Kussmaul breathing is a form of hyperventilation linked to metabolic acidosis, and is a compensatory attempt to decrease the CO2 in the blood via elimination through the lungs. Kussmaul breathing is generally well tolerated, provided the cause is effectively managed. However, some respiratory complications induced by the increased ventilatory demand have been described, with respiratory muscle fatigue being the most critical.1 Occasional incidences of pneumothorax and pneumomediastinum have also been reported.2
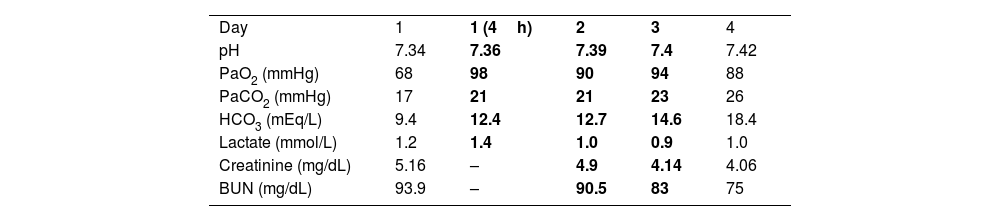
There is no specific treatment for Kussmaul breathing other than addressing the underlying metabolic problem and providing ventilatory support if respiratory fatigue occurs. Similar to acidemia induced by acute respiratory acidosis, dyspnea in acidemia engendered by metabolic acidosis can be described as “air hunger.” We recently observed this in an 81-year-old man with ischemic heart disease and renal failure caused by nephrolithiasis who was hospitalized because significant metabolic decompensation. He appeared very dyspneic, with regular hyperpnea – characteristic of Kussmaul breathing – but with an oxygen saturation of 92% on room air and respiratory rate close to 40breaths/min. An arterial blood gas analysis revealed metabolic acidosis with an elevated anion gap attributed to the considerably elevated blood urea nitrogen levels (Table 1). Because an elevated alveolar to arterial oxygen gradient, the resident physician placed him on high-flow oxygen therapy (HFOT) via a nasal cannula, with an FIO2 of 50% and a flow rate of 60L/min. A few minutes after commencing HFOT, the patient was breathing more comfortably, with less distress, and in a more superficial respiratory pattern; his respiratory rate decreased to 28breaths/min. However, a control arterial blood gas analysis performed 4h into the HFOT showed that the acidotic profile persisted. That is, the patient exhibited the same hyperventilation anomaly but with less respiratory work. His respiratory situation and metabolic acidosis persisted for the next two days; subsequently, with adjustments to the administered parenteral solutions, he was eventually able to regain adequate metabolic control and was finally weaned off HFOT.
Four-day follow-up on arterial blood gas determinations.
| Day | 1 | 1 (4h) | 2 | 3 | 4 |
| pH | 7.34 | 7.36 | 7.39 | 7.4 | 7.42 |
| PaO2 (mmHg) | 68 | 98 | 90 | 94 | 88 |
| PaCO2 (mmHg) | 17 | 21 | 21 | 23 | 26 |
| HCO3 (mEq/L) | 9.4 | 12.4 | 12.7 | 14.6 | 18.4 |
| Lactate (mmol/L) | 1.2 | 1.4 | 1.0 | 0.9 | 1.0 |
| Creatinine (mg/dL) | 5.16 | – | 4.9 | 4.14 | 4.06 |
| BUN (mg/dL) | 93.9 | – | 90.5 | 83 | 75 |
The bold values indicate days during which the patient underwent HFOT. BUN: blood urea nitrogen.
A physiological effect of HFOT is a reduction in CO2 levels due to a washing-out effect in the airways that lowers CO2 rebreathing and functional dead space. In healthy subjects, this resulting decrease in CO2 levels is not as evident as in COPD patients with some degree of hypercapnia. However, an HFOT-induced decrease in minute volume is typically unaccompanied by a proportional increase in CO2 levels.3 Similarly, in patients with sepsis, increased respiratory drive and inspiratory effort induced by lactic acidosis are effectively modulated by HFOT without CO2 elevation.4 In our patient, we surmise that the HFOT maintained compensatory alveolar hyperventilation, with the advantage of abolishing Kussmaul breathing and the accompanying increased respiratory workload. The recommendations for treating respiratory distress in these cases are based on diabetic ketoacidosis guidelines. In diabetic ketoacidosis, HFOT is accepted as a very useful and safe alternative to removing CO2 and reducing dead space while facilitating compensation for metabolic acidosis.5
The use of HFOT is rapidly increasing in various clinical situations, but this is occurring without clear indications or clinical practice guidelines. Effects of HFOT on arterial blood gas parameters especially partial CO2 require further investigation to provide insight into the efficacy and safety of the treatment. We observed in the present case how HFOT can be beneficial while waiting to resolve the metabolic issue inducing Kussmaul breathing; it makes the hyperventilation – which is necessary for compensatory elimination of CO2 – more bearable and lowers the respiratory workload and risk of complications.
Informed consentConsent was obtained from the patient and family caregivers.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsEach of the authors contributed to the planning and writing of the manuscript, and each has read and approved all statements in it.
Conflicts of interestsThe authors state that they have no conflict of interests.