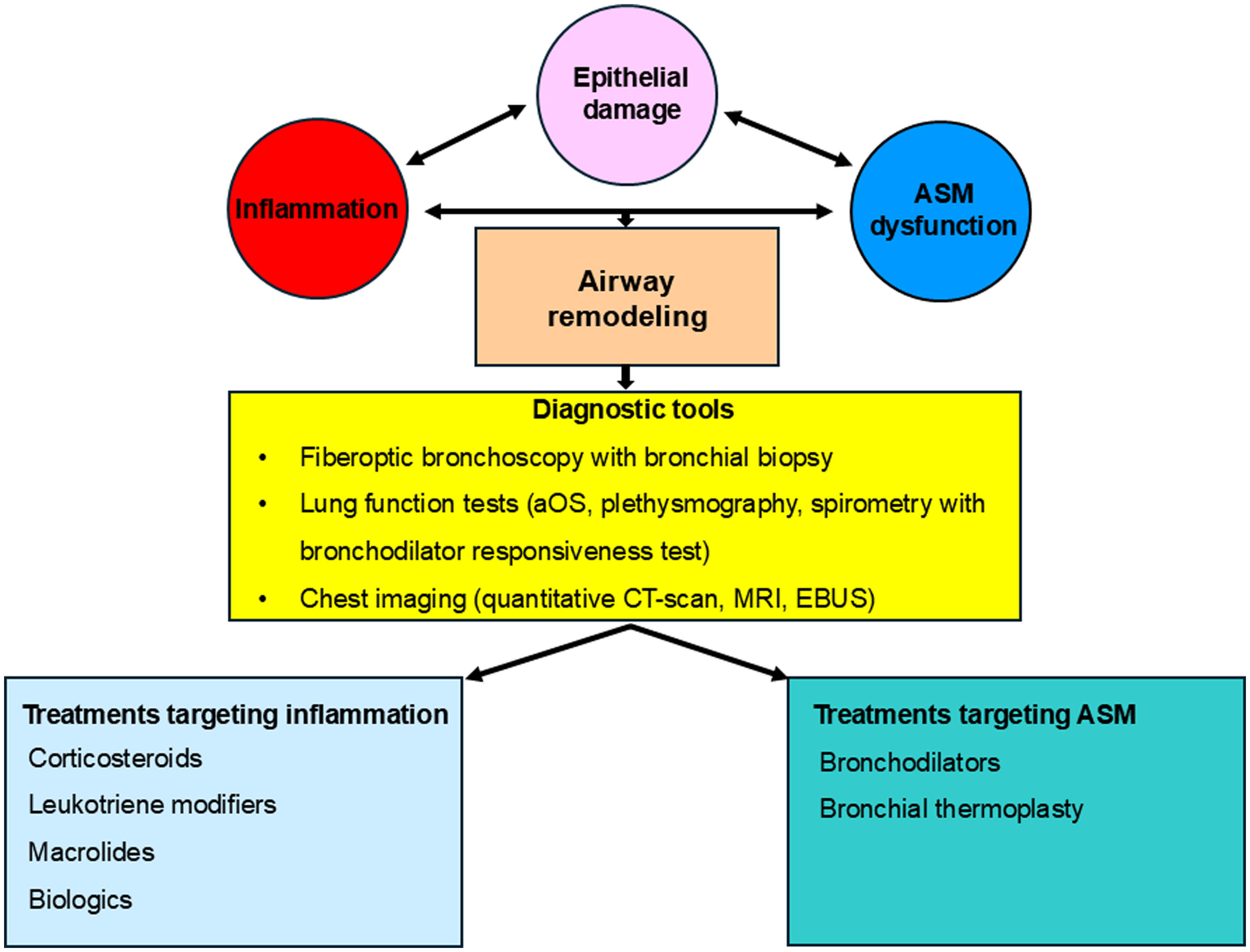
Airway remodeling (AR) and chronic inflammation are key features in asthma pathogenesis. AR involves changes in the airway wall structure such as hyperplasia of the smooth muscles, subepithelial collagen deposition with increased thickness of the reticular basement membrane (RBM), disruption of the epithelial barrier integrity with metaplasia of goblet cells inducing mucus hyperproduction, and angiogenesis.1
AR is associated with poor asthma outcomes, lower lung function, reduced response to treatments, and altered quality of life.2 The degree of AR correlates with asthma severity, but alterations of the bronchial wall architecture are also present in mild disease.3 Like inflammation, AR in asthma is heterogeneous and may contribute to different asthma phenotypes and endotypes.4 Most studies were focused on the role of inflammation in asthma pathogenesis, while understanding the mechanisms driving AR is crucial for developing disease-modifying therapeutic strategies.
Until recently, it was thought that AR is caused by chronic airway inflammation. In this model, the airway epithelial cells are “initiators” of AR, with secretion of growth factors (e.g. transforming growth factor beta) following tissue injuries and alarmins, that activate the immune cells who are “amplifiers” of the process but also the resident cells, notably fibroblasts and smooth muscle cells, who are “effectors” inducing structural changes in the bronchial wall. However, AR features were found in bronchial biopsies from pre-school children suggesting that the airway smooth muscle cells could be also the “initiators” of the process due to their hypercontractility status induced by altered calcium homeostasis and/or mitochondrial dysfunction, with epithelial damage because of excessive mechanical forces during bronchoconstriction.1,5
Bronchial biopsy is considered the gold standard of AR diagnosis, but this method is invasive, so it is difficult to be routinely performed in clinical practice. Fortunately, most of the currently available lung function tests and new generation multidetector computed tomography (MDCT) scans could evaluate indirectly but accurately the presence of AR in asthma patients.1
For the assessment of AR, performing at least a spirometry with responsiveness test to asthma medication is mandatory. The persistent airflow limitation despite treatment, reduced lung function, and decreased responsiveness to bronchodilator medications may suggest the presence of AR in asthma patients. Spirometry is also useful for monitoring disease progression in asthma, including AR development over time.6 The plethysmography assesses in addition the airway resistance directly related to AR and the presence of indirect signs of airway obstruction such as pulmonary hyperinflation. By measuring resistance, reactance, and impedance, airway oscillometry (aOS) could detect early changes in airway function even before airflow limitation.1 Patients developing irreversible airway obstruction (IRAO) due to AR represent half of patients with severe asthma. IRAO is usually defined as a forced expiratory volume in 1s (FEV1) <80% of predicted with the ratio of FEV1 and forced vital capacity <0.7.7 Factors frequently associated with IRAO are male gender, long-disease duration, smoking, mixed granulocytic inflammation in sputum, and high therapeutic pressure.8 Early identification of airway hyperresponsiveness (AHR) by bronchial challenge test allows for timely therapeutic intervention and prevention of AR.6
By applying minimal radiation levels, the MDCT-scan provides high-resolution lung imaging, during inspiratory and expiratory phases, with the possibility to extract quantitative data and evaluate bronchial thickness (lumen diameter – LD and lumen area – LA, bronchial wall thickness – WT and wall area –WA), the presence of mucus plugs in the airways, lung hyperinflation and air trapping. Strong correlations were found between AR features observed on MDCT images, pathological and functional examinations. Analysis of MDCT-scans allowed to identify distinct asthma phenotypes with different clinical outcomes.1 More advanced AR on MDCT-scan is associated with low lung function, increased peripheral resistance and reactance measured by aOS, high exacerbation rate, and worse response to standard therapy.9 The presence of mucus plugs is related to T2-inflammation, more severe asthma, frequent exacerbations and poor lung function.10 Longitudinal studies showed that MDCT-scan could help in identifying asthmatics prone to irreversible loss of lung function over time and assist in the assessment of response to biologics, becoming a useful diagnostic tool for personalized medicine in asthma management.1 The place of the other imaging methods in the evaluation of AR in asthma should be better defined.
Most current medications for asthma target airway inflammation rather than smooth muscle cell dysfunction and showed limited benefits on AR. Several data suggested that inhaled corticosteroids administered for at least 6 weeks helps in recovery of epithelial damage, decreases RBM thicknesses and collagen deposition, reduces goblet cell hyperplasia, AHR and angiogenesis in asthma patients with possible dose- and time-dependent effects. The association of long-acting β2-agonists to inhaled corticosteroids decreased goblet cell metaplasia and vessels density. The addition of glycopyrronium to this association reduced methacholine-induced bronchoconstriction, while tiotropium decreased airway WA and WT on MDCT-scan and improved airway obstruction. Administration of leukotriene modifiers improves AHR, decreases collagen deposition in airways in asthma children, and myofibroblast number in adults with mild atopic asthma.1 Azithromycin administration (250mg three days a week) during 8-months increased LA on MDCT-scan without significant change on the WT versus placebo.11 Biologics showed promising effects on AR in severe asthma patients. Omalizumab decreased AHR in allergic asthma, airway WT on MDCT-scan, RBM and fibronectin accumulation on bronchial biopsies if administered for at least 16-weeks.1,12 One-year treatment by mepolizumab significantly reduced airway WA on MDCT-scan, decreased RBM thickness, airway smooth muscle area, and extent of epithelial damage in patients with severe eosinophilic asthma and IRAO. Benralizumab reduced AHR, mucus plugs and ventilation defects on functional imaging, tissue myofibroblast number and smooth muscle mass in bronchial biopsies in patients with severe eosinophilic asthma after 3-months of treatment.1,13 Forty-eight weeks treatment with dupilumab decreased airway obstruction, mucus plugs and airway WT on MDCT-scan in patients with uncontrolled moderate-to-severe asthma.14 Tezepelumab reduced AHR and mucus plugs on MDCT-scan after 28-weeks of treatment. Other medications such as house dust mite sublingual immunotherapy, fevipiprant (a prostaglandin D2 type 2 receptor antagonist), and gallopamil (a calcium channel blocker) showed also some benefits on AR in asthma.1
The endoscopic treatment using radiofrequency energy, bronchial thermoplasty, decreased AR in 60% of adults with severe asthma by reducing the smooth muscle mass, RBM thickening, submucosal nerves, and epithelium neuroendocrine cells, the rearrangement of the extracellular collagen matrix, an improvement of the airway epithelial cells regeneration and a negative impact on fibroblasts/myofibroblasts proliferation without affecting vasculature. The response to treatment is independent of the bronchodilator responsiveness, but the reduction of the smooth muscle mass is greater in patients with poor lung function.1 The effect of treatment becomes visible after 3-months of treatment and persists >10 years after the procedure.15
Fig. 1 represents a summary of main contributors to AR in asthma, current diagnostic tools and therapeutic options.
Recognizing that AR may occur early in asthma pathogenesis and not just as a consequence of inflammation is crucial for developing new therapeutic strategies. Advances in imaging techniques and lung function testing allowed clinicians to accurately assess the presence of AR in asthma patients. Most of current therapies of asthma showed limited effects on AR by targeting inflammation and not resident cells dysfunction. More efforts should be deployed to understand the complex mechanisms driving AR in asthma and to identify new effective medications for the involved pathways.
FundingNone declared.
Authors’ contributionsA.T. wrote the editorial.
Conflicts of interestA.T. received honoraria as speaker for educational events from AstraZeneca, BMS, GSK, and Sanofi; as member of Advisory Board from AstraZeneca and Sanofi; support for attending meetings from AstraZeneca and Sanofi.