Chiari type I malformation (CM-I) is characterised by caudal ectopia of the cerebellar tonsils through the foramen magnum. This is associated with brain stem, high spinal cord, and cranial nerve compression phenomena. The most frequent symptoms are occipital headaches and dizziness. Less well-known symptoms are sleep disorders and nocturnal respiratory abnormalities.
SourcesMEDLINE and information from patients evaluated at the Neurosurgery and Clinical Neurophysiology Departments at Hospital Universitario Vall d’Hebron.
DevelopmentReview article based on data obtained from MEDLINE articles since 1966, using combinations of the following keywords: “Chiari malformation” or “Arnold-Chiari malformation” and “sleep apnea” or “sleep disorders”.
ConclusionsCM-I patients show a higher prevalence of sleep disorders than that observed in the general population. Some studies report a 50% prevalence of sleep apnea-hypopnea syndrome (SAHS), probably associated with sudden death in some cases. These results support analysing sleep respiratory parameters in these patients. Identifying SAHS symptoms may help optimise treatment, thereby improving quality of life and prognosis.
La malformación de Chiari tipo I (MC-I) se caracteriza por la existencia de una ectopia de las amígdalas del cerebelo que se sitúan por debajo del foramen mágnum, lo que puede asociarse a fenómenos compresivos del tronco del encéfalo, de la médula espinal alta y de los pares craneales. Las manifestaciones clínicas más frecuentes suelen ser las cefaleas occípitonucales y los mareos, aunque el cortejo sintomático puede ser muy extenso. Sin embargo, un aspecto menos conocido es la repercusión de la malformación sobre las alteraciones respiratorias nocturnas y los trastornos del sueño.
FuentesMEDLINE e información de pacientes con MC-I valorados en los servicios de neurocirugía y neurofisiología del Hospital Universitario Vall d’Hebron.
DesarrolloArtículo de revisión realizado a partir del análisis de todos los estudios publicados en MEDLINE a partir del año 1966, localizados a través del motor de búsqueda PubMed, utilizando combinaciones de las palabras clave: «Chiari malformation» o «Arnold-Chiari malformation» y «sleep apnea» o «sleep disorders».
ConclusionesLos pacientes con una MC-I presentan una mayor prevalencia de trastornos del sueño que la población general. En algunos estudios el 50% de los pacientes con MC-I presentan un síndrome de apnea-hipopnea del sueño (SAHS), habiéndose descrito incluso casos de muerte súbita probablemente relacionados con estos fenómenos. Estos resultados recomiendan incluir el análisis de los parámetros respiratorios nocturnos en el estudio de los pacientes con MC-I. Identificar la presencia de un SAHS contribuye a optimizar el tratamiento de estos pacientes, mejorando la calidad de vida y su pronóstico.
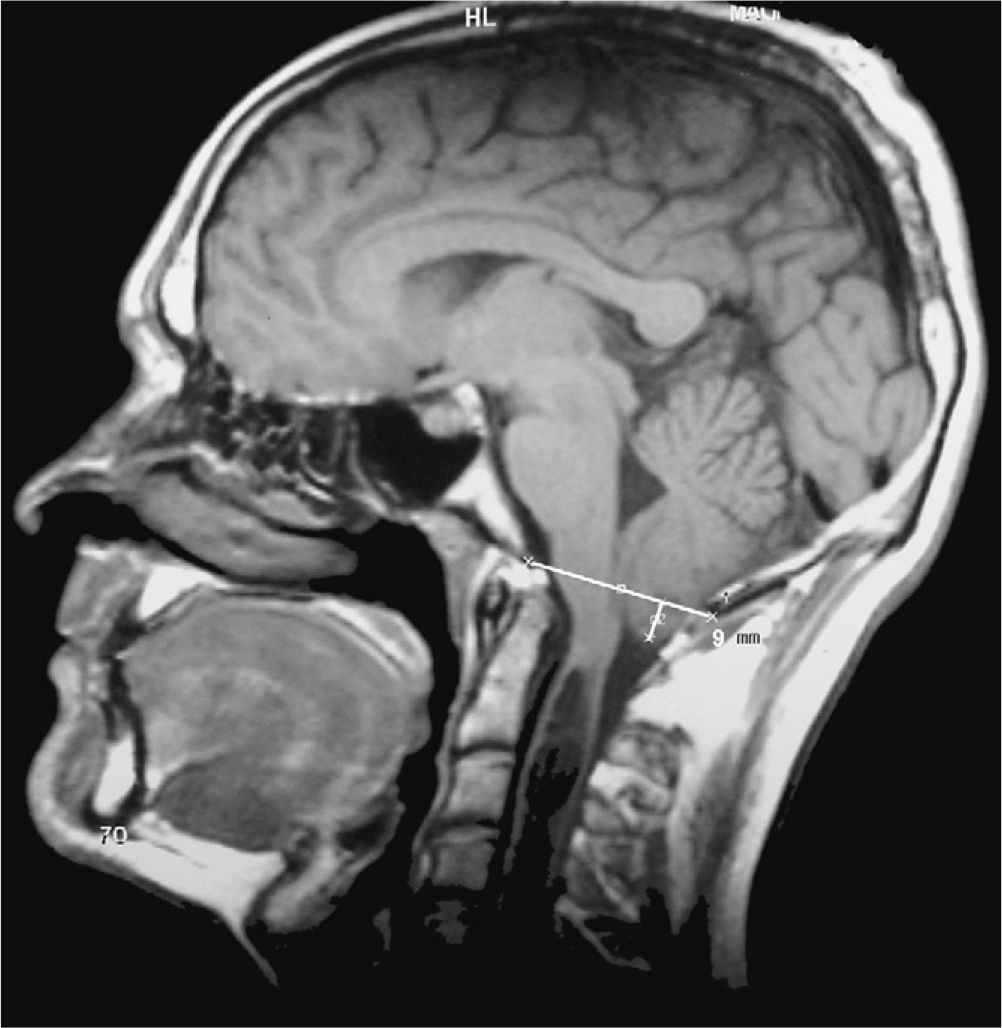
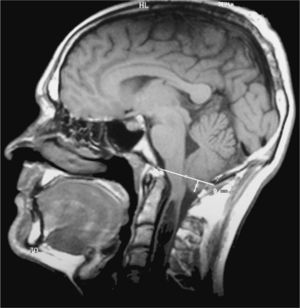
The eponyms ‘Chiari malformation’ and ‘Arnold-Chiari malformation’ are used as synonyms to indicate a series of malformations characterised by displacement of the cerebellar tonsils down through the foramen magnum. For reasons of historical fairness, the term ‘Chiari malformation’ has largely substituted ‘Arnold-Chiari malformation’. Doctors have historically listed 4 different types of Chiari malformations according to the associated anomalies. In any case, the most frequent is Chiari type 1 (CM1), in which the cerebellar tonsils protrude at least 3mm below the foramen magnum (Fig. 1).1 Nevertheless, significant clinical, aetiopathogenic and therapeutic debates remain regarding this malformation.
In patients with CM1, the displacement of the cerebellar tonsils causes a more or less significant obstruction at the craniocervical junction, which impedes the free circulation of cerebrospinal fluid (CSF). This situation explains why some patients develop associated syringomyelia or hydrocephalus.2 This malformation may also be associated with other bone anomalies of the craniocervical junction, arachnoiditis of the posterior fossa, scoliosis, and increases in intracranial pressure.3 CM1 has also been described in association with a smaller posterior fossa volume than that measured in healthy individuals.4–6 These structural anomalies may compromise normal function of the superior segment of the spinal cord and brainstem. As a result, they can alter the functions of sleep structures, the lower cranial nerves, and cardiorespiratory centres.
Clinical manifestations of patients affected by CM1 vary considerably. They depend on associated malformations and whether or not syringomyelic cavities and hydrocephalus are present at time of diagnosis. Headache, neck pain, and dizziness are the most frequent symptoms. While headaches are non-specific, they are located in the occipitocervical region and tend to intensify with Valsalva manoeuvres. The ‘dizziness’ reported by these patients is often a type of vertigo that is triggered by rotation of the head. These patients may also present dysphagia, ataxia, cervical radicular pain, sensory and/or motor abnormalities, and other symptoms. In recent years, numerous authors have highlighted a lesser-known aspect of this disease: the sleep disorders and sleep-related breathing disorders present in many of these patients and may result in respiratory failure7–15 or even sudden death.16–22
The American Academy of Sleep Medicine's revised International Classification of Sleep Disorders (ICSD-2)23 lists more than 81 sleep-related disorders. These fall into the categories of insomnia, sleep-related breathing disorders, hypersomnia, circadian rhythm sleep disorders, parasomnias, and sleep-related movement disorders. Despite the fact that many authors have observed different types of apnoea and hypopnoea in patients with CM1, there have been no studies that analyse sleep quality or circadian rhythm alterations related to this malformation. The purpose of this review is to provide updated information on sleep disorders that may be present in patients with CM1.
Types of sleep-related breathing disordersSleep-related breathing disorders are a very prevalent chronic illness. They are associated with sleep fragmentation and intermittent hypoxaemia. The ICSD-223 classifies breathing disorders as follows: (a) central sleep apnoea syndrome (CSAS); (b) obstructive sleep apnoea syndrome (OSAS); and (c) central alveolar hypoventilation syndrome (CAHS).
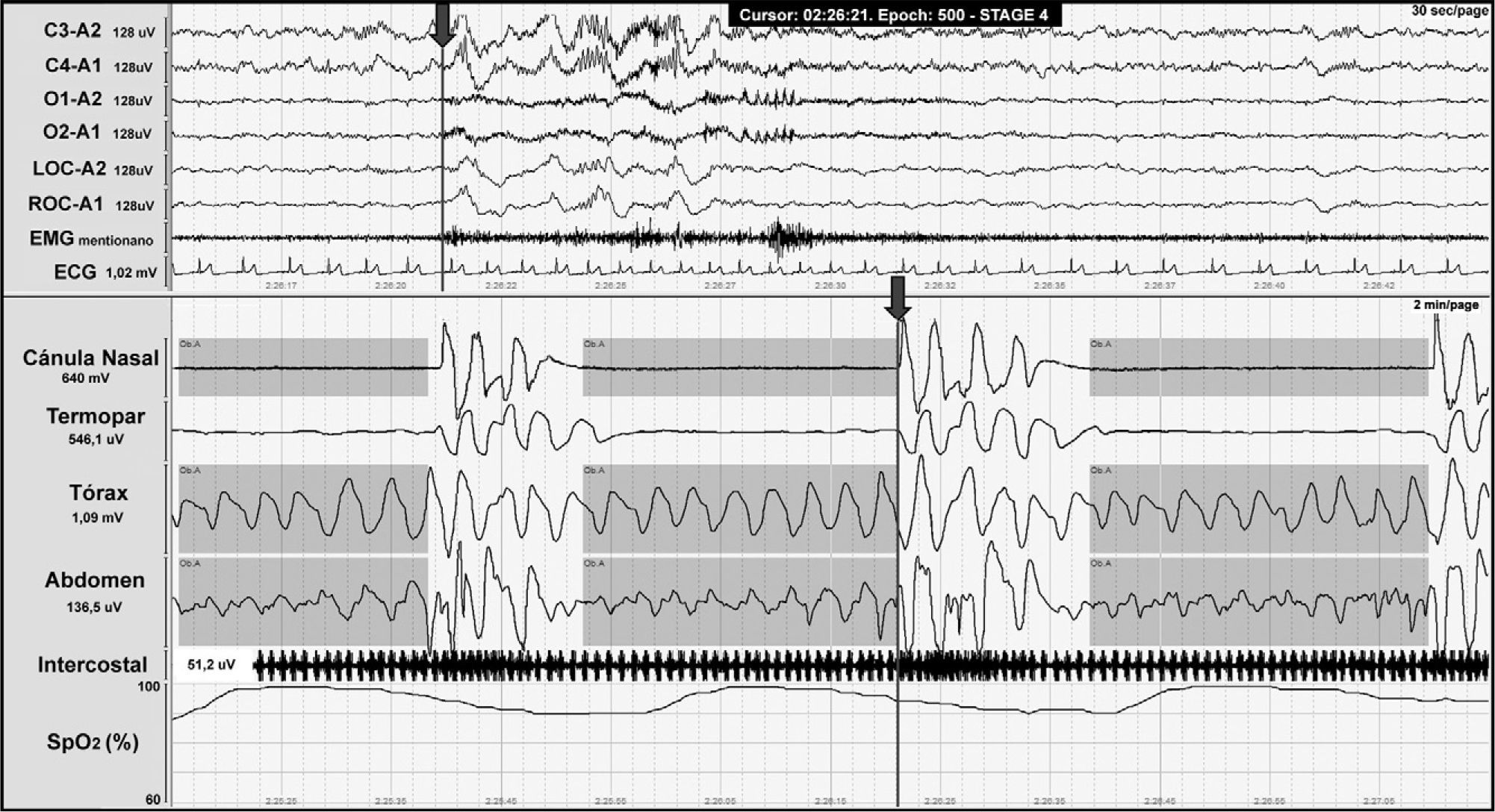
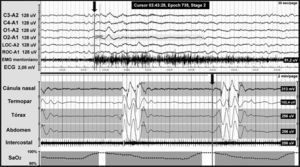
CSAS is characterised by a temporarily absent or decreased effort to breathe that may originate in the central nervous system or else appear as the result of muscular dysfunction. It is defined by a polysomnogram (PSG) showing 5 or more respiratory events with absence of respiratory effort lasting more than 10seconds associated with microarousal and oxygen desaturation (Fig. 2).
Central apnoea in a patient with Chiari malformation type 1. Night-time polysomnography record showing central apnoea (shown in grey bands). The grey line at the end of the respiratory event (arrow) indicates the onset of the cortical microarousal. This can be seen in the EEG channel results with a different time scale. The lower part of the figure shows the decrease in oxygen saturation secondary to apnoea.
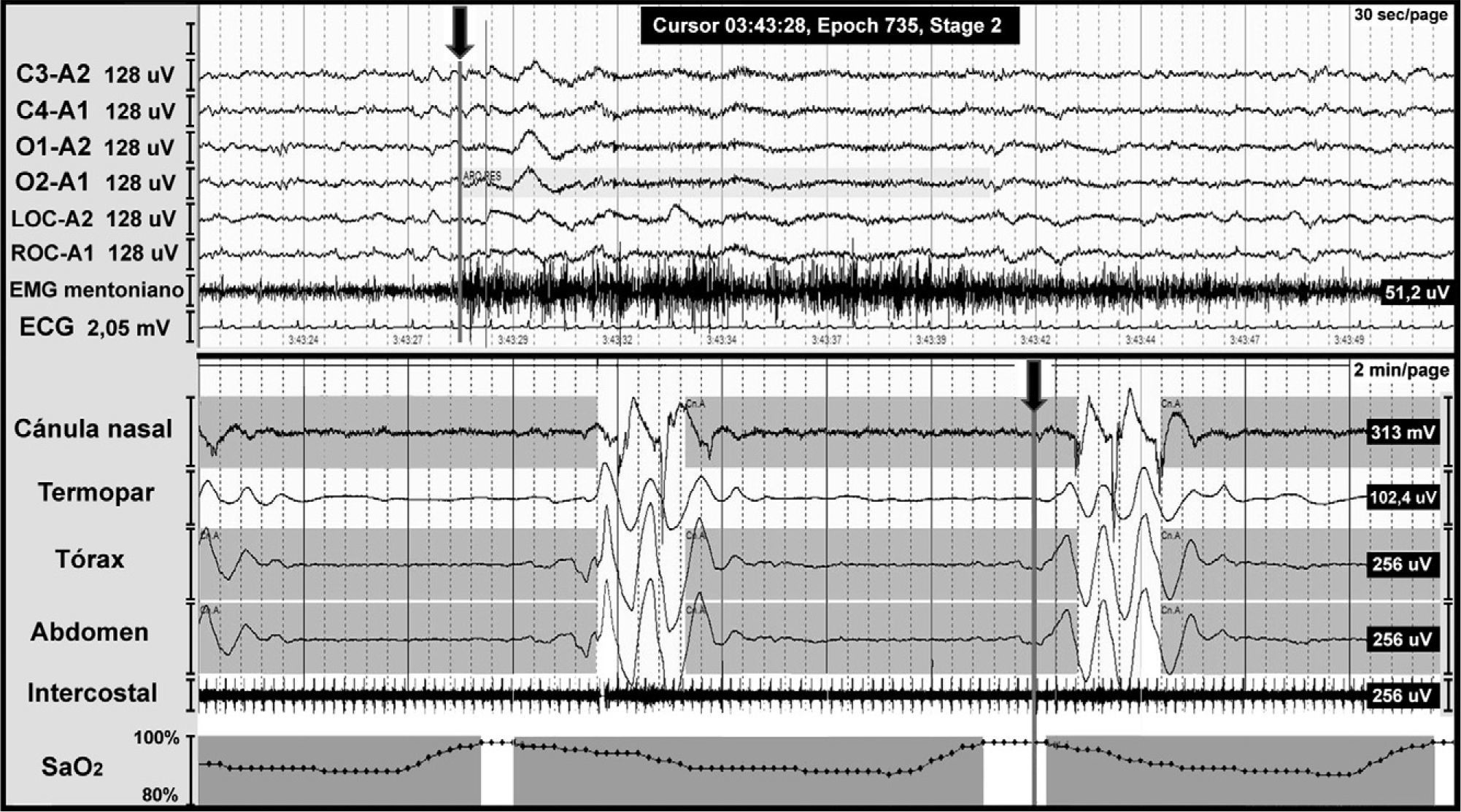
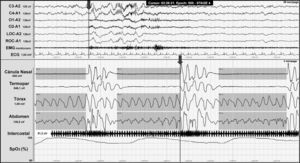
OSAS involves total or partial obstruction of the upper airway and it is defined by a PSG showing 5 or more respiratory events with presence of respiratory effort lasting more than 10seconds associated with microarousal and oxygen desaturation (Fig. 3).
Night-time polysomnography record showing obstructive apnoea (shown in grey bands) in a patient with Chiari malformation type 1. The grey line at the end of the respiratory event (arrow) indicates the onset of the cortical microarousal. This can be viewed in the EEG channel results and in the decrease in oxygen saturation.
CAHS is characterised by oxygen saturation of less than 90% during more than 5minutes with a nadir of 85%, or by oxygen saturation of less than 90% during 30% of the recording, or by arterial carbon dioxide partial pressure (PaCO2)>45% during the daytime.
Centres and pathways related to respiratory controlRespiration may be controlled automatically or voluntarily. Automatic respiration, which is indispensable during sleep, is controlled by a neural network located in the lower brainstem. This network coordinates activation of inspiratory, post-inspiratory, and expiratory neurons, which in turn are regulated by the sleep-wake system, central and/or peripheral chemoreceptors sensitive to PaCO2 and/or PaO2, and respiratory mechanoreceptors. Respiratory coordination is necessary not only in controlling breathing, but also in vocalising, swallowing, and vomiting.24
Automatic respiration originating in the brainstem is controlled by 3 interconnected groups of neurons: the pontine respiratory group (PRG), the ventral respiratory group (VRG), and the dorsal respiratory group (DRG).25,26
The PRG includes the parabrachial/Kölliker-Fuse respiratory complex located in the rostral and dorsal pons. The complex serves to control respiration, including coordination of the respiratory phase and integration of airway mechanoreceptor reflexes. In addition, the parabrachial nucleus transmits information from medullary respiratory neurons to the amygdala, hypothalamus, and other suprapontine structures.27
The VRG is a bilateral vertical column of neurons located in the ventrolateral region of the medulla oblongata. It extends from just below the facial motor nucleus to the cervical spinal cord (C1). The most rostral portion of the VRG includes the Bötzinger complex, which contains the expiratory neurons that inhibit inspiratory neurons in the VRG and project to the spinal cord. The pre-Bötzinger complex consists of propriobulbar neurons that play a key role in generating the respiratory rhythm.24 Caudally to the pre-Bötzinger complex, we find the rostral part of the VRG, located proximally to the nucleus ambiguus and containing the bulbospinal inspiratory neurons. The most caudal part of the VRG, the nucleus retroambiguus, extends from the C1 nerve root and contains the expiratory bulbospinal neurons that project to the intercostal and abdominal motor neurons.
The DRG, located in the nucleus of the solitary tract, is the first central recipient of stimuli from peripheral mechanoreceptors, chemoreceptors, and baroreceptors. It therefore serves as the first station for processing and integrating respiratory and cardiovascular reflexes.28
Automatic control over respiration is achieved through constant monitoring by afferent sensors and central chemoreceptors. Other factors in this process include plasma pH and the partial pressure of oxygen and carbon dioxide (PaO2 and PaCO2). When arterial blood gas levels and the plasma acid-base balance are abnormal, respiratory centres activate to increase or decrease the respiratory rate in order to correct these changes.29 Hypoxia activates the carotid chemoreceptors, which then trigger tonic activation of respiratory neurons in the brainstem by way of the nucleus of the solitary tract. Chemoreceptors located on the ventrolateral surface of the medulla oblongata are very sensitive to local changes in CO2. Small decreases in CO2 levels give rise to a slower respiratory rate, while increased CO2 levels increase that rate.
It is important to recall that each of the respiratory control centres is located proximally to the lower cranial nerve nuclei (VII, IX, X, XI, XII), most of which are responsible for innervating upper airway muscles. This would explain why certain structural changes in the lower brainstem or upper spinal cord would not only affect the respiratory control centres, but also result in changes in circulation, the function of peripheral chemoreceptors, and motor function of the nerves listed above.30
Respiratory control during sleepRespiratory control in a sleeping individual is solely dependent on automatic respiration. During sleep, the physiological response to the stimuli of hypoxia and hypercapnia is diminished; there is also a moderate increase in the resistance of the upper airway secondary to hypotonia of the dilator muscles. These phenomena explain why sleeping decreases the tidal volume and respiratory rate, resulting in discrete hypoventilation with an increase in PaCO2 of 2-7mmHg and a decrease in PaO2.
The respiratory pattern during sleep tends to be periodic in stages 1 and 2, much more regular during stages 3 and 4, and irregular during REM sleep. The respiratory pattern in REM sleep presents abrupt changes, with variations in both respiratory amplitude and rate, especially when rapid eye movements occur. The diaphragm is the only respiratory muscle to function during REM sleep, and changes affecting this structure may give therefore rise to profound hypoventilation during this phase of sleep.
Sleep-related breathing disorders in patients with Chiari malformation type 1Researchers have completed numerous studies on sleep-related breathing disorders in patients with CM1.31 Most of these studies have observed respiratory disorders with central features,32–43 while others have described both obstructive44–50 and mixed changes (Figs. 2 and 3).18,48,51–58 Dauvilliers et al.56 found that CAHS may be present in a low percentage of patients. Nevertheless, other studies presented a syndrome of hypoventilation and/or acute respiratory failure as the first and only symptom displayed by a patient with CM1.14,39
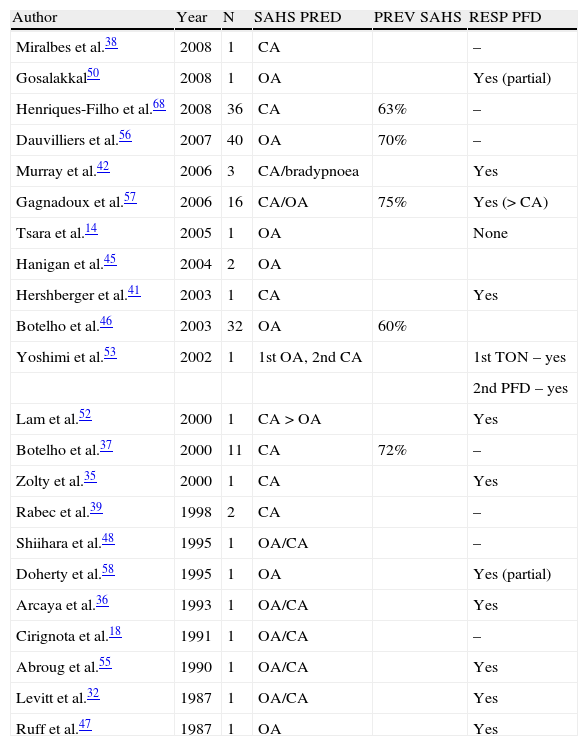
Only 4 articles have analysed sleep-related breathing disorders in a large group of patients with CM1. These studies observed that the prevalence of these changes may range from 60% to 72% of the study group, and that obstructive respiratory events are predominant.31,37,56,57
An analysis of all articles published to date studying respiratory disorders in patients with CM1 (Table 1) reveals that many are isolated case studies showing a tendency towards central and/or mixed respiratory events. Nevertheless, as increasingly larger case series of CM1 patients are being studied, this tendency towards central respiratory events is reversing and obstructive respiratory events are becoming predominant. Nevertheless, patients with CM1 and syringomyelia or basilar invagination will tend to have more central respiratory events.37
Published studies showing prevalence of sleep apnoea/hypopnoea syndrome (SAHS), apnoea type, and response to surgery among patients with Chiari malformation type 1.
| Author | Year | N | SAHS PRED | PREV SAHS | RESP PFD |
| Miralbes et al.38 | 2008 | 1 | CA | – | |
| Gosalakkal50 | 2008 | 1 | OA | Yes (partial) | |
| Henriques-Filho et al.68 | 2008 | 36 | CA | 63% | – |
| Dauvilliers et al.56 | 2007 | 40 | OA | 70% | – |
| Murray et al.42 | 2006 | 3 | CA/bradypnoea | Yes | |
| Gagnadoux et al.57 | 2006 | 16 | CA/OA | 75% | Yes (> CA) |
| Tsara et al.14 | 2005 | 1 | OA | None | |
| Hanigan et al.45 | 2004 | 2 | OA | ||
| Hershberger et al.41 | 2003 | 1 | CA | Yes | |
| Botelho et al.46 | 2003 | 32 | OA | 60% | |
| Yoshimi et al.53 | 2002 | 1 | 1st OA, 2nd CA | 1st TON – yes | |
| 2nd PFD – yes | |||||
| Lam et al.52 | 2000 | 1 | CA>OA | Yes | |
| Botelho et al.37 | 2000 | 11 | CA | 72% | – |
| Zolty et al.35 | 2000 | 1 | CA | Yes | |
| Rabec et al.39 | 1998 | 2 | CA | – | |
| Shiihara et al.48 | 1995 | 1 | OA/CA | – | |
| Doherty et al.58 | 1995 | 1 | OA | Yes (partial) | |
| Arcaya et al.36 | 1993 | 1 | OA/CA | Yes | |
| Cirignota et al.18 | 1991 | 1 | OA/CA | – | |
| Abroug et al.55 | 1990 | 1 | OA/CA | Yes | |
| Levitt et al.32 | 1987 | 1 | OA/CA | Yes | |
| Ruff et al.47 | 1987 | 1 | OA | Yes |
CA: central apnoea; TON: tonsillectomy; year: year of publication; OA: obstructive apnoea; PFD: posterior fossa decompression; N: number of patients included in the study; PREV SAHS: prevalence of sleep apnoea/hypopnoea syndrome (central and obstructive); RESP PFD: response to posterior fossa decompression; SAHS PRED: predominant type of sleep apnoea/hypopnoea syndrome.
Respiratory disorders are typically associated with other neurological changes, including syringomyelia, cranial nerve paralysis, ataxia, long pathway signs, diaphragmatic paralysis, and autonomic dysfunction. However, other authors stress that respiratory changes during sleep may constitute the first and only symptom of CM1, whether in adults or children; the latter will predominantly display central respiratory events.35,38,39,42,48,52 For this reason, some authors recommend taking a brain MRI of any patient presenting CSAS as initial symptom.50
It is also important to recall that in patients with CM1, respiratory changes have been linked with exacerbation of syringomyelia59 and intracranial hypertension.60,61
Origin of sleep-related breathing disorders in Chiari malformation type 1The pathophysiological mechanism underlying respiratory disorders in patients with CM1 is not yet fully understood, but it might be explained by brainstem dysfunction. Different studies have observed that the size and volume of the posterior fossa are diminished in patients with CM1.4,6,62 In these patients, lack of space and its potential compressive effect may affect the functioning of neural structures at these level, thereby causing breathing disorders that mainly occur while patients are asleep.
Dysfunction in central respiratory events is believed to exist due to compression of central respiratory centres, compression of cranial nerves IX and X, and/or changes in afferent nerves due to expansion of syringomyelia.63
Obstructive respiratory changes may appear in the context of cranial nerve hypofunction or dysfunction (nerves IX, X, XI, and XII) due to compression that would facilitate upper airway collapse. Nevertheless, we must not forget that patients with CM1 also present anatomical anomalies that may contribute to the appearance of obstructive events: macroglossia, large neck size, and retrognathism.64
Studies of syringomyelia, which is frequently associated with CM1, indicate that this abnormality may affect the functions of the solitary tract and the nucleus ambiguus and be accompanied by dysphagia and dysphonia. These circumstances may favour the appearance of obstructive apnoea.30
One additional mechanism that might explain the presence of sleep-related breathing disorders is hypercapnia. Even in healthy subjects, PaCO2 increases by 2-4mmHg during sleep. Hypercapnia is a potent vasodilator, and the resulting increase of bloodstream CO2 concentrations during sleep may lead to significant increases in intracranial pressure. This would exacerbate compression of neural structures located in the posterior fossa and craniocervical junction.62
Reversibility of respiratory disorders after treatmentSince CM1 is a structural and anatomical problem, it must be corrected surgically. The objectives of surgical treatment in these patients are as follows: (a) to increase the volume of the posterior fossa; (b) to lessen or eliminate the craniospinal CSF pressure gradient at the level of the foramen magnum; (c) to restore subarachnoid spaces to a normal anatomical state; (d) to eliminate the syringomyelic cavity if one is present together with CM1, and (e) to relieve pressure on the brainstem. The generally accepted surgical treatment for achieving these objectives in CM1 is posterior fossa decompression (PFD) or foramen magnum decompression.
PFD has been proved effective for treating the neurological symptoms associated with CM1, but some studies indicate that breathing parameters also improve following surgery (Table 1).4,9–11,14,32,35,36,42,43,52–55,65 Posterior fossa decompression contributes fundamentally to improving central respiratory parameters, whereas there have been mixed results for patients with obstructive apnoea (no changes after surgery, partial improvement, or complete resolution of presurgical abnormalities) (Table 1).
Two studies, one by Doherty et al.58 and another by Ely et al.51, present separate cases of OSAS recurrence 2 and 5 years after surgical decompression respectively. The authors therefore recommend monitoring these patients periodically. Recurrence of OSAS after surgery may be an early sign of recurrence of tonsillar herniation.57 Nevertheless, studies have described that in as many as 14% of all cases, sleep apnoea may appear immediately after CM1 surgery (in the first 5 days following the procedure). In some patients, this may be fatal.54,57 This phenomenon has been linked to the formation of a postsurgical oedema in the posterior fossa.54,57
Other sleep disorders in patients with Chiari malformation type 1HypersomniaDifferent researchers have found that patients with CM1 had a higher prevalence of daytime drowsiness than the healthy population. However, only three studies have used the Epworth sleepiness scale to measure this drowsiness. Gagnadoux et al.57 recorded daytime drowsiness (defined as a score >8 on the Epworth sleepiness scale) in 83% of their patients, while more than 50% of that population had scores above 10 points. Dauvilliers et al.56 recorded somnolence (Epworth >10) in 26% of the patients with a Chiari malformation, but this percentage rose to 48% in the group of adult patients only (age >18 years). Bothelo et al.46 described daytime drowsiness, expressed as an Epworth score greater than 9, in 72% of their patients with CM1. In these three studies, somnolence may be explained by the high prevalence of respiratory disorders registered during sleep in these patients.
Changes in sleep architectureFew studies have addressed changes in sleep architecture in patients with CM1. On the other hand, all the studies listed above feature very small patient series and their results cannot be extrapolated to the total population of patients with CM1. These studies show only moderate changes in sleep architecture. Dauvilliers et al.56 observed an overall decrease in total sleeping time, especially in patients older than 18, along with sleep efficiency of 74% to 76% and a decrease in deep slow-wave sleep and REM sleep. Bothelo et al.37 recorded an increase in sleep fragmentation with sleep efficiency of less than 90% in 81% of the patients, decreased REM sleep in 63%, and decreased deep slow-wave sleep in 36%. The changes observed in both studies may be explained by or linked to the sleep disorders present in these patients.
Parasomnias in patients with Chiari malformation type 1Of the many other types of parasomnia defined in ICSD-2, only REM sleep behaviour disorders have been observed in patients with CM1. No NREM parasomnias or other parasomnias during REM sleep have been detected.
REM sleep behaviour disorder (RSBD) is a parasomnia characterised by loss of muscular atony during REM sleep with concomitant motor disinhibition at the mesencephalic level. This causes motor hyperactivation, including movement and vocalisations, during REM sleep. These motor phenomena coincide with vivid dreams with frequently feature aggressive and violent content. RSBD has been linked to neurodegenerative diseases (Parkinson's disease), narcolepsy, agrypnia excitata, multiple sclerosis, cerebrovascular accident, and cerebellopontine tumours.66
Only two articles link RSBD to CM1. The first, written by Lapierre et al.67, states that RSBD may appear concomitantly with craniovertebral malformations, and it may be the first clinical sign of CM1 in an undiagnosed case. In the second study, Henriques-Filho et al.68 diagnosed RSBD in 19% of a patient group with CM1; 28% of these patients with RSBD also had associated sleep apnoea/hypopnoea. The pathophysiological mechanism proposed by these authors is brainstem dysgenesis resulting from chronic microtrauma to neural structures caused by bones of the craniocervical junction. The authors of this study highlighted the case of a patient treated with posterior fossa decompression whose symptoms of both apnoea and RSBD improved after the surgical procedure. These findings may provide a rationale for the pathophysiological mechanism the authors propose, but we still cannot rule out the presence of ‘pseudo-RSBD’ as described by Iranzo et al.69 According to that author, pseudo-RSBD is defined as exaggerated motor activity that may include vocalisations and which presents when waking during a respiratory event instead of during REM sleep.69
Sleep-related movement disordersWhile many movement disorders have been described in the literature, only two articles have been published on these phenomena in patients with CM1. Both studies focus on periodic limb movement disorder (PLMD).
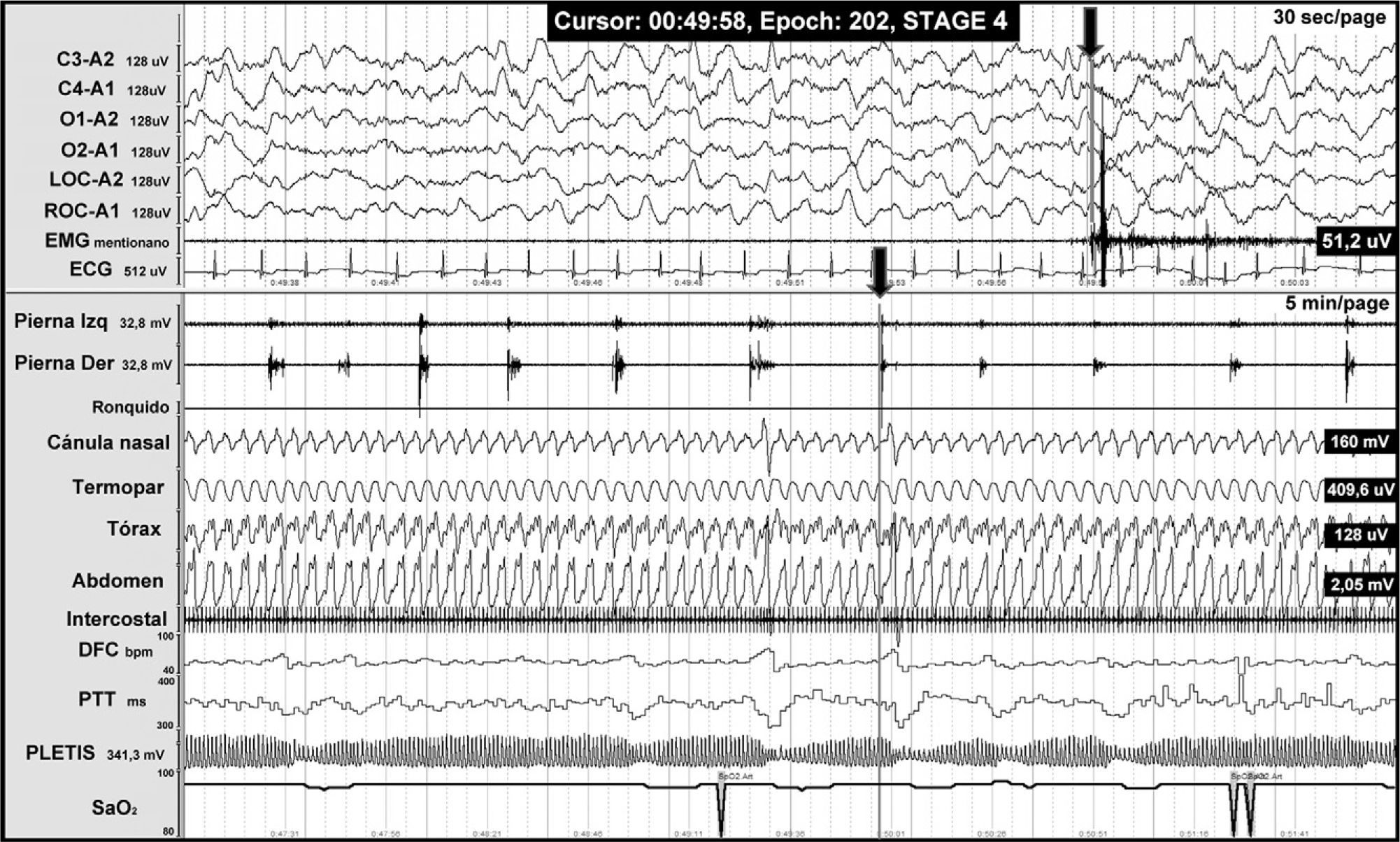
PLMD is characterised by repetitive and stereotypical dorsiflexion of the great toe, accompanied by ankle, knee, and hip flexion in 0.5-5 second bursts with a periodicity of 5-90seconds. Movements occur periodically in light slow-wave sleep and less frequently in waking hours (Fig. 4). PLMD has been linked to different spinal disorders including multiple sclerosis, Isaac syndrome, motor neuron diseases, cervical spondylosis, medullary lesions, tumours, and spinal anaesthesia.
Night-time PSG from a patient with Chiari malformation type 1, revealing periodic leg movements. The grey line at the end of one of the leg movements (arrow) is associated with sympathetic activation (seen in the following channels: heart rate [DFC], pulse transit time [PTT], pulse wave amplitude [PLETIS], and a cortical microarousal visible on the electroencephalography channels).
We have only found two references pointing to a significant increase in PLMD in patients with CM1; the condition is present in up to 62% of these patients, which is far higher than the prevalence of PLMD in the healthy population (5%).55,70 We should point out that all patients included in these studies presented associated syringomyelia.55,70
Only one study links CM1 to restless leg syndrome (RLS).71 Apart from CM1, these patients presented no other processes that might be responsible for RLS and all cases were resistant to normal pharmacological treatment (dopamine agonists). In this study, patients had a very high rate of PLMD in the PSG study, which confirmed diagnosis of RLS. However, these findings are insufficient to conclude that RLS is secondary to CM1.71
InsomniaAccording to ICDS-2 criteria, insomnia is defined as recurrent difficulty falling or remaining asleep, and/or waking early. These characteristics are associated with a sensation of non-restorative or poor-quality sleep which causes one or more daytime symptoms.
Only a few cases of insomnia and anxiety associated with CM1 have been reported in the literature.17,72,73 These studies suggest that CM1 may be the root cause of anxiety, or a factor exacerbating it. This symptom is difficult to control and responds poorly to treatment. However, authors report moderate improvement and better response to anxiety treatment after posterior fossa decompression, even when symptoms do not remit.72,73 These authors propose a combination of the following two theories as the pathophysiological basis of the condition: (a) compression of the brainstem would compromise the serotonergic pathways of the dorsal raphe nucleus and median raphe nucleus, which regulates acute and chronic states of anxiety, and of the locus coeruleus. The latter is responsible for providing noradrenaline to the cortex and therefore an important regulator of anxiety. (b) Anxiety could arise due to the symptoms associated with a Chiari malformation.73
Another aspect to consider is that sleep-related breathing disorders may also show insomnia and anxiety as their initial clinical signs, given that these respiratory events cause sleep fragmentation and chronic sleep deficit. For this reason, neurological disease should always be ruled out in patients with atypical psychiatric presentations.
Recommendations for assessing patients with Chiari malformation type 1The work-up for CM1, until more specific criteria are defined, should include clinical screening for respiratory disorders since their prevalence in this population is high. They may also be present without any other associated neurological anomalies. We highlight that early diagnosis and treatment of respiratory disorders are very important steps in managing patients with CM1. This is intended to minimise the risk of mortality and morbidity associated with these disorders. To this end, patients with CM1 who have suspected sleep-related breathing disorders should be assessed by polysomnography; this is also true in cases in which the patient is being evaluated for surgical indications.
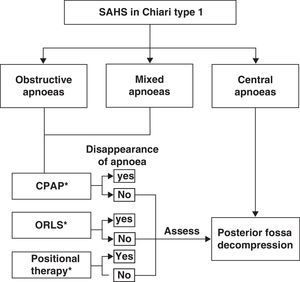
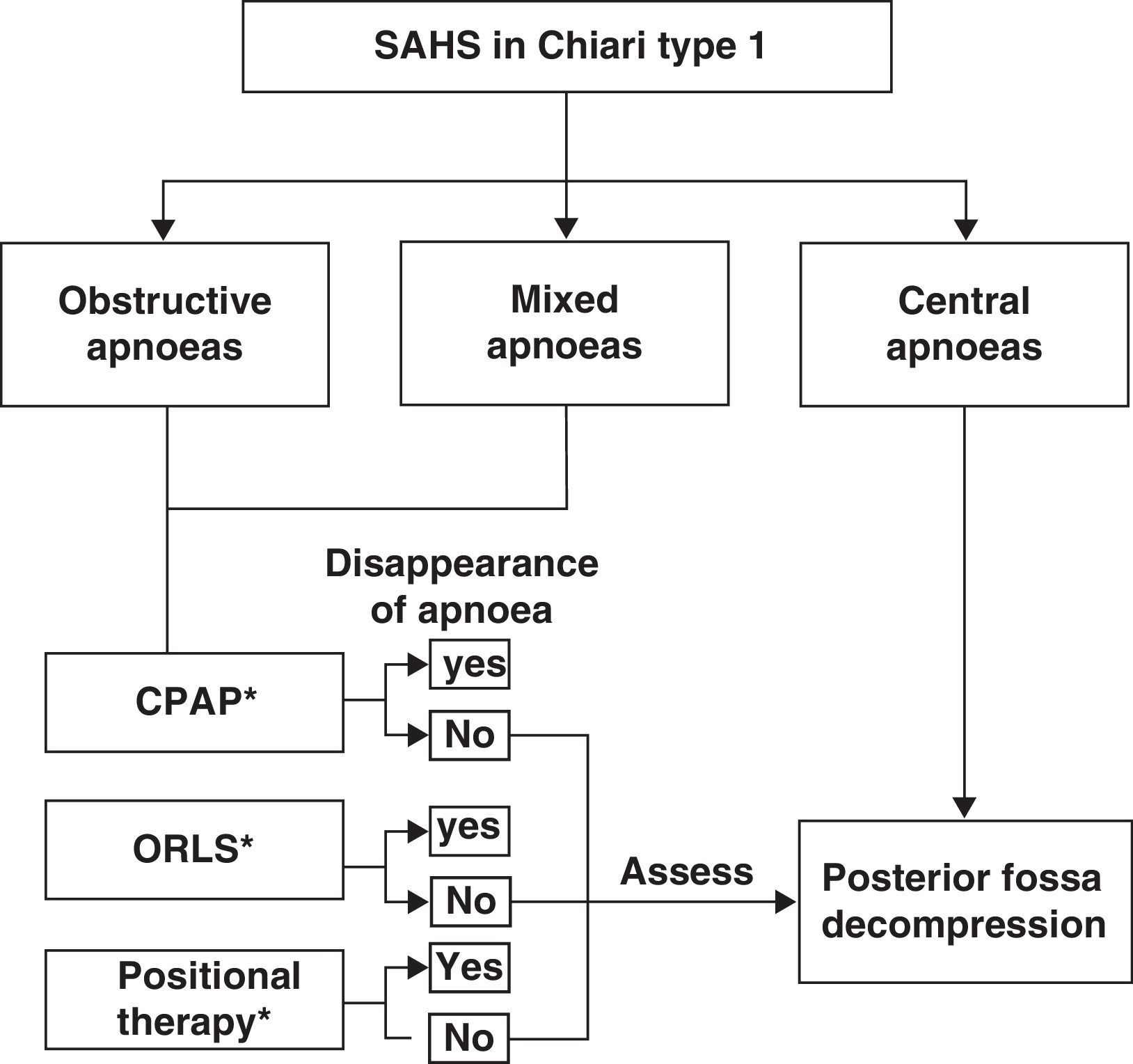
Among the breathing disorders these patients may present, it is also important to identify and distinguish between obstructive, central, and/or mixed apnoea so as to provide the patient with the appropriate treatment. A simplified example of a treatment strategy for respiratory disorders in these patients is as follows: (a) posterior fossa decompression in cases of central apnoeic events; (b) continuous positive airway pressure; (c) otorhinolaryngological surgery if a peripheral obstructive cause is diagnosed; (d) positional therapy in cases presenting postural OSAS, or (e) a combination of different treatment interventions.53,74,75 We should also recall that there have been cases of CM1 with obstructive or mixed apnoea or hypopnoea in which posterior fossa decompression improved or normalised respiratory symptoms once doctors had ruled out oropharyngeal obstruction problems. Fig. 5 shows a possible treatment algorithm that should be followed according to nocturnal respiratory findings in this patient group.
Treatment algorithm to follow in cases of patients with Chiari malformation type 1 and sleep-related breathing disorders. ORLS: otorhinolaryngological surgery; CPAP: continuous positive airway pressure; positional therapy: a system placed on the patient's back to prevent supine sleeping.
Patients with CM1 may present various sleep disorders, especially breathing disorders, which have been studied more than other types because of their high prevalence and important clinical repercussions. However, these patients may also present hypersomnia, insomnia, REM sleep behaviour disorder, PMLD, and RLS. Doctors should attempt to rule out these phenomena when taking the patient's medical history since such symptoms may go unnoticed by both doctors and patients. Early detection will permit starting appropriate treatment, improving sleep quality and quality of life, and decreasing morbidity and mortality associated with Chiari malformation type 1.
FundingThis review was partially funded by grant FIS PI07/0681 provided to M.A. Poca by Fondo de Investigación Sanitaria.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank all of our colleagues from both the sleep disorders unit and the neurosurgery department who showed their support and encouragement for our research.
Please cite this article as: Ferré Masó A, Poca MA, de la Calzada MD, Solana E, Romero Tomás O, Sahuquillo J. Alteraciones del sueño, un síndrome olvidado en los pacientes con malformación de Chiari tipo I. Neurología. 2014;29:294–304.





![Night-time PSG from a patient with Chiari malformation type 1, revealing periodic leg movements. The grey line at the end of one of the leg movements (arrow) is associated with sympathetic activation (seen in the following channels: heart rate [DFC], pulse transit time [PTT], pulse wave amplitude [PLETIS], and a cortical microarousal visible on the electroencephalography channels). Night-time PSG from a patient with Chiari malformation type 1, revealing periodic leg movements. The grey line at the end of one of the leg movements (arrow) is associated with sympathetic activation (seen in the following channels: heart rate [DFC], pulse transit time [PTT], pulse wave amplitude [PLETIS], and a cortical microarousal visible on the electroencephalography channels).](https://static.elsevier.es/multimedia/21735808/0000002900000005/v1_201406040014/S2173580814000467/v1_201406040014/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)