Theory of mind (ToM) is the human ability to perceive, interpret, and attribute the mental states of other people, and the alteration of this cognitive function is a core symptom of autistic spectrum disorder (ASD). In such other neurodevelopmental disorders as childhood-onset obsessive-compulsive disorder (OCD) and Tourette syndrome (TS) that can present with cognitive dysfunctions, ToM has been less extensively studied, especially in the young population. The aim of the study was to compare advanced ToM between groups of young people diagnosed with OCD, TS, or ASD and a control group.
MethodsClinical interviews were conducted with male patients aged between 11 and 17 years with a main diagnosis of OCD (n = 19), TS (n = 14), or ASD (n = 18), and a control group (n = 20). We administered instruments for estimating intelligence quotient and severity of psychiatric symptoms, and tasks to evaluate ToM (the "Stories from everyday life" task and the "Reading the mind in the eyes” test).
ResultsYoung people with TS and with ASD present similar difficulties in solving advanced ToM tasks, whereas patients with childhood-onset OCD present similar results to controls.
ConclusionsToM is altered in other neurodevelopmental disorders beyond ASD, such as TS.
La Teoría de la Mente (ToM) es la capacidad humana de percibir, interpretar y atribuir los estados mentales de las otras personas y la alteración de esta función cognitiva es un síntoma nuclear del Trastorno del Espectro Autista (TEA). Hay otros trastornos del neurodesarrollo como el Trastorno Obsesivo-Compulsivo de inicio en la infancia (TOC) y el Síndrome de Tourette (ST), que pueden presentarse con disfunciones cognitivas, y en los que la ToM ha sido menos estudiada, especialmente en población juvenil. El objetivo de este estudio fue comparar la ToM avanzada entre grupos de jóvenes con diagnóstico de TOC, ST o TEA y un grupo de controles sanos.
MétodosSe entrevistaron clínicamente a varones de entre 11 y 17 años con diagnóstico principal de TOC (n = 19), ST (n = 14), TEA (n = 18), y un grupo control de sujetos sanos (n = 20). Se les administraron instrumentos de estimación de cociente intelectual, severidad de los síntomas psiquiátricos, y las pruebas para evaluar la ToM: la tarea Historias de la Vida Cotidiana y el Test de la Mirada.
ResultadosLos jóvenes con ST presentan dificultades similares para resolver tareas de ToM avanzada al nivel de los pacientes con TEA, a diferencia de los pacientes con TOC de inicio en la infancia que presentan resultados similares a los controles sanos.
ConclusionesLa ToM está alterada en otros trastornos del neurodesarrollo más allá del TEA, como en el ST.
Social cognition is a construct encompassing a range of cognitive processes by which we store, process, and use information about other people.1 It includes multiple subdomains, such as the interpretation of social cues, emotional recognition, empathy, and theory of mind (ToM).2 ToM, defined as the ability to infer the feelings and emotions of other people, is a prerequisite for successful social interaction.3 The complexity of this cognitive function constitutes a challenge for its conceptual delimitation, which involves different levels and types of information processing, with more cognitive (inferring the motivations and beliefs of others) and more affective aspects (inferring the feelings and emotions of others).3 Furthermore, there is evidence of an association between ToM and other neurocognitive functions.4,5 Multiple tasks have been developed to assess ToM, such as false belief tasks, which explore the ability to differentiate between one’s own knowledge about one’s surroundings from what another person may believe about them.6 These tasks are divided into 2 levels of difficulty: first-order (e.g., what is this person thinking?), and second-order (e.g., what do you think that another person thinks about what the first person thought?).6 Some are designed for pre-school children,7 and assess first- and second-order false beliefs, but do not discriminate more complex ToM skills acquired at the age of 6–8 years in neurotypical individuals. To evaluate ToM after this ceiling effect occurs, so-called advanced ToM tasks have been developed that examine an individual’s capacity to generate attributions based on a social interaction in which they must correctly infer the mental or emotional state of a character shown interacting with others. Examples include the Happé’s8 Strange Stories task, which evaluates the attribution of intentions to others, such as sarcasm or white lies, or the test developed by Baron-Cohen et al.9 examining the ability to detect social faux pas. Another advanced theory of mind test, the “Reading the Mind in the Eyes” task (Eyes Task for short),10 differs from the previous tests in that subjects are only provided information from the eye area. In this case, attributions refer not only to emotions or mental status, but also to intentions, such as “wanting to play.” Finally, more complex ToM tasks address the ability to attribute beliefs, desires, and intentions, as well as the child’s ability to understand pragmatic language.11
ToM dysfunction is considered a key symptom in autistic spectrum disorders (ASD), both in adult and in paediatric populations.7,8,11,12 However, while less attention has been placed on ToM in other disorders, this dysfunction is not specific to ASD, and is also reported in other neurodevelopmental disorders.13,14 Specifically, childhood-onset obsessive compulsive disorder (OCD) and Tourette syndrome (TS) are neurodevelopmental disorders that, like ASD, can be severe and present in some patients with social difficulties, emotional outbursts, and such motor signs as repetitive movements.15,16
OCD is characterised by the presence of persistent undesired obsessions that cause elevated anxiety or ritualistic behaviours, or repetitive mental acts that are performed to alleviate the anxiety caused by the obsessions. A recent systematic review,17 including 10 articles on ToM in adult patients with OCD, reported that the majority of studies did not find statistically significant differences in ToM task performance between patients and controls.18–20 However, some studies did find evidence of ToM deficits in these patients, particularly in second-order false belief tasks,21,22 although the available evidence does not clearly show whether said deficits are related to the neurocognitive alterations that are also observed in some of these patients.14,23
TS is characterised by tics, repetitive movements, and vocalisations provoked by a sensory-cognitive premonitory urge. There is considerable evidence that patients with TS may have poorer ToM skills than healthy controls in the adult population.24–27 A study using a self-reported questionnaire to compare 95 patients with TS against 60 healthy controls of both sexes concluded that the TS groups presented differences in interpersonal reactivity with respect to controls, characterised by a reduced tendency to understand the perspectives of others and marked distress in response to intense emotional situations in others.25 Another study, including 18 adult patients diagnosed with TS and 10 healthy controls,24 reported that patients were less accurate than controls in interpreting sarcasm and metaphor in various ToM tasks. Neuroimaging studies have also identified differences in brain activity in somatosensory areas associated with the mirror neuron system during the performance of ToM tasks in patients with TS (e.g., at the temporoparietal junction, posterior cingulate).27 Patients with TS show greater activity than healthy controls in the lateral orbitofrontal cortex, posterior cingulate, right temporoparietal junction, and right amygdala.26
One of the main issues with the current scientific evidence on ToM difficulties in OCD and TS, unlike in other neurodevelopmental disorders, is the limited literature on adult populations and near-complete lack of research on paediatric populations. To our knowledge, only one study has compared different neurodevelopmental disorders, studying 4 patient groups: 35 with ASD, 33 with severe mood dysregulation, 32 with childhood-onset OCD, and 20 with TS; no control group was included. Patients were studied with the 65-item Social Responsiveness Scale, which dimensionally evaluates 5 domains of social reciprocity.28,29 Social reciprocity includes the following components: social communication (interaction), understanding of how to react in social situations (social awareness), the desire to interact with other people (social motivation), the ability to attribute a perspective to other people (social cognition), and proper management of atypical behaviours in social situations. No significant difference was observed between the OCD and TS groups in social cognition and communication scores. The ASD group showed the greatest deficits, followed by the severe mood dysregulation group.29
These neurodevelopmental disorders share certain neuroanatomical, cognitive, and behavioural characteristics that may explain the similarities between the ToM dysfunctions observed in the adult population. In the light of the fact that satisfactory interpersonal relationships constitute a protective factor for mental health, and are associated with an adequate capacity to perceive and interpret human behaviour, these deficits must be analysed in paediatric populations with specific, sensitive advanced ToM tasks.
ObjectiveThe primary objective of this study is to compare advanced ToM in groups of children and adolescents diagnosed with OCD, TS, and ASD, and in a group of healthy controls. The secondary objective is to examine how ToM is influenced by clinical characteristics and other aspects of neurocognitive function.
Patients and methodsPatientsWe gathered a sample of boys aged 11–17 years from the outpatient clinic of the paediatric psychiatry department of Hospital Clínic (Barcelona, Spain), with a main diagnosis of OCD (n = 19) or TS (n = 14), established according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). “Main diagnosis” refers to the diagnosis for which the patient and their family were consulting the department and which caused the greatest interference in their functioning (although comorbidities could also be diagnosed during follow-up). In addition to these samples, we used data gathered from participants in another study, from the same outpatient clinic,30 with the permission of the authors of that study. The sample from this second study included boys with a main diagnosis of ASD (n = 29) and a control group of healthy boys (n = 25). To enable age matching of samples, we only included 18 of the patients with ASD and 20 controls. The sample was restricted to male patients due to the low prevalence of ASD diagnoses in girls in our setting. The final sample included 19 patients with OCD, 14 with TS, 18 with ASD, and 20 healthy controls.
ProcedureThe study was approved by the Hospital Clínic ethics committee, and the parents or guardians of all patients gave written informed consent prior to assessment. All patient data were anonymised in compliance with Spanish data protection legislation. The test battery applied has an approximate duration of 3.5 hours, and includes the Kaufman Brief Intelligence Test (KBIT),31 a series of clinical questionnaires administered to parents/guardians (Autism Spectrum Screening Questionnaire [ASSQ]32) and patients (Children’s Yale-Brown Obsessive Compulsive Scale [CY-BOCS],33 Yale Global Tic Severity Scale [YGTSS]34), and advanced ToM tasks (the Stories from Everyday Life task [SEL]11 and the Eyes Task10). The 2 ToM tasks evaluate patients’ capacity to correctly infer a mental state based on a social interaction (SEL) or an image of the eye area (Eyes Task). All participants were evaluated by experienced specialists in paediatric clinical psychology or psychiatry from Hospital Clínic, with specific training in the administration of the SEL.11 In all cases, audio recordings were made during the SEL task, then anonymised for review and scoring by other members of the research team. To rule out psychopathology in members of the control group, we conducted a standardised, semi-structured interview with participants and their parents, applying the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version,35 an instrument used to diagnose psychiatric disorders in children and adolescents according to the criteria of the DSM-IV-TR. Clinical instruments were administered by 2 different research teams, at different times, although all professionals participated in a joint training session on the administration of the SEL. Furthermore, some instruments, such as the YGTSS, were included later, and therefore were not applied to the ASD group. In all groups, we excluded individuals with an intelligence quotient (IQ) below 70 or diagnosed with a psychotic disorder, substance use disorder, or disabling neurological disease. We also excluded patients with comorbid diagnoses of ASD plus OCD or ASD plus TS, according to data from their clinical records. Members of the research team who validated the Spanish-language version of the SEL30 administered the Autism Diagnostic Interview Revised36 to the main caregivers of patients in the ASD group to confirm the diagnosis. Comorbidity data for the OCD, TS, and ASD groups were provided by their regular physicians (psychiatrists or clinical psychologists) via clinical records, as screening for all mental disorders is performed during the first consultations.
Assessment instruments applied to parentsAutistic Spectrum Screening Questionnaire32:
The ASSQ is a screening instrument for the detection of high-functioning autistic spectrum disorders in individuals aged 7–17 years, for administration to parents. Data are presented as direct scores. A score of 22 based on information from parents indicates a need for more specific assessment of the children. In this study, the ASSQ was used to evaluate the severity of ASD symptoms in all participants, although only patients in the ASD group met criteria for this diagnosis. The administration time of the instrument is ≤ 10 minutes.
Assessment instruments applied to participantsChildren’s Yale-Brown Obsessive-Compulsive Scale33,37:
The CY-BOCS is the gold standard instrument for assessing the severity of OCD in children. It is a semi-structured clinical scale comprising 10 items (5 on obsessions and 5 on compulsion/rituals) that evaluate time, interference, distress, resistance, and control of symptoms. The maximum score for each item is 4 points (maximum total score of 40). Severity of OCD is classified as subclinical (≤ 10 points), mild (11–16), moderate (17–24), severe (25–32), or extreme (33–40). The validation study in a Spanish population found a Cronbach alpha of 0.87. Administration time ranges from 45 to 60 minutes.
Yale Global Tic Severity Scale34,38:
The YGTSS is the gold standard instrument for assessing the severity of TS. This semi-structured clinical scale begins with a semi-systematic inventory of tic symptoms, which the clinician marks as being present or absent over the past week and at the worst ever moment of the disease. Current motor and verbal tics are evaluated separately according to number, frequency, intensity, complexity, and interference, on a 6-point ordinal scale (0: absent; 1−5: level of severity), yielding 3 scores: total motor tic score, total verbal tic score, and total tic score (sum of the previous 2 scores, with a maximum of 50). Finally, the scale provides a global severity score (0–100), which is the sum of the total tic score (0–50) and the overall impairment rating (0–50). Severity is classified as subclinical (≤ 10 points), mild (11–20), marked (21–40), or severe (> 40). The validation study in a Spanish population found a Cronbach alpha of 0.99 for both dimensions (motor tics and phonic tics). Administration time ranges from 45 to 60 minutes.
Kaufman Brief Intelligence Test31:
The KBIT is a measure of verbal and non-verbal intelligence in children, adolescents, and adults. It includes 2 subtests, vocabulary and matrices. The vocabulary subtest comprises 2 parts, expressive vocabulary (including 45 elements) and definitions (37 elements). In the matrices subtest, all elements (48) are made up of drawings and abstract figures, reducing potential cultural influences. Results are presented as standardised scores with a mean of 100 and standard deviation of 15. The test takes 15-30 minutes to administer.
ToM task: Kaland’s Stories from Everyday Life11,30:
This task comprises a battery of 13 stories, and seeks to assess subjects’ capacity to make inferences about the mental, cognitive, and emotional state of characters in 13 interpersonal situations from everyday life. We used the abbreviated 7-item version (SEL-7) of the adapted, validated Spanish-language version30 of the original instrument.11 Stories are always presented in such a way that the first part describes a given situation to examine the subject’s ability to understand concrete facts (physical inferences), and the final part presents information on the mental state to be evaluated (mental inferences). The 7 phenomena evaluated are lies, white lies, figures of speech, misunderstandings, persuasion, contrary emotions, and empathy. The task yields 2 final scores, one for physical inferences and one for mental inferences; each score ranges from 0 to 14 points. Administering the test is a complex process; details are given in the Supplementary Material. The validation study in a Spanish population found a Cronbach alpha of 0.78 for physical inferences and 0.74 for mental inferences. Administration time ranges from 45 to 60 minutes.
ToM task: Eyes Task10:
This task evaluates the ability to recognise the cognitive and emotional mental state of a person by reading the expression of their eyes. The children’s version includes 28 black-and-white photographs of the upper part of the face (eyes and eyebrows) of individuals of both sexes. The subject must select from a list of 4 words the one that best reflects the emotional state of the person in the image. It assesses advanced ToM by providing the subject only with information on the eye area, requiring attributions including both emotional and mental states and intentions, such as “wanting to play,” “thoughtful,” or “satisfied.” As a control task, to rule out face processing disorders, the subject is asked to identify the sex of the person in the photograph. Administration time is ≤ 15 minutes.
Statistical analysisStatistical analysis was performed using the Stata statistics software, version 13. The nonparametric Kruskal-Wallis test was used for intergroup comparisons of quantitative variables. Given the potential influence of level of neurodevelopment and IQ on performance in ToM tests,39 a second analysis used a linear regression model including verbal IQ, non-verbal IQ, and age as covariates, with the final model including only those variables showing a significant effect (P < .05). Post hoc comparisons between groups were analysed with the Dunn test for multiple comparisons, and are presented without adjustment and after application of the Bonferroni correction. We calculated Pearson correlation coefficients between the ToM task and clinical and neurocognitive variables (age, verbal IQ, non-verbal IQ, CY-BOCS, YGTSS, ASSQ, number of comorbidities), with the threshold for significance set at .007 (.05/7).
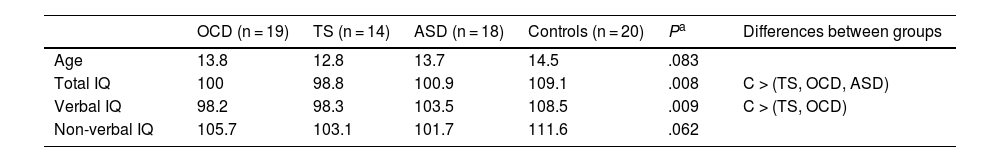
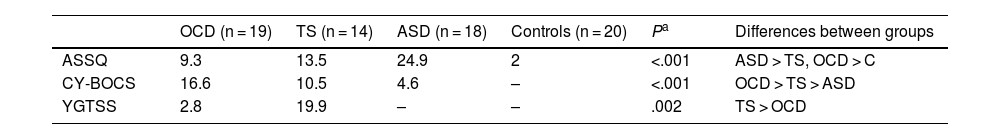
ResultsDescriptive statisticsTable 1 displays descriptive statistics from the sample. Mean age was lower in the TS group, although no statistically significant differences were observed between the 4 groups. Total and verbal IQ did show significant differences between groups, with the control group scoring better than the patient groups.
Descriptive statistics: age and estimated intelligence quotient.
| OCD (n = 19) | TS (n = 14) | ASD (n = 18) | Controls (n = 20) | Pa | Differences between groups | |
|---|---|---|---|---|---|---|
| Age | 13.8 | 12.8 | 13.7 | 14.5 | .083 | |
| Total IQ | 100 | 98.8 | 100.9 | 109.1 | .008 | C > (TS, OCD, ASD) |
| Verbal IQ | 98.2 | 98.3 | 103.5 | 108.5 | .009 | C > (TS, OCD) |
| Non-verbal IQ | 105.7 | 103.1 | 101.7 | 111.6 | .062 |
ASD: autistic spectrum disorder; IQ: intelligence quotient; OCD: childhood-onset obsessive-compulsive disorder; TS: Tourette syndrome. C > (TS, OCD, ASD): controls showed significantly higher total IQ than all other groups. C > (TS, OCD): controls showed significantly higher verbal IQ than the TS and OCD groups.
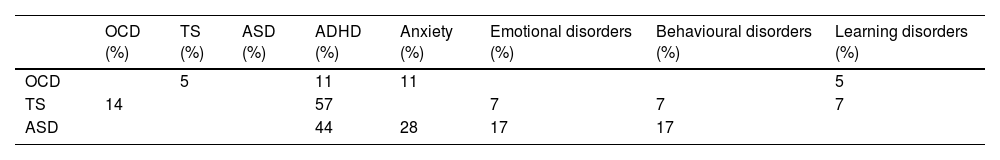
As shown in Table 2, the group scoring highest in the ASSQ was the ASD group, followed by the TS group; the OCD group scored higher in the CY-BOCS than the TS and ASD groups; and the TS group scored higher than the OCD group in the YGTSS. Both the TS and the ASD group presented more comorbidities than the OCD group (P = .05 and P = .008, respectively). Table 3 presents the comorbidities identified in the 3 patient groups as percentages; the most frequent were attention-deficit/hyperactivity disorder (ADHD) and anxiety disorders.
Clinical variables on symptom severity in the different study groups.
| OCD (n = 19) | TS (n = 14) | ASD (n = 18) | Controls (n = 20) | Pa | Differences between groups | |
|---|---|---|---|---|---|---|
| ASSQ | 9.3 | 13.5 | 24.9 | 2 | <.001 | ASD > TS, OCD > C |
| CY-BOCS | 16.6 | 10.5 | 4.6 | – | <.001 | OCD > TS > ASD |
| YGTSS | 2.8 | 19.9 | – | – | .002 | TS > OCD |
ASD: autistic spectrum disorder; ASSQ: Autism Spectrum Screening Questionnaire; CY-BOCS: Children’s Yale-Brown Obsessive-Compulsive Scale; OCD: childhood-onset obsessive-compulsive disorder; TS: Tourette syndrome; YGTSS: Yale Global Tic Severity Scale.
ASD > TS, OCD > C: patients with ASD scored significantly higher than the other 3 groups, and the TS and ASD groups scored significantly higher than the control group. OCD > TS > ASD: patients with OCD scored significantly higher than the TS group, and the TS group scored significantly higher than the ASD group. TS > OCD: the TS group scored significantly higher than the OCD group.
Comorbidities in the 4 groups in the study sample.
| OCD (%) | TS (%) | ASD (%) | ADHD (%) | Anxiety (%) | Emotional disorders (%) | Behavioural disorders (%) | Learning disorders (%) | |
|---|---|---|---|---|---|---|---|---|
| OCD | 5 | 11 | 11 | 5 | ||||
| TS | 14 | 57 | 7 | 7 | 7 | |||
| ASD | 44 | 28 | 17 | 17 |
ADHD: attention-deficit/hyperactivity disorder; ASD: autistic spectrum disorder; OCD: childhood-onset obsessive-compulsive disorder; TS: Tourette syndrome.
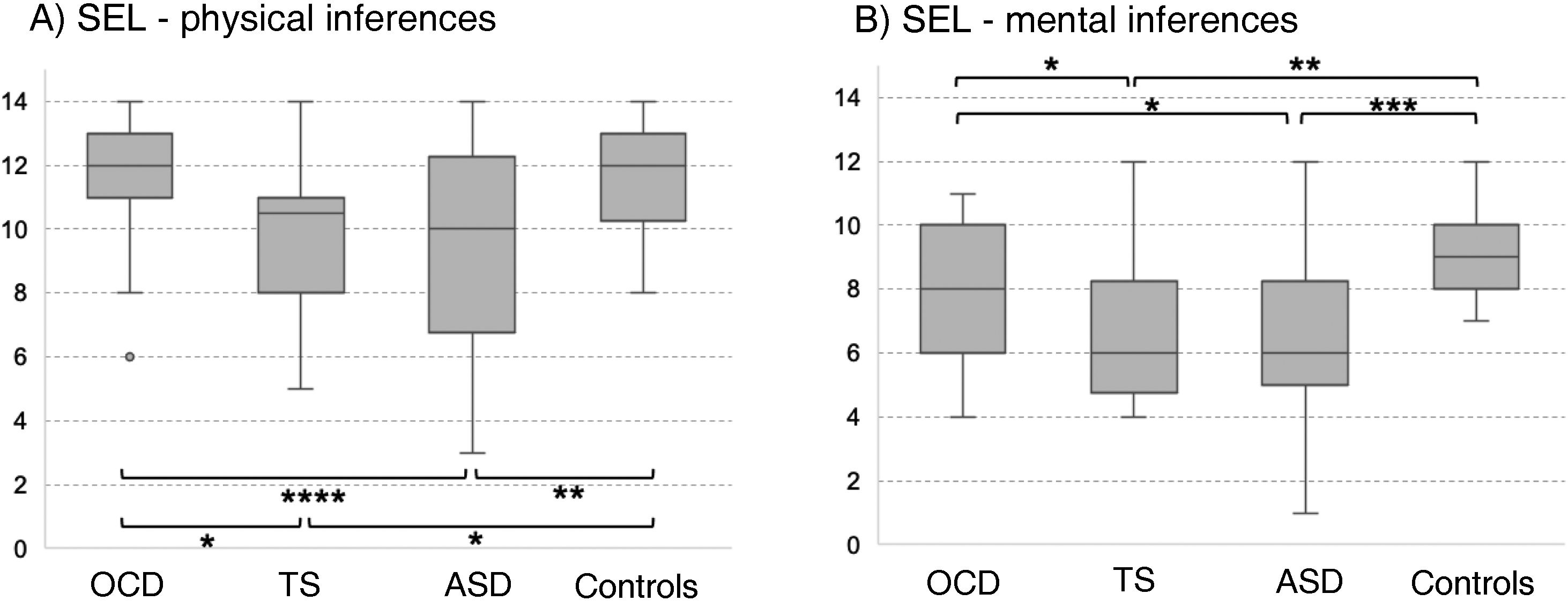
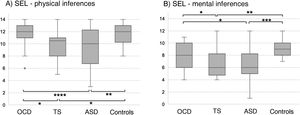
In the SEL, the ASD group scored lowest both for physical and for mental inferences (although no significant difference was observed with the TS group for median mental inference score), followed by the OCD group and control group. No significant differences between groups were observed for response times or the number of prompt questions. Significant differences in physical inference scores were observed between the ASD and the control group, between the ASD and the OCD group, between the TS and the control group, and between the TS and the OCD group (χ2 = 9.8; P = .02; Fig. 1A). Significant differences in mental inference scores were observed between the ASD and the control group, between the ASD and the OCD group, between the TS and the control group, and between the TS and the OCD group (χ2 = 15.29; P = .002; Fig. 1B). After application of the Bonferroni correction, the only significant differences were between the control and ASD groups, for mental and physical inferences (P ≤ .03) and between the control group and the TS group, for mental inferences (P = .002).
Theory of mind as evaluated with the Stories from Everyday Life task. (A) Physical inferences. (B) Mental inferences.
ASD: autistic spectrum disorder; OCD: childhood-onset obsessive-compulsive disorder; SEL: Stories from Everyday Life task; TS: Tourette syndrome.
*P < .05 for the intergroup comparison, before Bonferroni correction.
**P < .05 for the intergroup comparison, after Bonferroni correction.
***P < .05 for the intergroup comparison, after Bonferroni correction and inclusion of the covariate verbal intelligence quotient.
****P < .05 for the intergroup comparison, after Bonferroni correction and inclusion of the covariates verbal intelligence quotient and age.
After age and verbal IQ were added as covariates (P ≤ .003) in the analysis of physical inferences, the differences between the OCD and ASD groups remained significant (P = .004), whereas significance was lost for the difference between controls and patients with ASD (P > .01). In the analysis of mental inferences, after inclusion of verbal IQ as a covariate (P = .015), only the differences between controls and the ASD group remained significant (P = .017), with the differences between controls and patients with TS and between the OCD and ASD groups losing significance (P = .69 and P = .84, respectively).
Results of the analysis with the complete version of the test (13 stories) are included in the Supplementary Material.
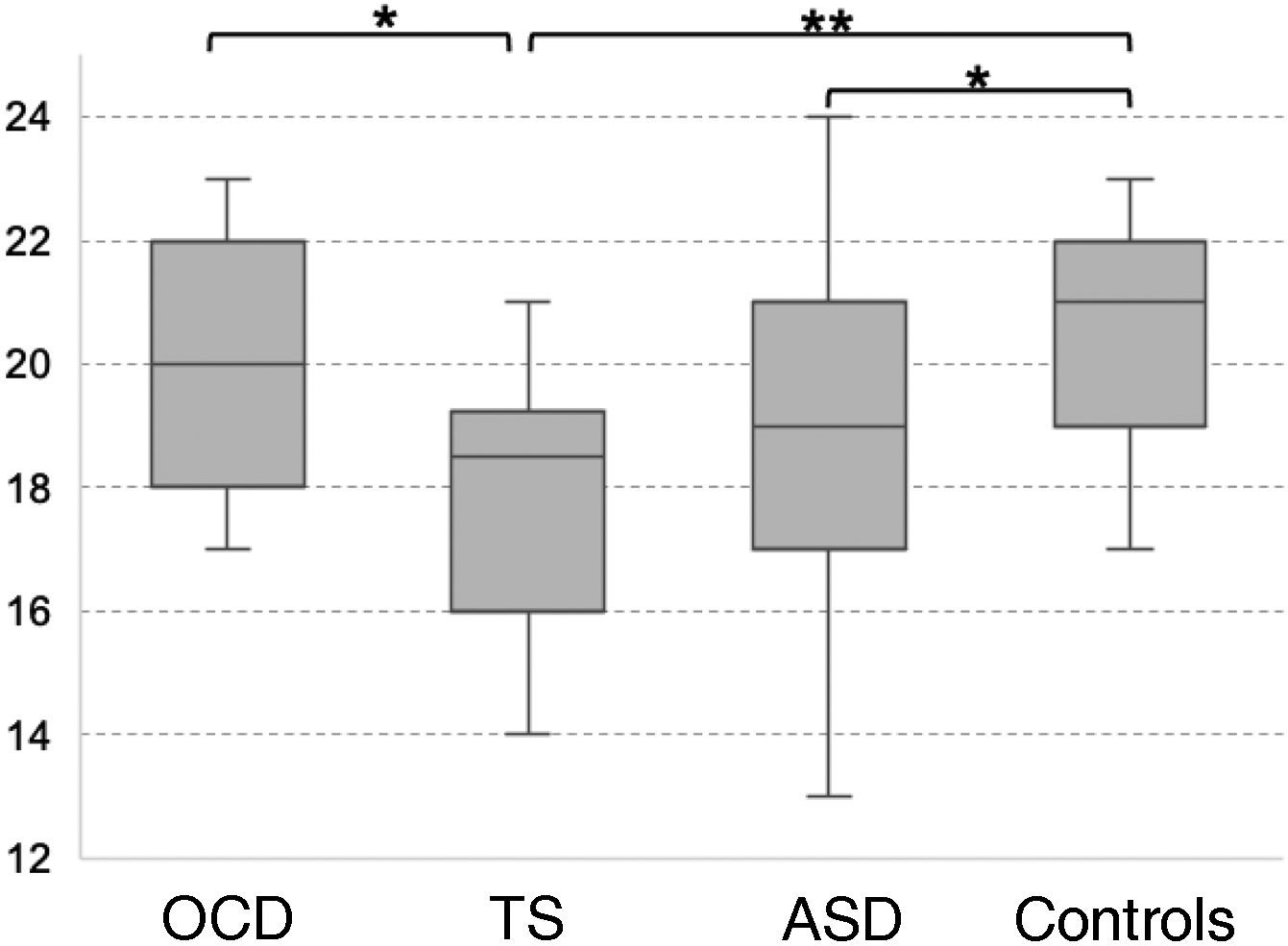
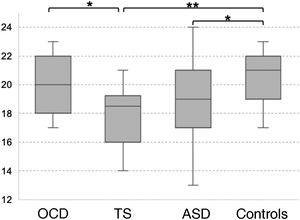
Theory of mind as evaluated with the Eyes TaskThe TS group scored the lowest in the Eyes Task, followed by the ASD, OCD, and control groups. Statistical analysis revealed significant differences between the control and the ASD group, between the control and the TS group, and between the OCD and the TS group (χ2 = 10.16; P = .017; Fig. 2). Only the differences between controls and patients with TS survived the Bonferroni correction (P = .009), with the difference between the OCD and TS groups showing a trend towards significance (P = .05). None of the covariates added to the model had a significant effect on the dependent variable (P ≥ .13).
Theory of mind as evaluated with the Eyes Task.
ASD: autistic spectrum disorder; OCD: childhood-onset obsessive-compulsive disorder; TS: Tourette syndrome.
*P < .05 for the intergroup comparison, before Bonferroni correction.
**P < .05 for the intergroup comparison, after Bonferroni correction.
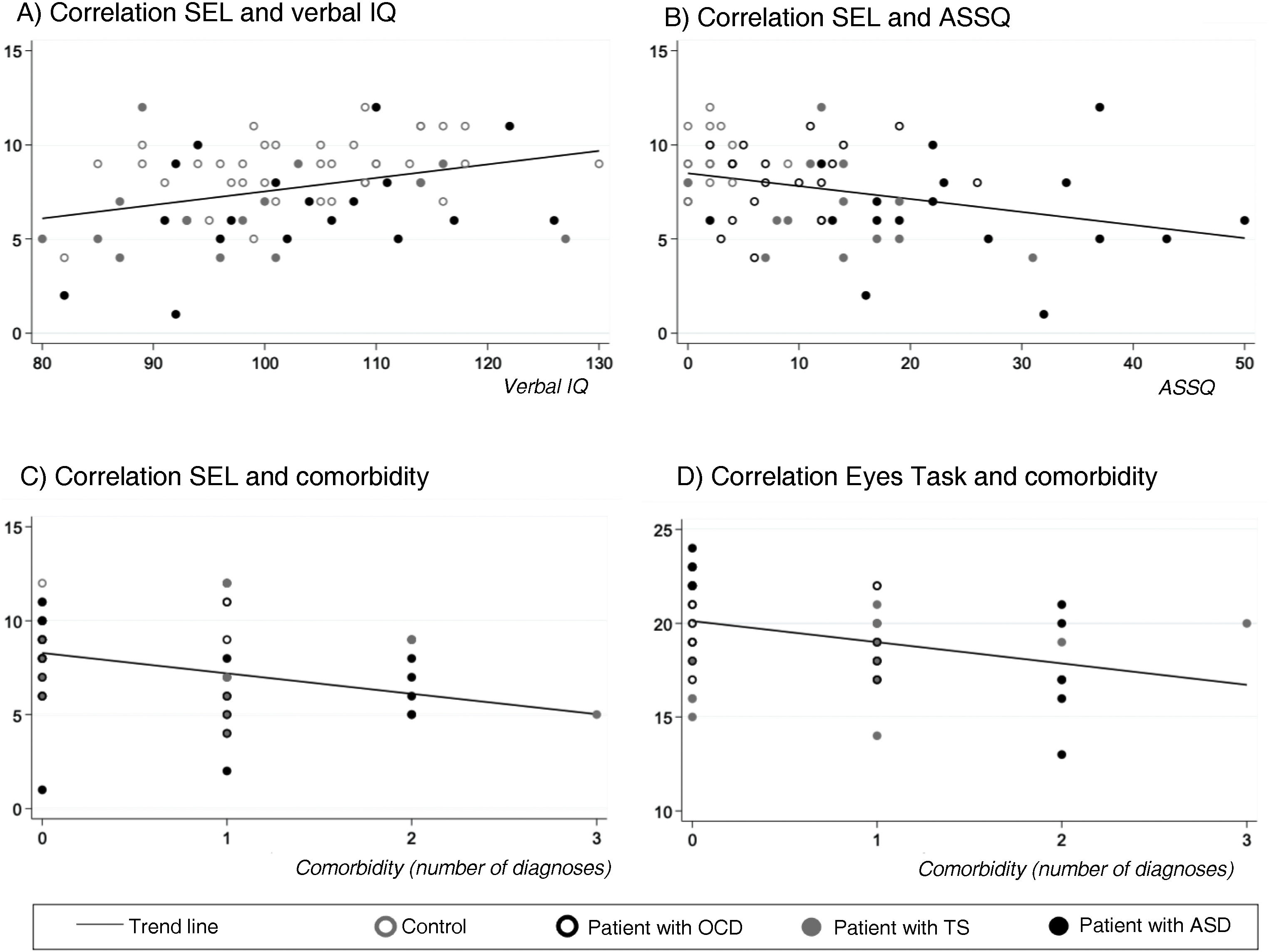
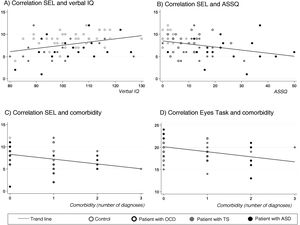
SEL-7 scores showed a positive correlation with verbal IQ (r = 0.34; P < .005) and a negative correlation with ASSQ (r = –0.33; P = .005) and number of comorbidities (r = –0.34; P < .005) in the entire sample; thus, SEL-7 scores were lower in patients with higher scores for ASD symptoms and higher numbers of comorbidities (Fig. 3). Eyes Task scores were negatively correlated with number of comorbidities, in the entire sample (r = –0.36; P = .004).
Correlations of theory of mind task performance with clinical and neurocognitive variables and with number of psychiatric comorbiditiesa.
ASD: autistic spectrum disorder; OCD: childhood-onset obsessive-compulsive disorder; SEL: Stories from Everyday Life task; TS: Tourette syndrome.
aPearson correlation coefficients.
The primary objective of this study was to compare advanced ToM in groups of children and adolescents diagnosed with OCD, TS, and ASD, and in healthy controls, using 2 different tests. Our main findings show that patients with TS have difficulty solving advanced ToM tasks as assessed with the SEL-7, performing similarly to patients with ASD.30 On the other hand, patients with childhood-onset OCD performed similarly to healthy controls. In the second ToM task, the Eyes Task, a surprising result was the greater difference observed between controls and patients with TS than between controls and patients with ASD. This may be explained by the impulsive behaviour of patients with TS40 and their difficulty inhibiting responses,41 which may lead them to give a less reflexive response than participants from the other groups. A systematic review including evidence on ToM and from neuroimaging studies suggests that among executive functions, inhibition is necessary but not sufficient for ToM processing, as several brain regions seem to contribute to mentalising, in addition to the regions supporting inhibitory control.42 One possible reason for these results is that patients with ASD were better trained in emotional recognition than those in the TS group. This may have been the case in our sample, as training in ToM tasks (e.g., emotional recognition) is commonly used in the therapeutic management of patients with ASD at our department, whereas patients with TS do not receive specific training in these skills. Another explanation may be unconventional responses mediated by emotional dysregulation in patients with TS, rather than difficulty attributing mental states.43
Our results are consistent with those reported in studies of adult populations with TS, which have shown that these patients have difficulties taking the perspective of others,25 as well as alterations in emotional aspects of advanced ToM.24
Our secondary objective was to examine how ToM is influenced by clinical characteristics and other aspects of neurocognitive function. The results show that comorbidities can also affect performance, particularly in the TS group, as shown in Table 3 (57% presented ADHD; 14% OCD, and 7% emotional, behavioural, and learning disorders). To our knowledge, no previous study of paediatric populations has addressed the effect of comorbidities in ToM task performance in patients with childhood-onset OCD or TS, although one study reported that anxiety influences ToM in patients with ASD,44 and a recent meta-analysis concluded that adolescent and pre-adolescent patients with ASD tend to present high levels of social anxiety symptoms.45 TS also shows a high level of comorbidity with anxiety disorders,46 and some patients may have presented symptoms of anxiety, but without sufficient interference for this diagnosis to be established. Furthermore, we did not evaluate comorbidities on a systematic, prospective basis; rather, these data were collected from the clinical records of each participant; therefore, some comorbidities may not have been identified.
The main limitation of this study is the small sample size, which reduces its statistical power. Furthermore, given its cross-sectional design and the correlations observed between ToM task performance and other variables that may be considered intermediaries of neurodevelopment (e.g., verbal IQ and age), the results presented must be interpreted with caution. While this effect was not observed with the SEL-7, scores on the complete version (13 stories) were positively correlated with age (Supplementary Material). Studies suggest that age influences cerebral responses during the performance of ToM tasks in younger subjects, particularly in tests of emotion attribution.47 The exact mechanism underlying this association is poorly understood, although it may reflect several processes that are not mutually exclusive, such as synaptic pruning,48 greater automatism in certain processes due to age,49 or increased neuronal efficiency due to practice.50 Another limitation is that ToM tasks were administered by 2 different research teams, at different times, although all professionals participated in a joint training session on the administration of the SEL. While patients with comorbid ASD were excluded from the OCD and TS groups, we did not rule out patients with OCD or TS as a comorbidity, which should also be taken into account in future studies. We also did not include questionnaires to screen for ADHD or assess symptom severity; therefore, ADHD symptoms may have acted as a confounder.51 Finally, we did not administer a complete neuropsychological battery beyond estimating IQ (e.g., executive dysfunction), despite the evidence on the relationship between these 2 areas of cognition.4,5 Furthermore, IQ was estimated using a screening tool, rather than the Wechsler Intelligence Scale for Children-V, which would have helped to better characterise the potential mediating role of IQ in the interpretation of ToM tasks. Another important consideration is that all participants were boys; therefore, our results do not present a sex bias, although they cannot be extrapolated to female patients.
In the light of all of the above, future studies should focus on administering ToM tasks to female patients with TS, OCD, or ASD, and seek to replicate the results of the present study in larger patient samples. It would also be valuable to use a semi-structured interview to identify all comorbidities. The most relevant clinical implication of our results is that at least some patients with TS may benefit from training in ToM skills as part of their therapeutic management, as is currently the case for paediatric patients with ASD. Future research could also use neuroimaging studies and neuropsychological tests in addition to these clinical assessments of advanced ToM, before and after application of specific treatments to improve ToM skills, in order to identify neuroanatomical changes or associations with other neurocognitive deficits. Finally, future studies might include a group of patients with ADHD and evaluate the influence of multicomorbidity in neurodevelopmental disorders.
Based on our results, we may conclude that patients with TS and ASD have greater difficulty in ToM tasks, compared to patients with childhood-onset OCD and healthy controls. Our findings also reflect the complex interaction and the reciprocal influence between different areas of cognition and neurodevelopmental disorders. Thus, ToM alterations are not an exclusive feature of ASD; rather, they constitute a marker of neurodevelopmental alterations as in other mental disorders with onset in childhood.
FundingA. Pérez-Vigil received a grant from the Alicia Koplowitz Foundation during the development of this study.
Conflicts of interestThe authors have no conflicts of interest to declare.