To decrease readmissions at 30 and 90 days post-discharge from a hospital admission for chronic obstructive pulmonary disease exacerbation (COPDE) through the home care model of the Ambulatory Chronic Respiratory Care Unit (ACRCU), increase patient survival at one year, and validate our readmission risk scale (RRS).
Materials and methodsThis was an observational study, with a prospective data collection and a retrospective data analysis. A total of 491 patients with a spirometry diagnosis of chronic obstructive pulmonary disease (COPD) requiring hospitalisation for an exacerbation were included in the study. Subjects recruited within the first year (204 cases) received conventional care (CC). In the following year a home care (HC) programme was implemented and of those recruited that year (287) 104 were included in the ACRCU, administered by a specialised nurse.
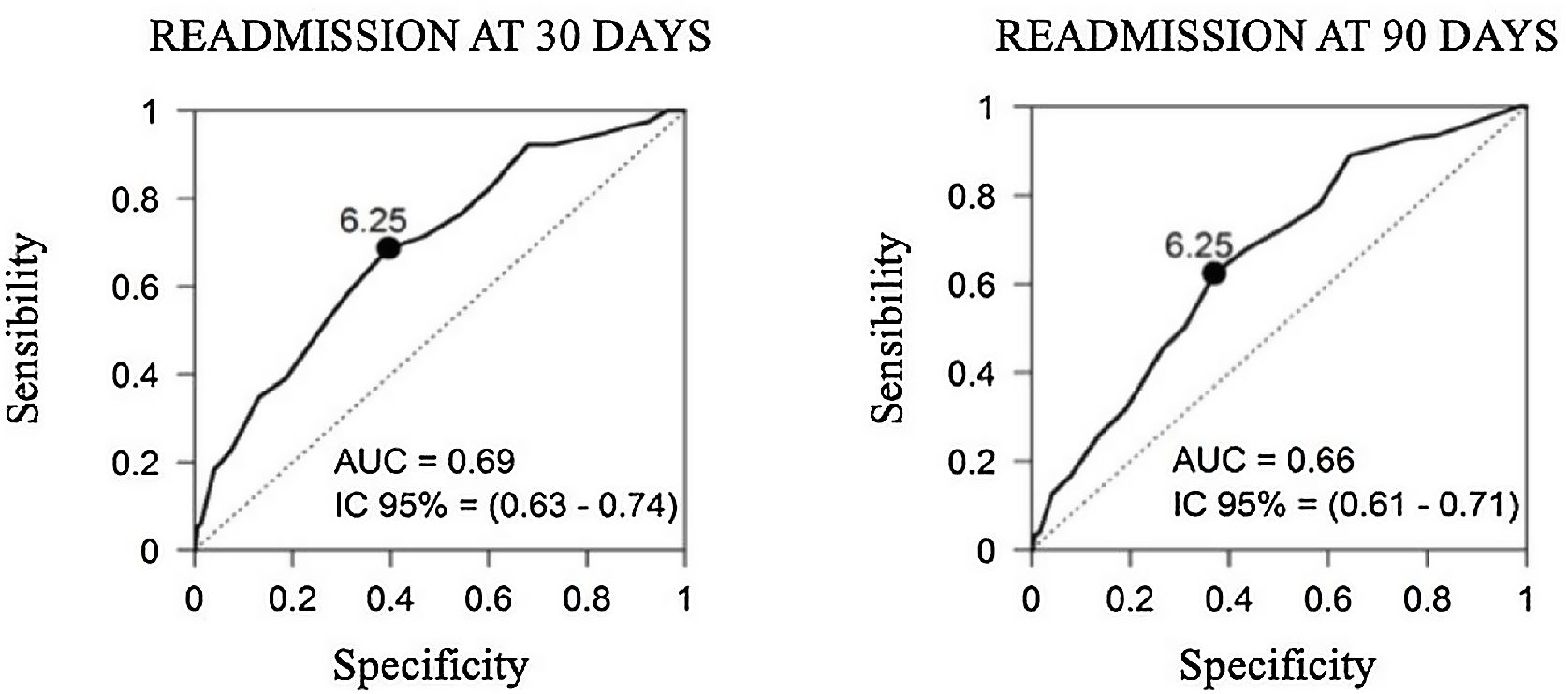
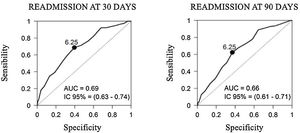
ResultsIn the group of patients included in the home care model of the Ambulatory Chronic Respiratory Care Unit (ACRCU) a lower number of readmissions was observed at 30 and 90 days after discharge (30.5% vs. 50%, p=0.012 and 47.7% vs. 65.2%, p=0.031, respectively) and a greater one-year survival (85.3% vs. 59.1%, p<0.001). The validation of our RRS revealed that the tool's capacity to predict readmissions at both 30 and 90 days was not high (AUC=0.69 and AUC=0.66, respectively).
ConclusionsThe inclusion of exacerbator or fragile COPD patients in the ACRCU could achieve a decrease in readmissions and an increase in survival. The number of episodes of exacerbation within the 12 months prior to the hospital admission is the variable that best predicts the risk of readmission.
Disminuir los reingresos a los 30 y 90 días tras el alta por un ingreso hospitalario por exacerbación de enfermedad pulmonar obstructiva crónica (EPOC) a través del modelo de atención domiciliaria de la Unidad de Cuidados Crónicos Respiratorios Ambulatorios (UCCRA), aumentar la supervivencia al año y validar nuestra escala de riesgo de reingreso (ERR).
Material y métodosEstudio observacional con recogida prospectiva de datos. Se incluyó en el estudio a un total de 491 pacientes con diagnóstico espirométrico de enfermedad pulmonar obstructiva crónica que requirieron hospitalización por una agudización. Los sujetos reclutados dentro del primer año (204 casos) recibieron atención convencional (AC). Al año siguiente se implementó un programa de atención domiciliaria (AD) y de los pacientes reclutados ese año (287), 104 fueron incluidos en la UCCRA con seguimiento de una enfermera especializada.
ResultadosEn el grupo de pacientes incluidos en el modelo de atención domiciliaria de la UCCRA se observó un menor número de reingresos a los 30 y 90 días tras el alta (30,5% vs 50%, p=0,012 y 47,7% vs. 65,2%, p=0,031, respectivamente) y una mayor supervivencia al año (85,3% vs. 59,1%, p<0,001). La validación de nuestra ERR reveló que la capacidad de la misma para predecir reingresos tanto a los 30 como a los 90 días no era alta (AUC=0,69 y AUC=0,66, respectivamente).
ConclusionesLa inclusión de pacientes con EPOC agudizadores o frágiles en la UCCRA podría conseguir una disminución de los reingresos y una aumento de la supervivencia. El número de agudizaciones en los 12 meses previos al ingreso hospitalario es la variable que mejor predice el riesgo de reingreso.
Chronic obstructive pulmonary disease (COPD) is a disease characterised by chronic airflow limitation that is not fully reversible and is associated with an abnormal inflammatory response to noxious particles and gases (mainly tobacco smoke).1,2 The prevalence of this condition is high (11.8% among subjects aged 40–80 years), but only one fourth of smokers develop the disease, thus adding to its underdiagnosis, which can reach up to 74.7% of cases.3 In addition, it is associated with high morbidity and mortality rates, with a recent World Health Organisation (WHO) projection identifying it as the third leading cause of death in 2030.4 As the disease progresses, patients experience episodes of exacerbation (COPDE)5 and have to be hospitalised, both of which are associated with a deterioration in their quality of life, greater mortality rates, and an increase in the use of healthcare resources, hospitalisations, and associated costs. In fact, COPD has an incredibly significant economic impact, as it accounts for 56% of all healthcare costs (38.6 billion euros). The long-term prognosis following hospitalisation for COPDE is poor, with an estimated five-year mortality rate of approximately 50%.6
Several scales, including the BAP-65 (blood urea nitrogen [BUN], altered mental condition, pulse>109bpm, and age ≥65 years)7 and DeCOPD (death in COPDE),8 are useful for measuring the severity of the COPDE and consider parameters such as the presence of uraemia, the degree of dyspnoea as measured by the modified Medical Research Council (mMRC) dyspnoea scale, the patients’ mental state (Glasgow Coma Scale), and their age.
Because these episodes of COPDE are a key point in the disease's evolution, we are urged to developed strategies aimed at treating them early to avoid hospitalisations and the negative consequences arising from them. For this purpose, we use home care (HC), defined as the set of resources aimed at providing support and care to patients in their own homes.9 Within HC, we can distinguish home hospitalisation (HH), considered an alternative to conventional hospitalisation and in which treatment of the same complexity, duration, and intensity than in an acute care hospital is administered, albeit without the need for continuing the patients’ hospital stay,9 and telemedicine (TLM), which can be defined as the provision of healthcare services in which distance is a critical factor overcome by the use of information and communication technologies (ICTs) and whose aim is to exchange data required to reach a diagnosis, recommend treatments, as well as prevent illnesses and accidents.4 Because the available literature describes a wide range of HC models, including HH, TLM, telemonitoring, etc. with varying results,10–13 we believe that conducting new studies providing more data on the benefits of HC is necessary.
Considering the above, we hypothesise that HC, after hospitalisation due to an episode of COPDE in patients with an exacerbator or fragile COPD phenotype, decreases the rate of readmissions due to this condition and increases patient survival. Therefore, the main objective of our study is to evaluate whether our HC model decreases the risk of hospital readmissions and improves survival following hospitalisation for a COPDE in patients with an exacerbator or fragile COPD phenotype. Its secondary objectives are to determine whether this model reduces the mean length of hospital stay and whether our readmission risk scale (RRS) is a useful tool for predicting readmissions in patients hospitalised for a COPDE.
Material and methodsStudy designThis was an observational study, with a prospective data collection and a retrospective data analysis, including patients admitted to the Pneumology service of a tertiary level hospital for a COPDE. Our hospital currently serves 458,545 inhabitants, in a district with an urban area of 33km2. The patients were recruited consecutively for two full years, with no data being collected for two months between the first and second year, as this was a transition period between the conventional care (CC) and the HC. The inclusion criteria were hospitalisation due to an episode of COPDE in patients with a previous diagnosis of COPD confirmed by a forced spirometry with a FEV1/FVC (forced vital capacity) ratio<0.70.6 Hospitalised patients without a prior diagnosis of COPD or a confirmed diagnosis of COPDE were excluded from the study.
The study was approved by the Research Ethics Committee of our clinic (EO 45/2016-FJD), and the patients’ rights were protected at all times by the provisions of the Declaration of Helsinki (October 2013 version) and the Principles of Good Clinical Practice. Written informed consent was not required from patients to participate in the study, as it was conducted following usual clinical practice.
Patients who were discharged after hospital admission for COPDE during the first year of the study received CC and were followed up by their Primary Care physician for 30 days after hospital discharge. The follow-up of these patients was performed according to Clinical Practice Guidelines (CPG), with no structured health programme or protocol established between Primary Care and Pulmonology. The other group in the study consisted of patients who, in the following year, were discharged after hospital admission for COPD. During this period, a home care programme, Ambulatory Chronic Respiratory Care Unit (ACRCU), was implemented, which was accessed by subjects who met the inclusion criteria and none of the exclusion criteria detailed in Table 1.
Inclusion and exclusion criteria of the ACRCU.
| Inclusion criteria |
| •Patients diagnosed with COPD by forced spirometry based on post-bronchodilator FEV1/FVC<0.70.•Patients of both sexes, older than 40 years and with a score on our readmission risk scale (RRS) greater than 7.•Presence of 2 or more hospitalisations for exacerbations of COPD in the last year.•Patients considered as “fragile”. |
| Exclusion criteria |
| •Patients who, at the investigator's discretion, did not meet any of the aforementioned inclusion criteria.•Patients who refuse to enter the HC programme.•Patients who have medical assistance in a nursing home or social-health centre, or who are in a social situation that prevents the HC.•Patients who do not belong to the care area of our hospital. |
COPD: chronic obstructive pulmonary disease; FEV1/FVC: relationship between forced exhaled volume in the first second (FEV1) and the forced vital capacity (FVC); HC: home care.
A COPDE was defined as an acute episode of clinical instability occurring throughout the natural course of the disease, characterised by sustained worsening of the patients’ respiratory symptoms beyond standard daily variability, and requiring a change of treatment.1 A fragile patient was defined as that presenting with a greater need for and risk of using social and healthcare resources, institutionalisation, deterioration in their quality of life, and death.14
During their hospital stay for COPDE, patients were assessed by the inpatient pulmonologist, who identified subjects meeting the inclusion criteria of our ACRCU, as determined by our RRS (Table 2). This scale was created by our team as a tool for predicting readmissions and consists in eight items (age, exacerbations requiring hospitalisation or not within the last 12 months, FEV1, chronic bronchitis profile, bacterial colonisation, Karnofsky index, and presence of a vascular comorbidity). Patients with an RRS score>7, at least two hospitalisations for a COPDE within the last year, and considered fragile15 were included in our ACRCU. Although a generic definition of frailty was established and the score obtained in the RRS was used, the final decision to include patients in the study depended on each physician.
RRS created in our centre for the inclusion of subjects in the ACRCU.
| Variables | Score | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | |
| Age | <50 | 50–65 | >65 | >75 | |||
| Non-hospital exacerbations in the last 12 months | 0 | 1 | ≥2 | ||||
| Hospitalisations last 12 months | 0 | ≥1 | |||||
| FEV1 | >60% | 50–59% | 49–49% | 30–39% | <30% | ||
| Chronic bronchitis profile | No | Sí | |||||
| Bacterial colonisation | No | Sí | |||||
| Karfnosky index | 70–100 | 50–69 | <50 | ||||
| Vascular comorbidity | No | Sí | |||||
FEV1: forced exhaled volume in the first second.
The patients hospitalised during the second year for COPDE and who met the criteria to be included in the ACRCU programme, after obtaining their verbal consent, were interviewed, as were their relatives and/or caregivers, by the HC nurse who provided them with information on the care that the patients would receive after hospital discharge. The first visit to the patients’ homes took place 24h after the discharge, and successive visits were completed during the following 30 days, at a frequency of every 2–3 days. During these visits, the nurse was responsible for collecting data on the patients’ environment, whether they had family members or caretakers helping them, as well as variables such as their blood pressure, oxygen saturation, heart rate, and respiratory rate; recording information on their pharmacological treatments (knowledge of the drugs taken, their dosage, and their usefulness); evaluating the presence of potential side effects from these drugs; re-evaluating the patients’ inhalation technique; and reviewing their non-pharmacological treatments (smoking cessation, oxygen therapy, non-invasive mechanical ventilation [NIMV], and respiratory physical therapy). The nurses performed these assessment from 08:00 to 20:00, Monday through Friday. If a patient required a medical evaluation, diagnostic testing, or therapeutic adjustments due to presenting with clinical worsening, they were transferred to our outpatient facility, where it was decided whether they had to be admitted to the hospital or continue the HC. Outside this centre's standard opening hours, the patients had to attend the Emergency Department.
Variables analysed and statistical analysisFollowing the patients’ inclusion in this study; that is, in the CC group during the first year and in the HC group throughout the second year, the following variables were measured and compared: sex; age; smoking habit and number of pack-years; presence of comorbidities (as per the crude Charlson Comorbidity Index, not adjusted by age16); pulmonary function parameters (FEV1, FEV1/FVC ratio, and bronchodilation percentage) measured through a spirometry test1; the classification of COPD in stable and exacerbated phase measured by GOLD (Global Initiative for Chronic Obstructive Lung Disease) scale17 and the classification of COPD according to phenotypes measured by GesEPOC (Spanish Guidelines for COPD),18 the BODEx (body mass index, obstruction, dyspnoea, and exacerbations) index,19 the BAP-65,7 and the DeCOPD8; the degree of dyspnoea as measured by the mMRC dyspnoea scale; the clinical profile of chronic bronchitis and the presence of cardiovascular comorbidities; the presence of bacterial colonisation (according to the SEPAR [Spanish Society of Pulmonology and Thoracic Surgery] regulations)20; the patients’ overall health and quality of life status, as measured using the Karnofsky index21 and the number of episodes of COPDE within the previous year (requiring hospitalisation or not). We also measured the mean length of hospital stay, the number of readmissions at 30 and 90 days, and the one-year survival after the patients’ discharge from the hospital following their admission for a COPDE.
Qualitative variables were summarised by absolute and relative frequencies, and quantitative variables by mean and standard deviation, or by median and interquartile range. Comparisons between CC and HC groups were performed regarding patient characteristics. Chi-square test or Fisher's exact test were used to compare qualitative variables, and Student's t test or Mann–Whitney U test were used for quantitative variables. The one-year survival was measured and compared between CC and HC groups. To make this comparison controlling for potential confounding factors, inverse probability weighting was used. Weights were calculated as the inverse of probabilities derived from logistic regression models, which regressed group variable (CC or HC) on potential confounding factors. Weighted survival curves were calculated and compared using weighted Cox regression models. Finally, to explore which RRS variables could predicted better readmissions, we used logistic regression models. Models were summarised by odds ratio (OR), 95% confidence interval (95%CI), and area under the ROC curve (AUC). Statistical analyses were performed using R 4.1.0 (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
ResultsOur study population was comprised of 491 patients with a COPDE, a mean age of 72.4 years, a diagnosis of severe COPD as determined by a spirometry test with a mean FEV1 of 48.6% (276 of the patients, 56.2% of the total, had a FEV1<50%), a GOLD class D, and a predominantly non-exacerbator phenotype prior to their admission to our clinic. More than 50% of these patients were ex-smokers (mean of 63.8 pack-years), 58.7% had one or more cardiovascular comorbidities, and 9% were diagnosed with bacterial colonisation. In the 12 months preceding their hospitalisation, the mean number of episodes of COPDE per patient was 2.16 (mean length of hospital stay of 7.14 days, and moderate severity according to the BAP-657 and DeCOPD8 classifications). In the total study population, 23.5% of the patients were readmitted within the first 30 days after their discharge from the hospital and 37.4% of them within the first 90 days, with a pooled one-year survival rate of 84.3% (Table 3).
Descriptive analysis of the total population under study.
| Variable | n | Average | SD |
|---|---|---|---|
| Age (years) | 491 | 72.4 | 10.1 |
| FEV1(%) | 491 | 48.6 | 17.2 |
| PYIa(pack-year index) | 424 | 63.8 | 33.6 |
| Total exacerbations | 491 | 2.16 | 2.61 |
| Hospitalisations | 491 | 1.70 | 2.14 |
| Nonhospitalisations | 491 | 0.46 | 1.18 |
| Days of hospital stay | 491 | 7.14 | 5.24 |
| BODExa | 480 | 4.14 | 2.24 |
| Karfnosky index | 491 | 78.7 | 15.4 |
| Comorbidities (Charlson)a | 489 | 5.81 | 2.12 |
| DeCOPDa | 484 | 2.47 | 2.20 |
| BAP-65a | 487 | 2.39 | 0.88 |
| Variable | n | % |
|---|---|---|
| Sex | ||
| Male | 360 | 73.3 |
| Female | 131 | 26.7 |
| mMRC | ||
| 0 | 22 | 4.5 |
| 1 | 100 | 20.7 |
| 2 | 136 | 28.1 |
| 3 | 115 | 23.8 |
| 4 | 111 | 22.9 |
| GOLDa | ||
| A | 72 | 14.8 |
| B | 76 | 15.7 |
| C | 78 | 16.1 |
| D | 259 | 53.4 |
| GesEPOCa | ||
| Non-exacerbator | 200 | 41.1 |
| Asthma-COPD | 6 | 1.2 |
| Emphysema exacerbator | 167 | 34.3 |
| Bronchitis exacerbator | 114 | 23.4 |
| Chronic bronchitis profile | 140 | 28.5 |
| Smokinga | ||
| Never smoker | 11 | 2.3 |
| Current smoker | 167 | 34.4 |
| Ex-smoker | 308 | 63.4 |
| Vascular comorbidities | 288 | 58.7 |
| Bacterial colonisation | 44 | 9.0 |
| Readmission at 30 days | 115 | 23.5 |
| Readmission at 90 days | 183 | 37.4 |
| Survival | 414 | 84.3 |
Description of quantitative variables by means of mean and standard deviation (m and SD) and qualitative variables by percentages (%); n: study population.
FEV1: forced exhaled volume in the first second; mMRC: modified scale of the Medical Reasearch Council; COPD: chronic obstructive pulmonary disease.
Missing data: PYI: 67; BODEx: 21; Comorbidities (Charlson): 2; DeCOPD: 7; BAP-65: 4; mMRC: 7; GOLD: 6; GesEPOC: 4; Smoking: 5.
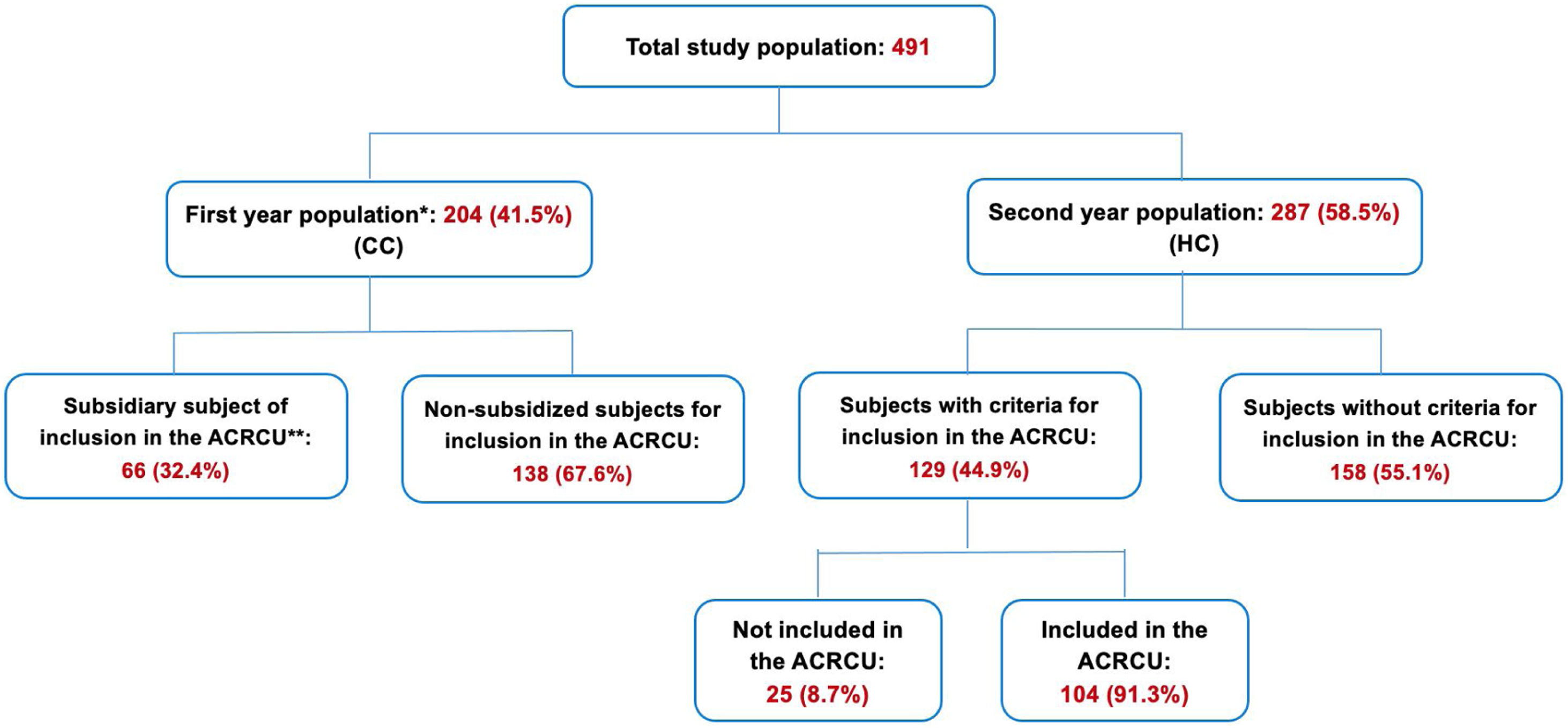
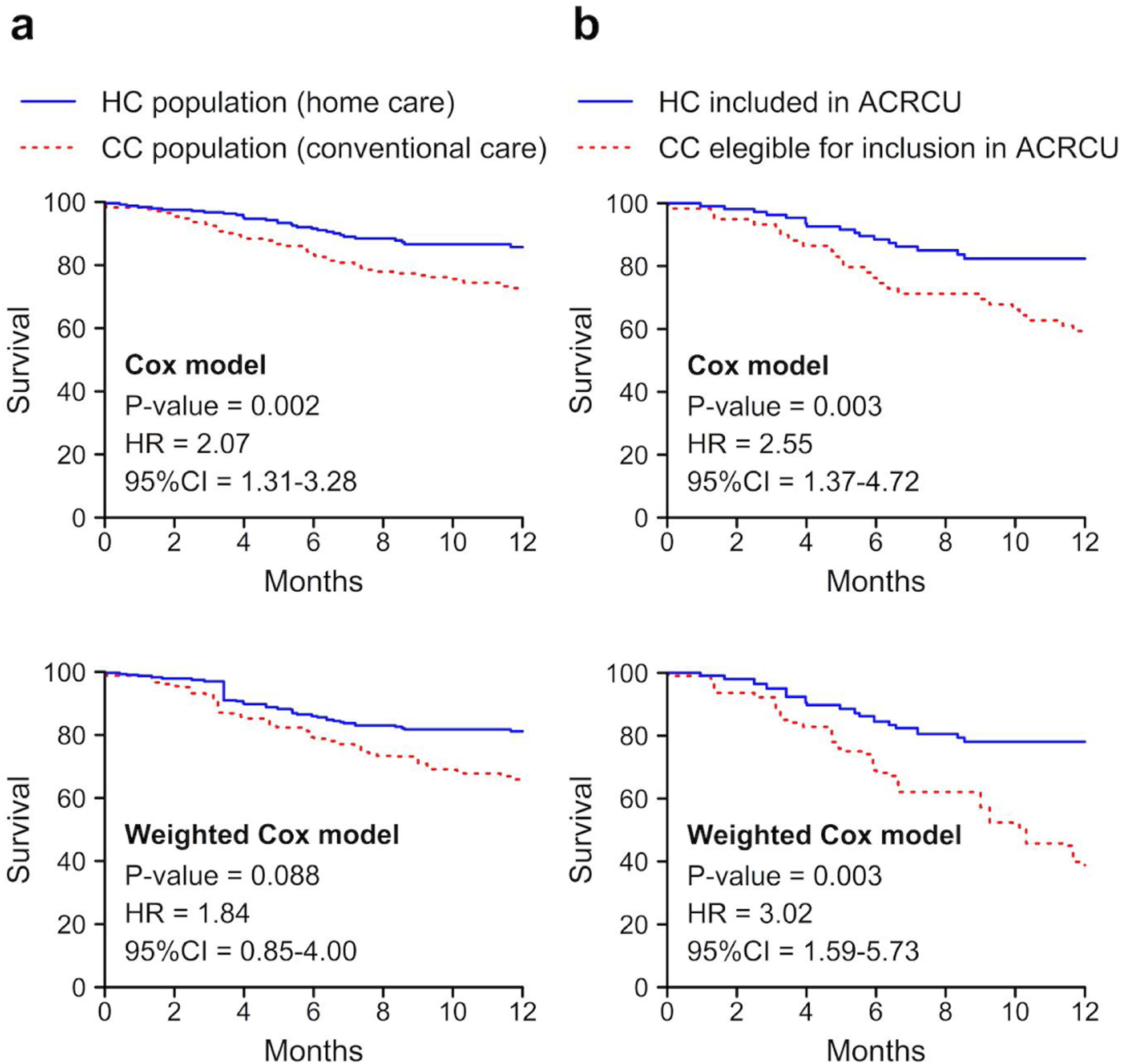
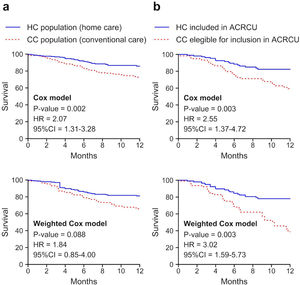
A descriptive and comparative analysis was then performed between the 204 patients included in the CC group and the 287 subjects of the HC group (Fig. 1). The subjects of both study groups were predominantly of male sex and ex-smokers, with a greater number of pack-years in the CC group and a greater Karnofsky index21 in the HC group (p<0.05). Statistically significant differences were found between both groups in the degree of dyspnoea (mMRC), the presence of bacterial colonisation, and the COPD classification (predominance of the non-exacerbator phenotype in the CC group and of the exacerbator with emphysema phenotype in the HC group). Although no differences were found in the total number of COPDEs, there were differences in the mean length of hospital stay and the number of ambulatory exacerbations (p<0.05). We found a lower percentage of readmissions at 30 days in the HC population compared with the CC group (19.9% vs. 28.6%, p=0.035), in addition to a higher one-year post-discharge survival rate (84.4% vs. 73.5%, p=0.005) (Fig. 2, Table 4).
Outline of the total study population.
CC: conventional care; HC: home care; ACRCU: Ambulatory Respiratory Chronic Care Unit.
* Patients included in the study in the first year who were discharged after hospital admission for COPDE and received CC.
** Patients included in the study in the first year who could have been included in the ACRCU if it had been implanted in this period.
Descriptive and comparative analysis of the CC and HC populations.
| Variable | CC | HC | p |
| Age (years) | 73.1±9.7 | 71.9±10.3 | 0.178 |
| FEV1(%) | 49.2±17.6 | 48.1±17.0 | 0.489 |
| PYI (pack-years index) | 60.0 (45.0) | 53.0 (39.3) | 0.003 |
| Total exacerbations | 1.00 (3.00) | 1.00 (3.00) | 0.158 |
| Hospitalisations | 1.00 (3.00) | 1.00 (2.00) | 0.456 |
| Nonhospitalisations | 0.00 (0.00) | 0.00 (1.00) | 0.045 |
| Days of hospital stay | 6.61±4.12 | 7.52±5.88 | 0.045 |
| BODEx | 4.04±2.51 | 4.20±2.03 | 0.459 |
| Karfnosky index | 76.0±15.8 | 80.7±14.9 | 0.001 |
| Comorbilidities (Charlson) | 5.84±2.12 | 5.79±2.12 | 0.772 |
| DeCOPD | 2.00 (2.00) | 2.00 (2.00) | 0.056 |
| BAP-65 | 2.42±0.90 | 2.38±0.87 | 0.575 |
| Variable | CC | HC | p |
|---|---|---|---|
| Sex | 0.001 | ||
| Male | 166 (81.4%) | 194 (67.6%) | |
| Female | 38 (18.6%) | 93 (32.4%) | |
| mMRC | 0.007 | ||
| 0 | 16 (8.1%) | 6 (2.1%) | |
| 1 | 45 (22.7%) | 55 (19.2%) | |
| 2 | 44 (22.2%) | 92 (32.2%) | |
| 3 | 48 (24.2%) | 67 (23.4%) | |
| 4 | 45 (22.7%) | 66 (23.1%) | |
| GOLD | <0.001 | ||
| A | 52 (26.1%) | 20 (7.0%) | |
| B | 41 (20.6%) | 35 (12.2%) | |
| C | 20 (10.1%) | 58 (20.3%) | |
| D | 86 (43.2%) | 173 (60.5%) | |
| GesEPOC | <0.001 | ||
| Non-exacerbator | 113 (56.2%) | 87 (30.4%) | |
| Asthma-COPD | 0 (0%) | 6 (2.1%) | |
| Emphysema exacerbator | 31 (15.4%) | 136 (47.6%) | |
| Bronchitis exacerbator | 57 (28.4%) | 57 (19.9%) | |
| Chronic bronchitis profile | 66 (32.4%) | 74 (25.8%) | 0.137 |
| Smoking | <0.001 | ||
| Never smoker | 4 (2.0%) | 7 (2.5%) | |
| Current smoker | 44 (21.8%) | 123 (43.3%) | |
| Ex-smoker | 154 (76.2%) | 154 (54.2%) | |
| Vascular comorbidities | 125 (61.3%) | 163 (56.8%) | 0.368 |
| Bacterial colonisation | 33 (16.2%) | 11 (3.8%) | <0.001 |
| Readmissions at 30 days | 58 (28.6%) | 57 (19.9%) | 0.035 |
| Readmission at 90 days | 79 (38.9%) | 104 (36.4%) | 0.631 |
| Survival | 150 (73.5%) | 248 (86.4%) | 0.001 |
Description of quantitative variables by mean standard deviation (m±SD) or by median and interquartile range in parentheses. Description of qualitative variables by frequencies and percentages.
CC: conventional care; HC: home care; FEV1: forced exhaled volume in the first second; mMRC: modified scale of the Medical Research Council; COPD: chronic obstructive pulmonary disease.
Only 104 of the 129 patients meeting the inclusion criteria were included in our ACRCU (HC) (Fig. 1). The remaining 25 patients were not admitted to this unit due to the patients’ refusal, access to care in a nursing home or social-healthcare centre, homeless condition, or the fact that they did not belong to the healthcare area covered by our hospital.
To compare patients with a similar COPDE and severity, we conducted a comparative analysis between CC subjects who met the required criteria to have been eligible for inclusion in the ACRCU unit had it been implemented (66 patients) and the 104 HC subjects who were included in the ACRCU throughout the second year of the study. We found no differences in terms of the patients’ sex, mean age, or number of cardiovascular comorbidities. However, we did observe statistically significant differences in the number of pack-years, the total number of episodes of COPDE within the previous year (including those requiring hospitalisation), the severity of the exacerbation according to the DeCOPD, the Karnofsky index, and the presence of bacterial colonisation (Table 5). According to the GesEPOC, the exacerbator with chronic bronchitis phenotype predominated in the CC population, whereas the exacerbator with emphysema phenotype was more prevalent in the HC group (p<0.05) (Table 5). We observed a lower rate of readmissions at both 30 and 90 days post-discharge (30.5% vs. 50%, p=0.012 and 47.7% vs. 65.2%, p=0.031, respectively) and a greater one-year survival (85.3% vs. 59.1%, p<0.001) among the patients included in our ACRCU (HC). The results of survival when the comparison of the total population is made and when the comparison is made between the patients with an indication of being included in the ACRCU, are shown in Fig. 2. In case (a) we can see that, although when carrying out adjusted propensity score statistical significance is lost, the difference between the curves remains quite similar. The difference between the curves can be quantified with the Hazard ratio (HR). In this way, we see that in case (a) the HR is slightly reduced, but remains quite high, and in case (b) the HR even increases.
Descriptive and comparative analysis of the CC patients eligible to be included in the ACRCU and the HC patients included in the programme.
| Variable | Subsidiaries of ACRCU (CC) | Included in ACRCU (HC) | p |
|---|---|---|---|
| Age (years) | 73.23±8.19 | 73.57±9.78 | 0.810 |
| FEV1 (%) | 44.17±15.19 | 44.60±15.71 | 0.852 |
| PYI (pack-years index) | 65.00 (42.00) | 55.00 (36.50) | 0.010 |
| Total exacerbations | 3.00 (4.00) | 3.00 (3.00) | 0.024 |
| Hospitalisations | 3.00 (3.00) | 2.00 (2.00) | 0.002 |
| Non hospitalisations | 0.00 (1.00) | 0.00 (1.00) | 0.207 |
| Days of hospital stay | 8.05±4.24 | 8.77±6.99 | 0.367 |
| BODEx | 5.44±2.09 | 4.74±1.91 | 0.021 |
| Karfnosky index | 72.27±16.44 | 77.05±13.72 | 0.033 |
| Comorbidities(Charlson) | 6.48±2.35 | 5.94±1.92 | 0.086 |
| DeCOPD | 3.00 (2.00) | 2.00 (1.00) | <0.001 |
| BAP-65 | 2.70±0.70 | 2.48±0.77 | 0.059 |
| Variable | Subsidiaries of ACRCU (CC) | Included in ACRCU (HC) | p |
|---|---|---|---|
| Sex | 0.065 | ||
| Male | 57 (86.4%) | 95 (73.6%) | |
| Female | 9 (13.6%) | 34 (26.4%) | |
| mMRC | 0.361 | ||
| 0 | 1 (1.5%) | 2 (1.6%) | |
| 1 | 10 (15.2%) | 19 (14.8%) | |
| 2 | 14 (21.2%) | 42 (32.8%) | |
| 3 | 15 (22.7%) | 30 (23.4%) | |
| 4 | 26 (39.4%) | 35 (27.3%) | |
| GOLD | 0.131 | ||
| A | 2 (3.0%) | 2 (1.6%) | |
| B | 8 (12.1%) | 6 (4.7%) | |
| C | 9 (13.6%) | 28 (21.9%) | |
| D | 47 (71.2%) | 92 (71.9%) | |
| GesEPOC | <0.001 | ||
| Non-exacerbator | 13 (19.7%) | 22 (17.2%) | |
| Asthma-COPD | 0 (0.0%) | 1 (0.8%) | |
| Emphysema exacerbator | 18 (27.3%) | 75 (58.6%) | |
| Bronchitis exacerbator | 35 (53.0%) | 30 (23.4%) | |
| Chronic bronchitis profile | 23 (34.8%) | 38 (29.5%) | 0.545 |
| Smoking | 0.117 | ||
| Never smoker | 1 (1.5%) | 4 (3.1%) | |
| Current smoker | 13 (20.0%) | 42 (33.1%) | |
| Ex-smoker | 51 (78.5%) | 81 (63.8%) | |
| Vascular comorbidities | 44 (66.7%) | 80 (62.0%) | 0.630 |
| Bacterial colonisation | 25 (37.9%) | 8 (6.2%) | <0.001 |
| Readmission at 30 days | 33 (50.0%) | 39 (30.5%) | 0.012 |
| Readmission at 90 day | 43 (65.2%) | 61 (47.7%) | 0.031 |
| Survival | 39 (59.1%) | 110 (85.3%) | <0.001 |
Description of quantitative variables by mean and standard deviation (m and SD) or by median and interquartile range in parentheses. Description of qualitative variables by frequencies and percentages.
CC: conventional care; HC: home care; FEV1: forced exhaled volume in the first second; mMRC: modified scale of the Medical Research Council; COPD: chronic obstructive pulmonary disease; ACRCU: Ambulatory Respiratory Chronic Care Unit.
The validation of our RRS revealed that the tool's capacity to predict readmissions at both 30 and 90 days was not high (AUC=0.69 and AUC=0.66, respectively) (Fig. 3). In the multivariable analysis used to examine all variables, we found that only the number of prior hospital admissions due to a COPDE had an acceptable capacity to predict readmissions at 30 and 90 days (Table 6).
Readmission at 30 and 90 days.
| Readmission | Variable | OR | (95% CI) | p | AUC |
|---|---|---|---|---|---|
| Age | 1.006 | (0.986–1.028) | 0.552 | 0.520 | |
| Exacerbations | 1.158 | (0.984–1.371) | 0.075 | 0.548 | |
| Hospitalisations | 1.405 | (1.275–1.558) | <0.001 | 0.703 | |
| At 30 days | FEV1 | 0.987 | (0.974–0.999) | 0.040 | 0.563 |
| Chronic bronchitis profile | 0.844 | (0.520–1.029) | 0.481 | 0.482 | |
| Bacterial colonisation | 3.070 | (1.614–5.792) | 0.001 | 0.555 | |
| Karnofsky index | 0.981 | (0.968–0.994) | 0.005 | 0.417 | |
| Vascular comorbidity | 1.035 | (0.678–1.589) | 0.873 | 0.504 | |
| Age | 0.996 | (0.978–1.014) | 0.646 | 0.488 | |
| Exacerbations | 1.224 | (1.039–1.476) | 0.023 | 0.539 | |
| Hospitalisations | 1.428 | (1.291–1.591) | <0.001 | 0.704 | |
| At 90 days | FEV1 | 0.983 | (0.972–0.994) | 0.002 | 0.581 |
| Chronic bronchitis profile | 0.823 | (0.544–1.021) | 0.353 | 0.479 | |
| Bacterial colonisation | 2.158 | (1.158–4.065) | 0.016 | 0.533 | |
| Karnofsky index | 0.987 | (0.975–0.999) | 0.033 | 0.440 | |
| Vascular comorbidity | 0.865 | (0.597–1.255) | 0.445 | 0.482 |
Odds ratio together with their 95% confidence intervals, the “p” value and the area under the ROC curve (AUC) of the model; FEV1: forced expiratory volume in the first second.
The implementation of a post-discharge HC programme, such as the ACRCU, in patients hospitalised for a COPDE could reduce readmissions at both 30 and 90 days and improves one-year survival. Numerous HH programmes have been implemented with the same purpose of avoiding readmissions after hospitalisation for COPDE.22–27 While some of these studies yielded results similar to those found in the present study in terms of the decrease in the number of readmissions, others failed to achieve this outcome.22 It should be noted that, as was the case in our ACRCU, in most other studies reporting a decrease in the rate of readmissions, a specialised nurse was in charge of following the patients at their home after their discharge from the hospital while maintaining continuous contact with the pulmonologist responsible for their case.
Other working groups applied TLM programmes,28–31 including Vitacca et al.,28 Polisena et al.,29 and the PROMETE study,30 with a reduction in the rate of readmissions being observed in all cases. Marcos et al.31 recently obtained comparable results, observing a decrease in the rate of readmissions at one year in a group of COPD patients included in a telemonitoring programme. In contrast, in the PROMETE II project32 it was concluded that home telemonitoring did not lead to a decrease in hospital admissions compared with standard practice. Moreover, in a study carried out in the United States, Hamadi et al.33 observed a significant increase in the rate of readmissions at 30 days of COPD patients included in a telemonitoring programme. As demonstrated by the above, there is disparity in the results obtained by these programmes depending on the method used by each working group. The existence of a standardised definition of HC would help to better define the model and methodology to be applied in subsequent studies, in addition to facilitating the development of universal measurement indicators.
Another objective is to reduce the mean length of hospital stays. In our study, the HC population included in our ACRCU did not have a shorter mean length of hospital stay compared with patients not included in this programme, which we believe could be explained by a greater severity of the COPD and the presence of a higher number of comorbidities in these patients. Similar data were yielded by a study performed by Jurado et al.,25 who limited their study population to patients with a diagnosis of COPD, under the age of 75, and without severe comorbidities (criteria of lesser frailty that we did not apply). Hernando et al.24 analysed the mean length of hospital stay (2.82 days) and, on the other hand, the mean length of home stay (7.29 days), with the sum of both parameters being lower in their study population than in the population included in our ACRCU. Díaz et al.23 achieved a decrease in the mean length of hospital stay in the patients included in their HH programme (12.2–9.2 days, p<0.05). A decrease in the length of the hospital stay was also achieved in the TLM group of the PROMETE study,30 but this goal was not reached in the PROMETE II study.32
One-year post-discharge survival in the group of patients included in our ACRCU was greater than in the population not included in such unit. Caplan et al.27 and Hernando et al.24 found comparable results to ours, although it should be noted that their home follow-up period was shorter than ours. Marcos et al.31 also associated a telemonitoring programme with reduced one-year mortality after a severe COPDE. In contrast, in other studies,34 TLM or home telemonitoring were not found to have an impact on patient survival, even in the study conducted by Puebla et al.,35 in which case 30-day mortality increased in the group of patients included in the follow-up programme.
The severity of the COPD of the subjects included in our ACRCU, as measured by the airflow limitation, corresponded to severe obstruction (mean FEV1 of 44.6%), with our results being similar to those of the PROMETE study (FEV1<50%)30 as well as the research carried out by Jurado et al. (40.4%)25 and Davies et al. (36.1%).26 In addition, the severity of our patients’ disease was classified as GOLD class D, and, according to the GesEPOC, the prevalent phenotype was exacerbator with emphysema. In the study carried out by Jurado et al.,25 55% of the patients included in the intervention group had a condition classified as stage 3 or 4 according to the GOLD 2011 revision. These results highlight the idea that patients who should be included in HH programmes are mainly those with more severe and exacerbator conditions.
In our study, we identified a greater severity of the COPD (as determined by a worse FEV1), a GOLD class D, or an exacerbator with emphysema phenotype as factors associated with hospital readmission after a COPDE. As seen in other studies, this is to be expected considering that patients with a more severe condition are the most susceptible to experiencing exacerbations and, therefore, to requiring readmission to the hospital.1 The capacity of our RRS to predict readmissions at 30 and 90 days post-discharge from a hospital admission for a COPDE was not as expected, and, in line with the results of the study carried out by Hart et al.,36 we found that only the number of hospital admissions within the previous 12 months had an acceptable predictive capacity. The fact that our ERR does not have a good predictive capacity may be due to the excess of variables used since. Perhaps, according to some studies,36 it would have been sufficient to use only the number of previous exacerbations. Despite the non-efficacy of our RRS, this should not influence the final results since the variable considered “optimal” (number of previous exacerbations) was already included. In addition, our RRS has received neither internal validation, following the methodology of using separate derivation and validation cohorts, nor external validation.
One of the limitations of our study is that we were unable to include certain patients in the ACRCU due to their social situation or their refusal to enter the programme. Another one is a potential selection bias dependent on the physician analysing each particular case (despite there being a standard definition for frailty, this concept is subject to subjective assessment) and/or a nursing intervention bias (the home visits were carried out by two different nurses who could have differed in their perception and management of symptoms, action guidelines applied, etc.). In addition, the schedule established for the home visits (08:00–20:00, Monday through Friday) could have increased the number of Emergency Room visits on certain occasions, which would have increased the risk of readmission. An additional limitation was the failure to include patients who would have benefited from the programme due to the severity of their COPD, but who were not due to not having an RRS score >7, their social situation, or their own refusal to enter the programme. We attempted to correct this limitation by performing the comparative analysis of similar severity between those subjects who were admitted to the ACRCU and those who were included in the CC group despite meeting the inclusion criteria for such programme, but we continued observing a lower rate of readmissions and a higher survival rate in the first group of patients receiving HC. When we compared the population included in the ACRCU with that of CC susceptible to inclusion in the same programme if it had been implanted, we appreciate that these are not similar in certain variables such as smoking, previous exacerbation or the Karfnosky index, which means that the results should be interpreted with caution.
Finally, we can conclude that, in patients with exacerbator or fragile COPD requiring hospitalisation due to an exacerbation, our HC (ACRCU) model could be useful for reducing the rate of readmissions at 30 and 90 days, and managed to increase their one-year post-discharge survival. In short, patients with severe COPDEs and prior hospital admissions are those at a higher risk of requiring readmission.
Informed consentWritten informed consent was not required from patients to participate in the study, as it was conducted following usual clinical practice.
FundingThere was no financial support for the conduct of the research and/or preparation of the article.
Authors’ contributionsDS-M, FV-Á and GPB provided study design and guidance of the project. IM-F performed the statistical analysis. DS-M, FV-Á, IFO, ANP, RAF and TGPM was involved patient recruitment, data collection and analysis and bibliographic review. All authors contributed in writing of the final paper. All authors read and approved the final manuscript.“
Conflict of interestFV-Á has attended or participated in activities organised or financed by the pharmaceutical companies Almiral, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Esteve, Ferrer, Menarini, Novartis, Mundipharma, Orion, Pfizer, Teva and Zambon.
GPB has received grants, contracts and honoraria for talks from Zambon, Menarini, Chiesi, GSK, Boehringer Ingelheim, and Novartis.
The rest of the authors declare no conflict of interest.
FV-Á is part of the Editorial board of Open Respiratory Archives and declare that they have remained outside the evaluation and decision-making process in relation to this article.