Systemic sclerosis (SSc) is an autoimmune connective tissue disease, which affects several organs through inflammation, vascular injury and lung fibrosis.1 In the past century, the primary cause of death among SSc patients had been renal crisis, but the rate of this event decreased from 42% to 6% due to advances in treatment.2 Recent data show that associated interstitial lung disease (ILD) and pulmonary hypertension are the most frequent SSc-related cause of death, with a reported mortality rate of 33–35% for ILD.2,3
Some recommended medication incorporated for the SSc-ILD treatment includes immunosuppressive therapy (cyclophosphamide,4 mycophenolate mofetil5), and hematopoietic stem cell transplantation.4 Despite lung transplantation is a relative contraindication for pulmonary scleroderma, post-transplant survival rates are similar to those patients with other causes for lung transplantation.6 Antifibrotic therapy with pirfenidone7 and nintedanib8,9 is currently approved for idiopathic pulmonary fibrosis, showing a reduction in forced vital capacity (FVC) decline and acceptable tolerability profile. At present, nintedanib is also approved for SSc-ILD in Europe, US and Canada.
Interestingly, the recent results from the SENSCIS clinical trial have demonstrated that nintedanib slows-down the FVC decline in patients with SSc-ILDs.10 Furthermore, randomized controlled trials have evaluated the effect of nintedanib and pirfenidone on lung function decline of these patients.11,12 Exclusion criteria for these trials include airway obstruction, severe lung function impairment (FVC<40% predicted or diffusing lung capacity for carbon monoxide (DLCO)<30% of predicted), lung transplant requirement in the following year and severe pulmonary hypertension.8,13,14 We hypothesized that patients with severe progressive fibrotic SSc-ILD that were evaluated for lung transplant could also benefit from nintedanib treatment. Therefore, descriptive data about safety and effects in functional decline were obtained from patients in which nintedanib was initiated as an off-label treatment use at the same time they started lung transplant evaluation.
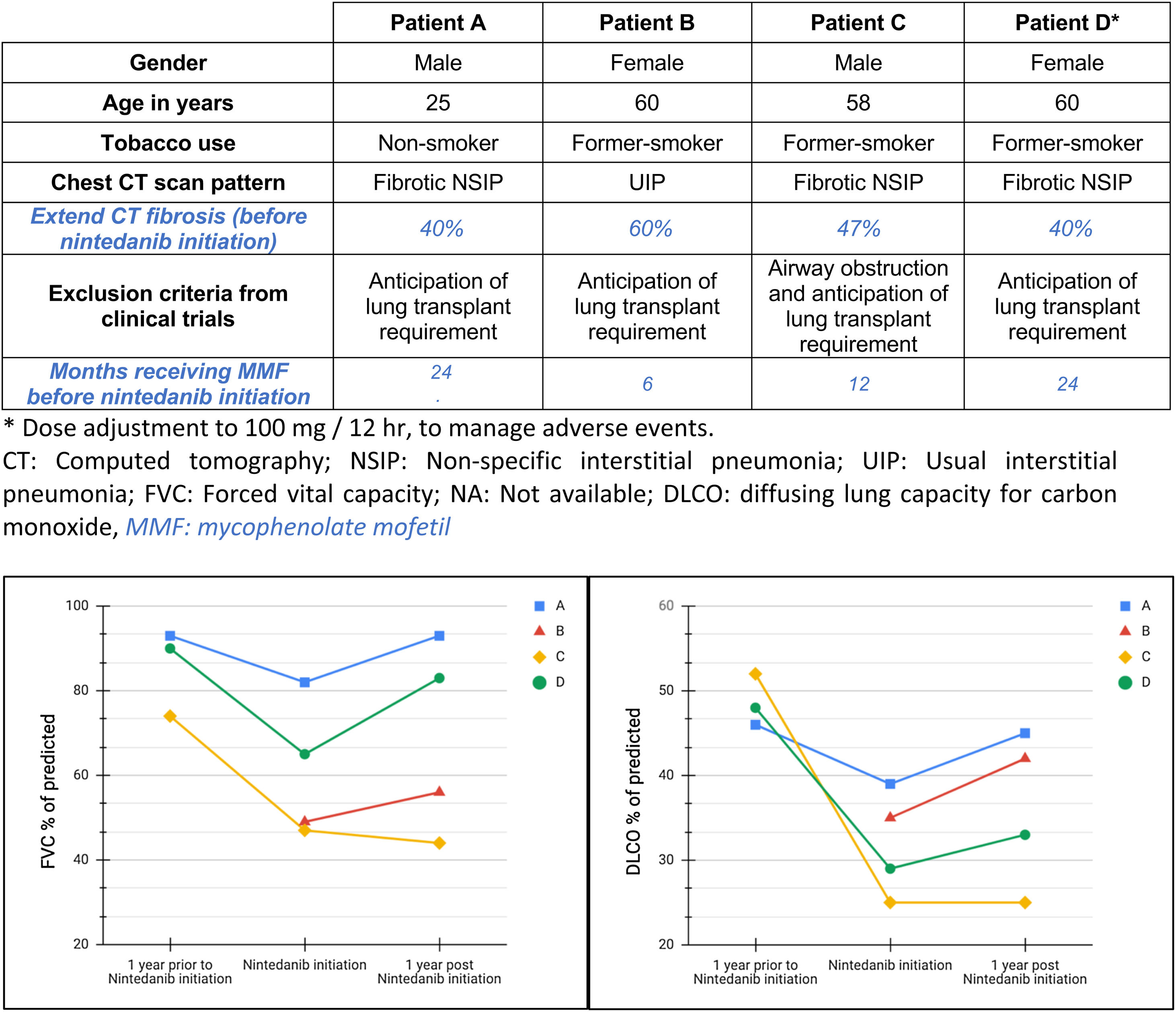
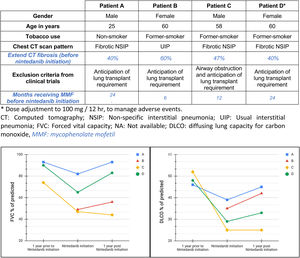
We report a descriptive series of patients with progressive fibrotic SSc-ILD who were evaluated for lung transplant (Fig. 1) and received off-label treatment with nintedanib, focused on effects in safety and functional decline. These patients couldn’t be included in clinical trial because anticipation of lung transplant requirement after two years.8,10,14 All participants signed an informed consent form before being treated with nintedanib. Other forms of connective tissue disease or ILDs were excluded. Clinical and functional data was recorded at the time of treatment initiation, one year prior and after one-year under treatment, except for the last subject included whose most recent follow-up visit was at 3 months.
Four patients were included. At the time of SSc-ILD diagnosis, 3/4 of patients had cough, all of them had dyspnea (3/4 severe and 1/4 moderate), 3/4 presented with inspiratory crackles and 2/4 with finger clubbing. High-resolution computed tomography (HRTC) of the chest showed a predominant pattern of fibrosis, one of them with mild associated emphysema in upper lobes and all of them had hiatal hernia. One patient had cardiovascular comorbidity and pulmonary hypertension treated with sildenafil and bosentan. One subject presented with obesity, depression and obstructive sleep apnea treated with CPAP. No familiar history of ILD was reported. Regarding serological markers, 4/4 had positive antinuclear antibodies, 1/4 had positive anti-Scl-70 and 2/4 had others serological markers. All patients were treated with nintedanib (150mg/12h) and mycophenolate (720mg/12h), but three of them also received low-dose corticosteroids (≤10mg/day prednisone). Oxygen therapy was required for one subject before starting antifibrotic therapy. All patients had a clinical and radiological progressive pulmonary fibrosis in the previous year, 3 of them with significant FVC decline. However, at the last follow up after initiating nintedanib, mean FVC and DLCO was stabilized or increased (Fig. 1).
The progression of %FVC and %DLCO before and after nintedanib treatment is summarized in Fig. 1. Mean time from symptoms occurrence to nintedanib treatment was 1128 days (SD±510 days). Three patients reported digestive intolerance, 2/4 weight loss, 1/4 asthenia and 1/4 presented some liver function abnormality. Treatment related adverse events were mild and no treatment discontinuation was required. One patient underwent lung transplantation 249 days after antifibrotic treatment initiation.
This limited series of four cases with progressive fibrosing SSc-ILD patients treated with nintedanib showed an overall reduction in lung function impairment and good tolerability. In two patients, FVC and DLCO decline slowed down, and in the other two a significant FVC improvement was observed. These results are consistent with case report which used pirfenidone as off-label antifibrotic drug, showing improvement in lung function tests.15 Also, as it was observed in tolerability and safety trials,10 the use of nintedanib in our patients did not relate to the occurrence of severe adverse events.
A limitation of this series of cases is that only 4 patients were analyzed. However, it should be considered that this is a rare disease and only those patients with severe pulmonary fibrosis that were not able to be included in clinical trials since anticipating lung transplant requirement have been included. In addition, patient B initiated the MMF 6 months before Nintedanib, and as the MMF can improve SSc-ILD until 12 months, the benefit could also be attributed to the MMF. Besides, this descriptive series can add value to future clinical practice. The results are hypothesis-generating and that should be confirmed in further research projects.
Despite the fact that the pathophysiology mechanism of SSc-ILD fibrosis progression is not well known, clinical trials have shown that anti-fibrotic medication seems to inhibit this fibrotic process. Therefore, antifibrotic therapy with nintedanib could be a useful option for patients with fibrotic SSc-ILD that worsen instead of receiving commonly used medication in the real-world clinical practice.
Financial disclosureBoehringer-Ingelheim Spain has supported financially the costs required for publishing the work. The authors received no compensation related to the development of the manuscript.