parallel with age, although differences were not statistically significant. This trend is consistent with results observed in the literature, with numerous studies reporting an increase in the prevalence of obesity among patients with PTCS in the postpubertal stage.8,9,14
Unlike other series, in which 15% to 22% of patients are asymptomatic at diagnosis,10,11,15 ours did not include any asymptomatic cases. These fluctuations may be linked to the greater difficulty faced by paediatric patients in describing their symptoms.
The most frequent comorbidity in our patients was overweight/obesity (39.3%), followed by migraine (28.6%) and asthma (10.7%); these findings are consistent with those reported in the literature.12 Several studies have suggested that obesity causes systemic inflammation, which may explain its association with asthma and play a role in the pathogenesis of PTCS.12,16
All patients presented papilloedema, except 4, in whom pseudopapilloedema was suspected; in these patients, the presence of drusen does not rule out PTCS. For example, in the series published by Gospe et al.,17 up to 48% of patients with PTCS presented optic disc drusen. Other recent retrospective studies have reported a prevalence of 9% in patients with PTCS without papilloedema.12,18 The fact that our series did not include patients without papilloedema may be explained by the low frequency of this patient profile, as well as the stricter diagnostic criteria in use since 2013, according to which diagnosis of PTCS requires the presence of sixth cranial nerve palsy; in the absence of this symptom, diagnosis can only be established if the patient presents at least 3 of the following head MRI findings: empty sella syndrome, DPSS, globe flattening, and transverse sinus stenosis.2
The frequency of MRI signs of intracranial hypertension varies greatly between studies. For instance, Görkem et al.19 reported DPSS in 88%, globe flattening in 56%, and reduced pituitary gland size in 64%, while other series include patients displaying no neuroimaging alterations.11,15 One possible explanation may be the preferential mechanism of CSF reabsorption in each patient: while patients with predominantly lymphatic outflow present DPSS, optic nerve tortuosity, or globe flattening, these findings are less prevalent in patients with predominantly venous outflow.20 This may be due to the fact that the cerebral lymphatic system in the dural venous sinuses drains into the sheaths of such cranial nerves as the optic nerve, with CSF overflow causing the previously mentioned MRI alterations. Furthermore, the predominance of one CSF outflow pathway or the other is influenced by age, with venous outflow being more frequent in the prepubertal stage.21 This may explain the lower prevalence of radiological signs in our series.
The increase in the size and number of Virchow-Robin spaces, or perivascular spaces, a finding observed in 3 of our patients, may be linked to glymphatic system dysfunction, which has recently been linked to the pathogenesis of PTCS.4,5 Lenck et al.20 proposed that glymphatic system congestion in PTCS translates into increased CSF volume in the subarachnoid space, brain interstitium, and perivascular spaces. Based on this hypothesis, we may conclude that the presence of enlarged Virchow-Robin spaces on MRI is a sign of PTCS; however, this finding has not been reported in studies describing the radiological characteristics of PTCS.22
As in our series, other authors report presence of sinusitis before or concomitantly with PTCS in 26.3% of patients.15 The mechanism by which sinusitis causes PTCS is unknown. The syndrome may be associated with cerebral venous sinus thrombosis; however, the diagnostic criteria for secondary PTCS only regard sinusitis as a cause when it is associated with cerebral venous abnormalities.2 In other series, the aetiology of the syndrome is identified in up to 77% of cases.23 This variability in the prevalence of secondary PTCS in the paediatric population may be linked to differences in the application of the diagnostic criteria proposed by Friedman et al.2 According to these criteria, neither vitamin D deficiency nor infectious processes constitute secondary causes, whereas other series do consider them as potential triggers.15
The percentage of patients with secondary PTCS in our series was 15% higher in prepubertal patients than in postpubertal patients (31.8% vs 16.7%); although this difference is not statistically significant, further research should aim to rule out trigger factors in this age group.
OCT may help to detect peripapillary drusen and papilloedema. The fact that 23.8% of our patients presented normal OCT results may be because the study was performed after onset of medical treatment. Furthermore, although visual field testing is useful for monitoring visual function in this age group, it is limited by the poor cooperation of our patients, with results being unreliable in 38% of cases.
In our series, 32.2% of the patients received corticosteroid treatment; all but one of these had been diagnosed with PTCS before 2014. Although the administration of corticosteroids reduces CSF production through inhibition of the 11β-HSD1 enzyme,16 corticosteroid therapy is not currently indicated for these patients due to the risk of symptom recurrence after treatment withdrawal, and the high prevalence of adverse reactions.24
Surgical treatment is recommended in the event of refractory visual dysfunction; the available techniques include CSF shunting, optic nerve sheath fenestration, and venous sinus stenting, depending on the case and each centre’s experience. In our series, 4 patients underwent CSF shunting, with complications only being observed in one patient.
In patients with PTCS and persistent headache despite medical treatment, it is important to determine whether the headache is due to intracranial hypertension or, rather, it is a chronic condition secondary to resolved intracranial hypertension in patients with a pre-existing primary headache disorder.25,26 The International Headache Society’s International Classification of Headache Disorders includes diagnostic criteria for headache attributed to idiopathic intracranial hypertension, emphasising worsening of pre-existing headache and the association with papilloedema and/or pulsatile tinnitus.27 Supplementation of antihypertensive treatment is not indicated in the absence of visual deterioration without worsening of papilloedema. In these cases, we must first rule out analgesic overuse and psychiatric comorbidities, and consider the administration of such drugs as topiramate or zonisamide, 2 medications for chronic migraine prophylaxis that promote weight loss and reduce CSF production.24–26
Visual function prognosis was poorer in our sample than in previous series: visual field defects were found in 61% of the 18 patients with visual field test results (vs 20% in other series), VA alterations in 23% (vs 20%), optic atrophy/optic nerve pallor in 21% (vs 13%), and chronic papilloedema in 25%.13,17 However, despite the high prevalence of visual alterations, these defects were mild in most of our patients, with only one patient presenting VA < 0.8 and 3 patients presenting moderate-to-severe visual field defects. The subjective distinction between normal results and a mild visual field defect or small VA fluctuations (0.9–1.0) may explain these differences between our results and those of previous studies.
The recurrence rate in our sample was 19.2%, a similar rate to those reported in the literature.6,12,28 All patients presenting recurrence were in the prepubertal group; furthermore, recurrence was 13% more frequent among patients in whom a possible cause for the syndrome had been identified, although these differences were not significant.
Treatment for recurrence depends on severity, trigger factors, baseline treatment, and treatment tolerance. The standard procedure at our centre is to restart or intensify treatment with acetazolamide, adding such complementary drugs as topiramate or furosemide, depending on tolerance. Surgery (CSF shunting) is considered in the event of treatment resistance or progression of visual deterioration.
Our study presents several limitations, such as its retrospective design. Furthermore, the classification of patients as prepubertal or postpubertal was established according to age, rather than based on the Tanner staging classification system. Due to this limitation and the small size of the postpubertal subgroup, our data should be interpreted with caution.
PTCS includes a spectrum of diseases manifesting with abnormal CSF flow; the factors inducing this alteration in prepubertal patients may be different from those in postpubertal or adult patients. In prepubertal patients, the higher prevalence of secondary causes and the lower prevalence of excessive weight suggest a different aetiopathogenic mechanism, probably linked to changes in the hormonal environment.
FundingThis study received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.