The use of systemic corticosteroids in severely ill patients with coronavirus disease 2019 (COVID-19) is controversial. We aimed to evaluate the efficacy and safety of corticosteroid pulses in patients with COVID-19 pneumonia.
MethodsA quasi-experimental study, before and after, was performed in a tertiary referral hospital, including admitted patients showing COVID-19-associated pneumonia. The standard treatment protocol included targeted COVID-19 antiviral therapy from 23rd March 2020, and additionally pulses of methylprednisolone from 30th March 2020. The primary outcome was a composite endpoint combining oro-tracheal intubation (OTI) and death within 7 days.
ResultsA total of 24 patients were included. Standard of care (SOC) (before intervention) was prescribed in 14 patients, while 10 received SOC plus pulses of methylprednisolone (after intervention). The median age of patients was 64.5 years and 83.3% of the patients were men. The primary composite endpoint occurred in 13 patients (92.9%) who received SOC vs. 2 patients (20%) that received pulses of methylprednisolone (odds ratio, 0.02; 95% confidence interval, 0.001 to 0.25; p=0.019). Length of hospitalization in survivors was shorter in the corticosteroids group (median, 14.5 [8.5–21.8] days vs. 29 [23–31] days, p=0.003). There were no differences in the development of infections between both groups. There were 3 deaths, none of them in the corticosteroids group.
ConclusionsIn patients with severe pneumonia due to COVID-19, the administration of methylprednisolone pulses was associated with a lower rate of OTI and/or death and a shorter hospitalization episode.
El uso de corticosteroides sistémicos en pacientes gravemente enfermos por enfermedad coronavírica de 2019 (covid-19) es controvertido. Nuestro objetivo fue evaluar la eficacia y la seguridad de los pulsos de corticoesteroides en los pacientes con neumonía por covid-19.
MétodosSe realizó un ensayo cuasiexperimental, tipo antes y después, en un hospital terciario de referencia que incluyó a pacientes ingresados por neumonía asociada a covid-19. El protocolo de tratamiento estándar incluía un tratamiento antiviral dirigido contra el virus de la covid-19 desde el 23 de marzo de 2020 y añadió pulsos de metilprednisolona desde el 30 de marzo de 2020. El resultado primario fue un criterio combinado compuesto por la intubación orotraqueal y el fallecimiento durante los siguientes siete días.
ResultadosSe incluyó un total de 24 pacientes. El protocolo de tratamiento (antes de la intervención) se prescribió en 14 pacientes, mientras que 10 recibieron el protocolo de tratamiento además de los pulsos de metilprednisolona (después de la intervención). La edad media de los pacientes fue de 64,5 años y el 83,3% de los pacientes eran hombres. El resultado combinado primario tuvo lugar en 13 pacientes (92,9%) que recibieron el protocolo de tratamiento frente a 2 pacientes (20%) que recibieron los pulsos de metilprednisolona (odds ratio=0,02; intervalo de confianza del 95%=0,001-0,25; p=0,019). La duración de la hospitalización en los supervivientes fue más corta en el grupo que recibió corticoesteroides (media=14,5 [8,5-21,8] días frente a 29 [23-31] días, p=0,003). No hubo diferencias en el desarrollo de infecciones entre ambos grupos. Hubo tres fallecimientos, ninguno de ellos en el grupo que recibió corticoesteroides.
ConclusionesEn los pacientes con neumonía grave por covid-19, la administración de pulsos de metilprednisolona se asoció a unas tasas menores de intubación orotraqueal y/o muerte y a episodios de hospitalización más cortos.
Coronavirus disease 2019 (COVID-19) is caused by a new beta-coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), firstly identified in Wuhan, China, in late December 2019 has been declared by the World Health Organization as a pandemic for its rapid spread and potential lethality.1,2 Current data support the hypothesis that inflammatory response is the main responsible for lung damage and the consequent mortality.3 Patients with more severe manifestations of COVID-19 have higher levels of pro-inflammatory cytokines that lead to lung damage typical of acute respiratory distress syndrome (ARDS).4,5 In this context, the role of anti-inflammatory treatments, such as systemic corticosteroids, has been raised. However, systematic reviews of observational studies conducted in SARS and MERS-CoV did not find an improvement in terms of survival with the use of corticosteroids, but a delay in viral clearance at the lung level.6,7 These findings justified that World Health Organization (WHO) discouraged the use of systemic corticosteroids in the treatment of COVID-19 pneumonia outside of clinical trials.8
However, in the last months, many published studies have been focus on this objective in order to clarify the role of corticosteroids as a treatment for lung damage due to COVID-19 infection. The first studies were observational. Some of them identified a better prognosis in patients receiving corticosteroids, which has led some authors to recommend the use of systemic corticosteroids to treat inflammation and secondary ARDS in patients with COVID-19 pneumonia.9,10 More recently, lot of evidence has been published on this field supporting the use of corticosteroids in COVID-19 pneumonia.11,12 Still, the doses and the type of corticosteroids used are different among the studies, and the use in this population it's has not been already set.
Our aim during the first months of the COVID-19 pandemic was to evaluate the efficacy and safety of corticosteroid pulses in patients with SARS-CoV-2 associated pneumonia and ARDS, by conducting a quasi-experimental preliminary before and after study.
MethodsDesign and settingsWe designed a quasi-experimental, before and after study, between March 23rd and April 9th at the Virgen del Rocio University Hospital, Seville, Spain. Independent ethical committee approved the study (internal code: 1014-N-20).
PatientsHospitalized patients with a SARS-CoV-2 infection confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) were candidates to enter into the study if they met the following inclusion criteria: (1) over 18 years of age at the time of inclusion; (2) pneumonia confirmed by chest imaging; (3) candidates to intensive care unit (ICU) in case of deterioration; (4) at least, one raised biomarker of the following: C-reactive protein, D-dimer or ferritin; (5) more than 5 days since the onset of symptoms; and (6) peripheral capillary oxygen saturation (SpO2) of 94% or less while they were breathing room air or acute distress respiratory syndrome (ADRS) defined as ratio of the partial pressure of oxygen (PaO2) divided to the fraction of inspired oxygen (FiO2) (PaO2: FiO2) below 300mg Hg and more than 100mm Hg. PaO2:FiO2 ratio was assessed indirectly by SpO2:FiO2 ratio, as previously described.13 Exclusion criteria were: (1) presence of multi-organ failure, (2) hemodynamic instability, (3) acute exacerbation of chronic obstructive pulmonary disease (COPD), (4) PaO2:FiO2 less than 100mm Hg, (5) previous long-term oxygen therapy or ventilatory support for chronic lung disease, (6) respiratory acidosis (pH<7.35 and PaCO2>50mm Hg), (7) Glasgow Coma Scale of 12 points or less, (8) abnormal mental status, (9) urgent need for oro-tracheal intubation or (10) non-subsidiary to the intensive care unit.
InterventionsPatients were systematically treated as per local protocol, which included antiviral and supportive treatment, from 23rd March 2020 to 29th March 2020, and additionally pulses of methylprednisolone from 30th March 2020 to 9th April 2020. In the corticosteroid group, all patients received at least one pulse of 250mg of intravenous methylprednisolone. The day after, patients were assessed to decide the need of receiving another pulse, until a maximum of three. After this pulse or pulses, 40mg bid of methylprednisolone were administrated to complete a total of 5 days with corticosteroids. High flow nasal cannula (HFNC) was used in all patients with a gas flow rate set at 60 L/min and a FiO2 adjusted to maintain pulse oximetry at 92% minimum with a dedicated device (AirvoTM 2, Fisher and Paykel Healthcare, Auckland, New-Zealand) equipped with a heated humidifier (MR850, Fisher and Paykel Healthcare, Auckland, New-Zealand).
Patient in both groups were closely monitored. They were admitted to ICU if they had persistent or worsening signs of respiratory failure. Intubation criteria included a respiratory rate of >40 breaths per minute, signs of increased breathing effort, SpO2 of <90% despite high FiO2 or acidosis with a pH of <7.35, occurrence of hemodynamic instability or deterioration of neurologic status, as previously described.14
Clinical and laboratory monitoringClinical parameters, respiratory rate, pulse oximetry and signs of increased breathing effort were assessed, at least, at inclusion, 1–2h and 12–24h after initiation of oxygenation strategies. PaO2/FiO2 ratio was assessed indirectly by SpO2/FiO2 ratio, as previously described.13
To evaluate the failure of pulses of methylprednisolone and poor clinical outcome with HFNC, we used the ROX index.15 As previously published, we considered HFNC failure if ROX index was less than 2.85, 3.47, and 3.85 at 2, 6, and 12h of HFNC initiation, respectively.16 Laboratory tests were performed every 48–72h and included blood count, assessment of liver and renal function, coagulation, D-dimer, C-reactive protein and ferritin. Radiological evaluations were performed by plain chest radiography and reviewed by an expert and instructed radiologist at the hospital centre.
Study outcomesThe primary endpoint was a composite outcome, defined as oro-tracheal intubation (OTI) or death at 7 days. Secondary outcomes included OTI or death at 14 days, the duration of mechanical ventilation, length of hospitalization in survivors and clinical status as assessed with the seven-category ordinal scale on days 7 and 14. The seven-category ordinal scale has been recently used in a trial with COVID-1917 and was also recommended by the WHO R&D Blueprint expert group.18 This scale consisted of the following categories: 1, not hospitalized with resumption of normal activities; 2, not hospitalized, but unable to resume normal activities; 3, hospitalized, not requiring supplemental oxygen; 4, hospitalized, requiring supplemental oxygen; 5, hospitalized, requiring nasal high-flow oxygen therapy, non-invasive mechanical ventilation, or both; 6, hospitalized, requiring ECMO, invasive mechanical ventilation, or both; and 7, death.
Safety outcomes included proportion of patients in each group who developed glucocorticoid-related side effects, defined as new onset diabetes mellitus or infections.
Sample size estimationWe estimated that 85% of mild or moderate ADRS had OTI or death at 7 days. This number provided the study with a power of 80 per cent to detect a reduction in primary outcome from 85% to 30% with the use of methylprednisolone. Assumptions included the use of a one-tailed test and a 5 per cent level of significance. The sample size calculation was 9 patients.
Statistical analysisFor the descriptive analysis, the absolute frequency (N) and the relative frequency (%) have been calculated for the qualitative variables. In the case of quantitative variables, the median and the interquartile range (P25–P75) have been obtained. For the analysis of the qualitative variables, the Chi-square test has been calculated to see if there is any type of relationship (dependency) between the variables, through the crossed tables. In 2×2 tables, the “continuity correction” is applied, obtaining Fisher's exact statistic.
For quantitative variables, normality tests have been performed with the Shapiro–Wilk statistic. In the event that the behaviour of the variables follows a normal distribution, the T test is applied for independent samples. Otherwise, the Mann–Whitney U test is applied for independent samples. To know the evolution of the variables over time, the T test for related samples has been applied if the variables are related to normality, or the Wilcoxon test of the signed ranges for related samples if they do not follow a normal distribution.
A 95% confidence level has been taken into account, so the experimental p-value has been compared with a significance level of 5%. Statistical analysis was performed using the IBM SPSS Statistics 22 statistical package.
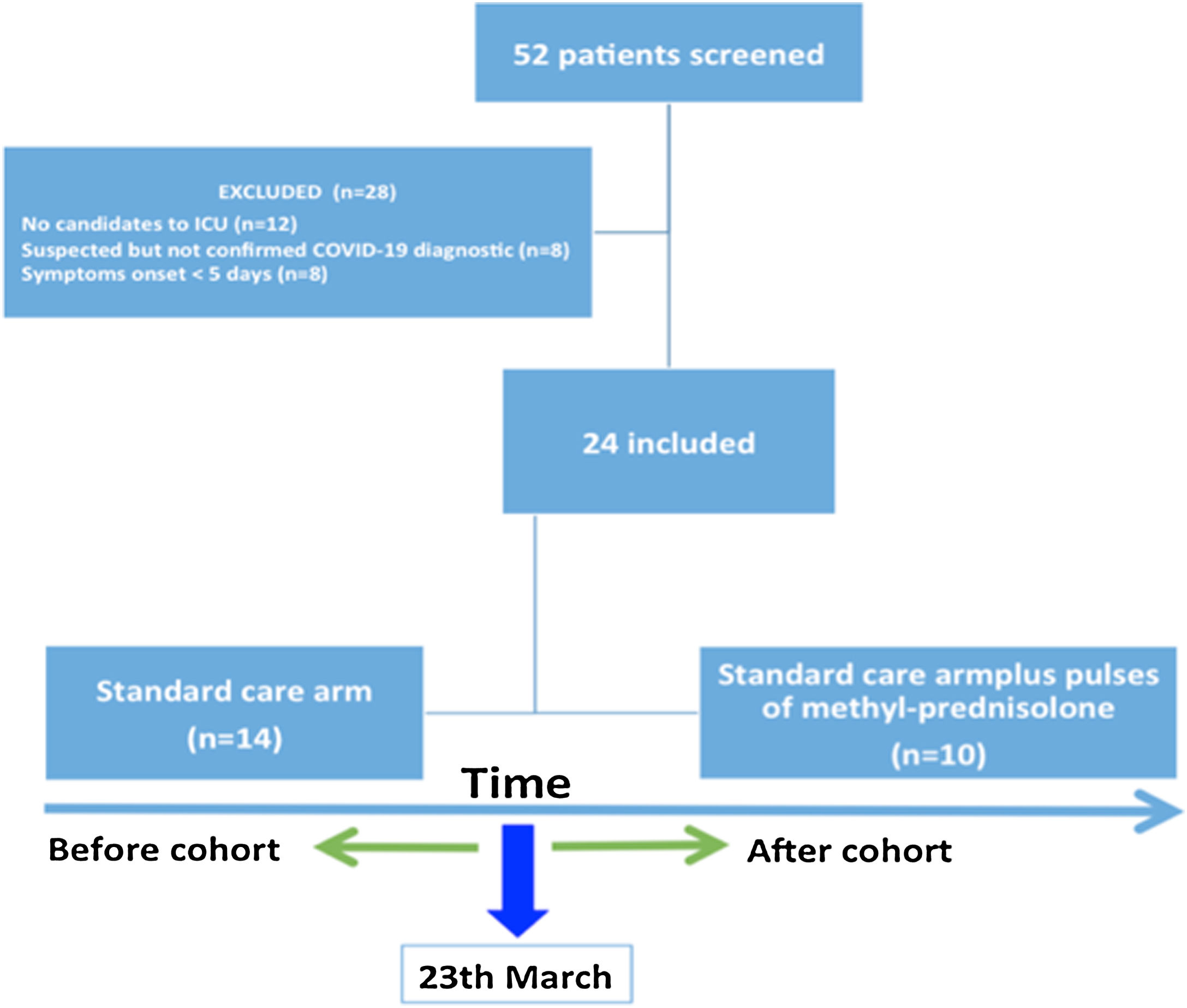
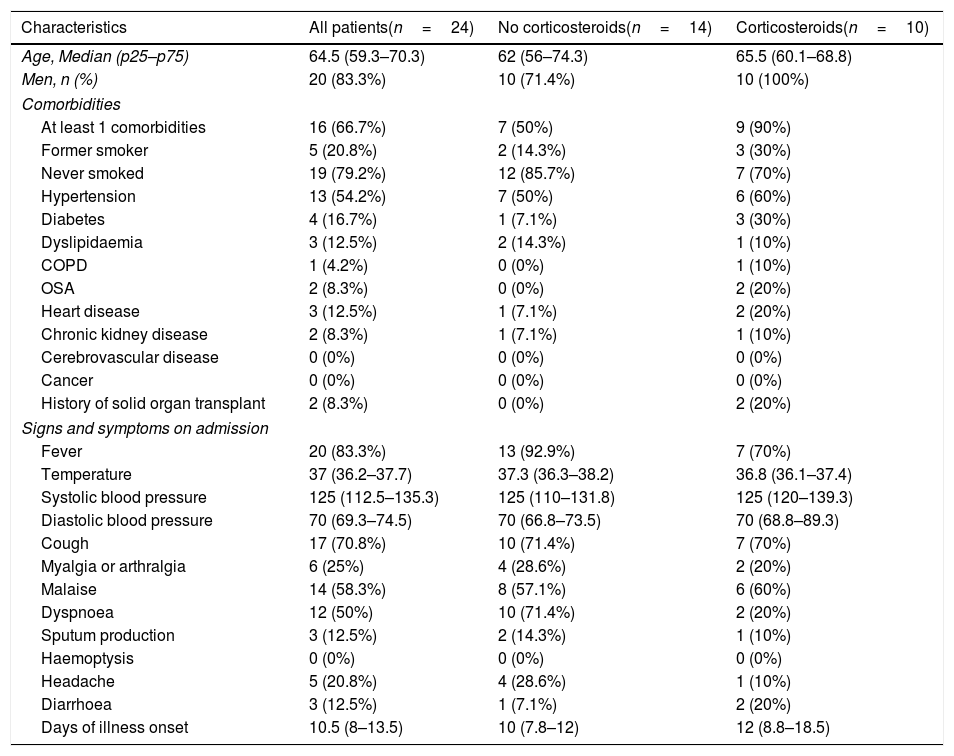
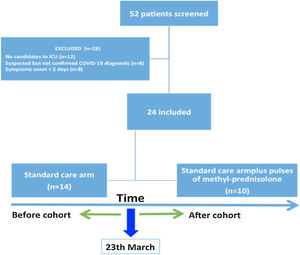
ResultsPatientsOf the 52 patients evaluated by a pulmonologist with a suspicion of COVID-19, 24 patients were finally included, 14 received standard care (SOC) (before intervention) and 10 received SOC plus pulses of methylprednisolone (after intervention) (Fig. 1). The characteristics of both groups were similar in terms of age and comorbidities. The median age of patients was 64.5 years (P25–P75: 59.3–70.3), and 83.3% of the patients were men (Table 1). More than half had hypertension (54.2%), and 12.5% present dyslipidemia, 12.5% heart disease and 8.2% chronic kidney disease. Two patients had history of solid organ transplantation, both included in the corticosteroids group.
Demographic and clinical characteristics of the patients at baseline.
| Characteristics | All patients(n=24) | No corticosteroids(n=14) | Corticosteroids(n=10) |
|---|---|---|---|
| Age, Median (p25–p75) | 64.5 (59.3–70.3) | 62 (56–74.3) | 65.5 (60.1–68.8) |
| Men, n (%) | 20 (83.3%) | 10 (71.4%) | 10 (100%) |
| Comorbidities | |||
| At least 1 comorbidities | 16 (66.7%) | 7 (50%) | 9 (90%) |
| Former smoker | 5 (20.8%) | 2 (14.3%) | 3 (30%) |
| Never smoked | 19 (79.2%) | 12 (85.7%) | 7 (70%) |
| Hypertension | 13 (54.2%) | 7 (50%) | 6 (60%) |
| Diabetes | 4 (16.7%) | 1 (7.1%) | 3 (30%) |
| Dyslipidaemia | 3 (12.5%) | 2 (14.3%) | 1 (10%) |
| COPD | 1 (4.2%) | 0 (0%) | 1 (10%) |
| OSA | 2 (8.3%) | 0 (0%) | 2 (20%) |
| Heart disease | 3 (12.5%) | 1 (7.1%) | 2 (20%) |
| Chronic kidney disease | 2 (8.3%) | 1 (7.1%) | 1 (10%) |
| Cerebrovascular disease | 0 (0%) | 0 (0%) | 0 (0%) |
| Cancer | 0 (0%) | 0 (0%) | 0 (0%) |
| History of solid organ transplant | 2 (8.3%) | 0 (0%) | 2 (20%) |
| Signs and symptoms on admission | |||
| Fever | 20 (83.3%) | 13 (92.9%) | 7 (70%) |
| Temperature | 37 (36.2–37.7) | 37.3 (36.3–38.2) | 36.8 (36.1–37.4) |
| Systolic blood pressure | 125 (112.5–135.3) | 125 (110–131.8) | 125 (120–139.3) |
| Diastolic blood pressure | 70 (69.3–74.5) | 70 (66.8–73.5) | 70 (68.8–89.3) |
| Cough | 17 (70.8%) | 10 (71.4%) | 7 (70%) |
| Myalgia or arthralgia | 6 (25%) | 4 (28.6%) | 2 (20%) |
| Malaise | 14 (58.3%) | 8 (57.1%) | 6 (60%) |
| Dyspnoea | 12 (50%) | 10 (71.4%) | 2 (20%) |
| Sputum production | 3 (12.5%) | 2 (14.3%) | 1 (10%) |
| Haemoptysis | 0 (0%) | 0 (0%) | 0 (0%) |
| Headache | 5 (20.8%) | 4 (28.6%) | 1 (10%) |
| Diarrhoea | 3 (12.5%) | 1 (7.1%) | 2 (20%) |
| Days of illness onset | 10.5 (8–13.5) | 10 (7.8–12) | 12 (8.8–18.5) |
COPD: chronic obstructive lung disease; OSA: obstructive sleep apnoea.
The main symptom at admission was fever, present in 83.3% of the patients, followed by cough (70.8%), malaise (58.3%) and dyspnoea (50%). The median interval time between symptom onset and pulmonologist evaluation was 10 days (8-14). Regarding radiological findings, the predominant patterns were interstitial abnormalities (54.2%), with bilateral reticular nodular opacities presented in 41.7% and ground glass opacities in 33.3% (Table A.1 in the Appendix). Median SpO2:FiO2 was 179 (p25–p75: 156–217), with an estimated PaO2:FiO2 of 214 (195–246). The variables included at baseline evaluation (SpO2, FiO2, respiratory rate, SpO2:FiO2, Rox Index, and SOFA score), laboratory test at evaluation, and antiviral treatments are included in Tables A.2–A.4 in the Appendix.
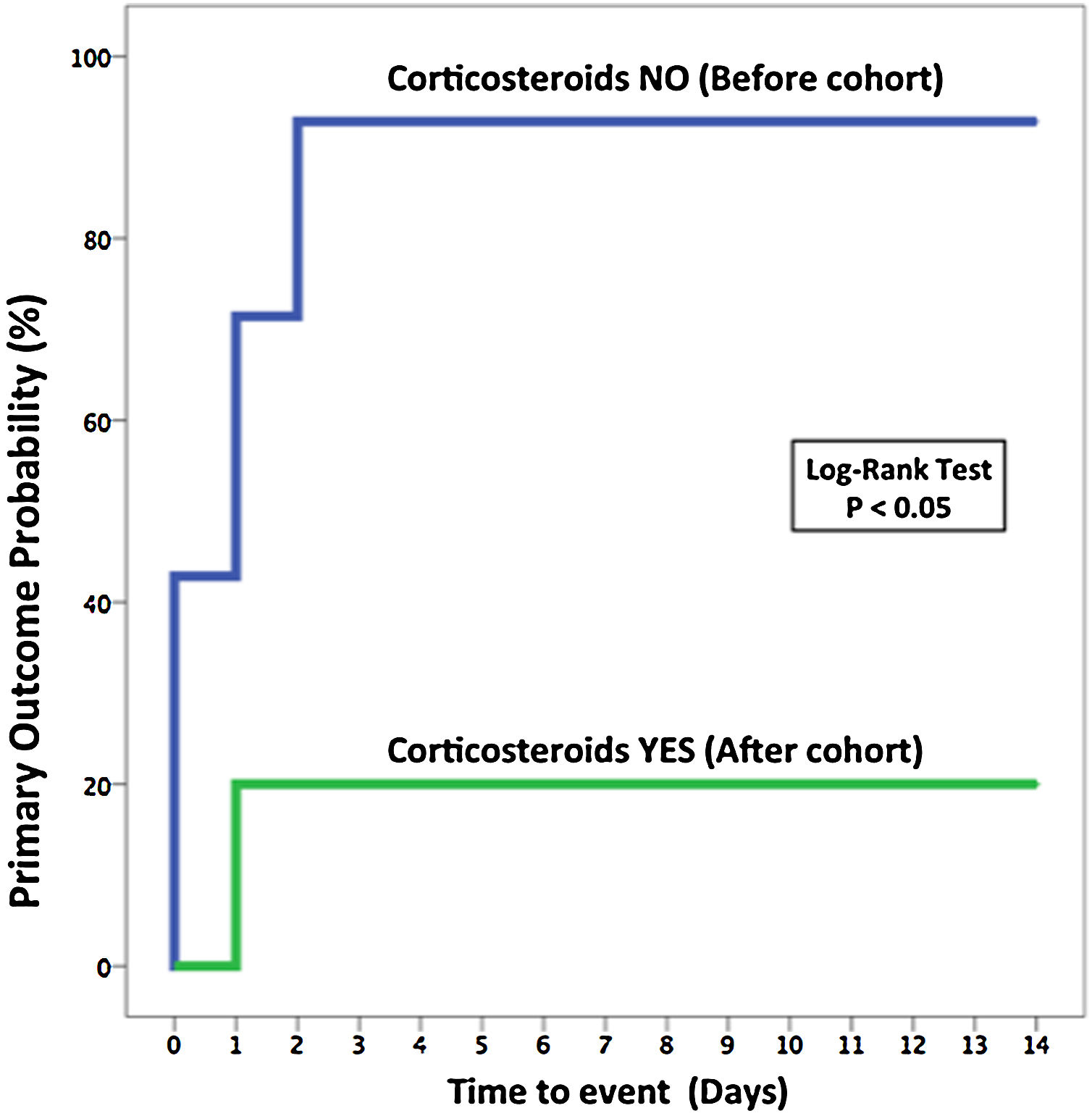
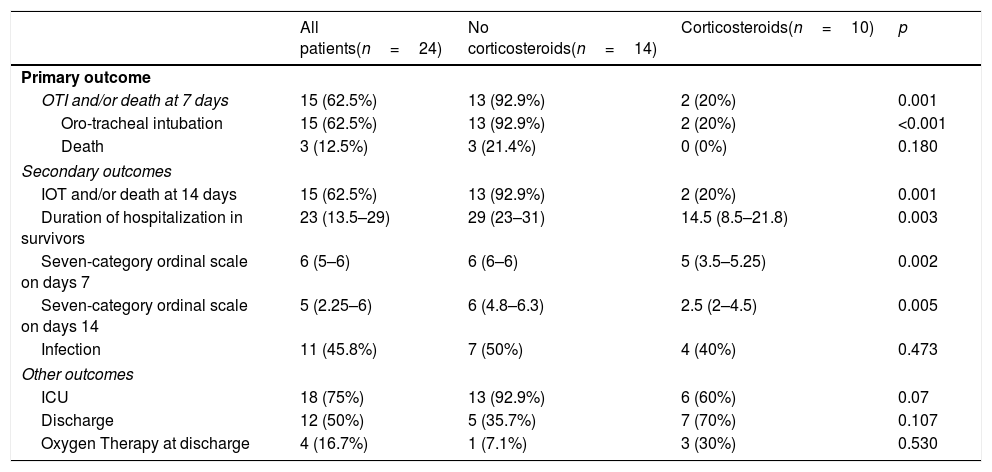
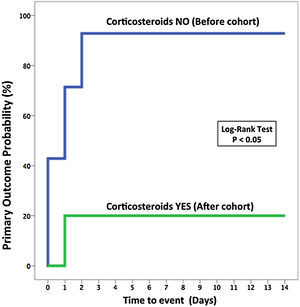
Primary and secondary outcomesPatients that received pulses of methylprednisolone had a better primary outcome (OTI and/or death at 7 days) than those who received SOC (20% vs. 92.9%; odds ratio [OR] for primary outcome, 0.02; 95% confidence interval [CI], 0.001–0.25; p=0.019) (Table 2 and Fig. 2). Regarding the length of hospitalization in survivors, the corticosteroids group had a shorter stay than patients included in the standard treatment group (median, 14.5 [8.5–21.8] days vs. 29 [23–31] days, p=0.003). The seven-category ordinal scale at 7 and 14 days was lower in the corticosteroids group comparing to the SOC group (5 [3.5–5.25] vs. 6 [6–6], p=0.002 at 7 days and 2.5 [2–4.5] vs. 6 [4.8–6.3], p=0.005 at 14 days) (Tables A.5 in the Appendix). There was no difference in the development of infections between both groups (40% in corticosteroids group vs. 50% in standard treatment group; OR, 0.76; 95% CI, 0.15–3.96; p=0.47), Table 2.
Primary and secondary outcomes.
| All patients(n=24) | No corticosteroids(n=14) | Corticosteroids(n=10) | p | |
|---|---|---|---|---|
| Primary outcome | ||||
| OTI and/or death at 7 days | 15 (62.5%) | 13 (92.9%) | 2 (20%) | 0.001 |
| Oro-tracheal intubation | 15 (62.5%) | 13 (92.9%) | 2 (20%) | <0.001 |
| Death | 3 (12.5%) | 3 (21.4%) | 0 (0%) | 0.180 |
| Secondary outcomes | ||||
| IOT and/or death at 14 days | 15 (62.5%) | 13 (92.9%) | 2 (20%) | 0.001 |
| Duration of hospitalization in survivors | 23 (13.5–29) | 29 (23–31) | 14.5 (8.5–21.8) | 0.003 |
| Seven-category ordinal scale on days 7 | 6 (5–6) | 6 (6–6) | 5 (3.5–5.25) | 0.002 |
| Seven-category ordinal scale on days 14 | 5 (2.25–6) | 6 (4.8–6.3) | 2.5 (2–4.5) | 0.005 |
| Infection | 11 (45.8%) | 7 (50%) | 4 (40%) | 0.473 |
| Other outcomes | ||||
| ICU | 18 (75%) | 13 (92.9%) | 6 (60%) | 0.07 |
| Discharge | 12 (50%) | 5 (35.7%) | 7 (70%) | 0.107 |
| Oxygen Therapy at discharge | 4 (16.7%) | 1 (7.1%) | 3 (30%) | 0.530 |
OTI: oro-tracheal intubation; UCI: intensive care unit.
This quasi-experimental before and after study found that pulses of methylprednisolone, in addition to SOC, were associated with fewer OTI and/or death at 7 days in patients with COVID-19-associated pneumonia and ADRS. The hypothetical corticosteroids benefit in patients at the inflammatory phase of COVID-19 is based on their ability to modulate the inflammatory response, reducing immunopathological damage and the associated ARDS suffered by these patients.19 Use of corticosteroids in pneumonia and viral infections is controversial, probably due to the great heterogeneity in clinical studies focused on this aspect, but in severe pneumonia, as presented in our study, a reduction of mortality and morbidity has been observed.20 Stern et al. published a Cochrane search that included randomised controlled trials (RCT) assessing systemic corticosteroid therapy, given as adjunct to antibiotic treatment, versus placebo or no corticosteroids for adults and children with pneumonia. They included 17 RCT (n=2264), concluding that corticosteroids significantly reduced mortality in adults with severe pneumonia (RR 0.58, 95% CI 0.40–0.84). Comparable findings were found in SARS infection. When patients with severe SARS infection are distinguished from patients with non-severe infection, it is observed that individuals receiving corticosteroids had lower mortality and a shorter hospital stay.21 Many analyses on the usefulness of corticosteroids in COVID-19-associated pneumonia seem to be in the same line. In a retrospective study by Wu et al., the risk of death in patients showing ARDS was lower when they were taking methylprednisolone (HR, 0.38; 95% CI, 0.20–0.72).22 Improvement in respiratory symptoms and lung lesions has also been observed when methylprednisolone was used at later ARDS stages and rapid disease progression, but it did not increase overall survival.9
Most recently studies published about the efficacy of Corticosteroids in COVID-19-associated pneumonia shows similar results.
The clinical trial conducted by the RECOVERY Collaborative Group recruited almost 6500 patients. In this study, 2104 patients received dexamethasone and 4321 received standard care. In the corticosteroids group, the incidence of death among patients who underwent to invasive mechanical ventilation was lower than in the control group (29.3% vs. 41.4%; rate-ratio, 0.64; 95% CI, 0.51–0.81). Authors concluded that the use of dexamethasone resulted in lower 28-day mortality in patients who were receiving either invasive ventilation or oxygen alone at the time of randomization without differences in patients who didn’t need respiratory support.12 Moreover, in the controlled clinical trial published by Edalatifard M et al.11 performed in 68 patients who were randomized in standard care with methylprednisolone pulse (intravenous injection, 250mgday−1 for 3 days) or standard care alone, they showed that the methylprednisolone group had better outcomes (reduced time to discharge and to improvement) compared to patients in the standard care group, and lower mortality rate.
With regards to doses of corticosteroids used, we chose moderate-to-high doses with a short duration (≤ 5 days). The reason of using high doses was due to the theoretical benefit of glucocorticoids acting through the non-specific-non-genomic route. We decided a short treatment regimen, because of the increased risk of perpetuating viral replication associated with prolonged treatments.23 Similar experiences are derived from studies conducted in SARS, where administration of high pulse of methylprednisolone involved a significant benefit, such as reducing ICU admission, reducing mechanical ventilation and mortality rate, and showed to be safer compared with lower dosages.24,25 However, this aspect is controversial and other authors advocate the use of low-to-moderate doses (25–150mg/day of methylprednisolone).26,27
The study has several strengths. First, an adequate selection of patients, with 5–10 days of symptoms and early ARDS. Second, the regimen chosen to administer methylprednisolone boluses was the minimum dose necessary to saturate all cytosolic receptors, which is generally 250mg, thus ensuring the speed of the effect. Third, the short duration of treatment to avoid the activation of the genomic pathway and, therefore, the side effects of its use in the medium and long term. As proposed by Siddiqui et al., the use of corticosteroids too early may not be necessary and could promote prolonged viral replication, but in a second stage of the disease, where patients develop lung inflammation, and in the presence of hypoxia, corticosteroids may be useful.28 In our cohort, median of symptoms was 10.5 days (8–13.5) and were accompanied by raised inflammatory biomarkers (ferritin, D-dimer, and C-reactive protein). Similar conclusions were drawn from a previous study focused in SARS patients in Guangzhou.21 Whilst the analysis of the 401 confirmed cases did not show any benefit of corticosteroid on the death rate and hospitalization days, when patients with critical illness were chosen (n=152), those patients that received corticosteroids had a significant beneficial effect on mortality and shorter hospitalization days without being associated with significant secondary lower respiratory infections or other complications. In opposition, the use of early corticosteroids might be harmful. In H5N1 infection, it has already been confirmed that early hydrocortisone administration initiated within the first 7 days of illness was associated with a higher plasma viral load at second and third weeks.29
The most undesirable adverse events from the use of corticosteroids are the enhancement in viral replication and development infections. In our study, once we analyzed the number of infections in both groups, we did not find significant differences between the groups treated with or without corticosteroids (40% vs. 50%, p: 0.473).
This study has several limitations. First, the sample size was low and no randomization was considered, which could imply a bias, although it should be noted that there were no differences in the clinical characteristics of both groups, and this aspect is essential in a non-randomized study. Even so, we provide evidence of performing the same treatment protocol for both cohorts (before–after), except for using methylprednisolone in the second cohort of patients. Second, all patients were treated with multiple other agents (including antiviral medications), and although treatment received was very similar, it is not possible to determine whether the improvement observed could have been related to therapies other than pulses of methylprednisolone.
In conclusion, we found that pulses of methylprednisolone, added to the standards of care, were associated with fewer OTI and/or deaths at 7 days in patients with COVID19-associated pneumonia and ADRS. These data provided a proof of concept study, and open a new scenario that will require confirmation in forthcoming clinical trials.
Authors’ contributionsConception and design of the study: Michelle Espinosa-Solano, Demetrio Gonzalez-Vergara, Marta Ferrer-Galvan, Maria Isabel Asensio-Cruz, Jose M Lomas, Cristina Roca-Oporto Maria Dolores Navarro-Amuedo, Maria Paniagua-Garcia, Cesar Sotomayor, Nuria Espinosa, Manuel Garcia-Gutierrez, Jose Molina Gil-Bermejo, Manuela Aguilar-Guisado, Manuel Poyato, Julia Praena-Segovia, Elisa Cordero, Candela Caballero-Eraso, Luis Jara-Palomares.
Writing the core content of the study: Michelle Espinosa-Solano, Demetrio Gonzalez-Vergara, Marta Ferrer-Galvan, Maria Isabel Asensio-Cruz, Candela Caballero-Eraso, Luis Jara-Palomares.
Analysis and interpretation of data: Michelle Espinosa-Solano, Demetrio Gonzalez-Vergara, Marta Ferrer-Galvan, Maria Isabel Asensio-Cruz, Jose M Lomas, Cristina Roca-Oporto Maria Dolores Navarro-Amuedo, Maria Paniagua-Garcia, Cesar Sotomayor, Nuria Espinosa, Manuel Garcia-Gutierrez, Jose Molina Gil-Bermejo, Manuela Aguilar-Guisado, Manuel Poyato, Julia Praena-Segovia, Elisa Cordero, Candela Caballero-Eraso, Luis Jara-Palomares.
Drafting the article or revising it critically for important intellectual content: All authors.
Preparation and critical review of the manuscript: Michelle Espinosa-Solano, Demetrio González-Vergara, Marta Ferrer-Galván, María Isabel Asensio-Cruz, José M Lomas, Cristina Roca-Oporto María Dolores Navarro-Amuedo, María Paniagua-García, Cesar Sotomayor, Nuria Espinosa, Manuel Garcia-Gutierrez, José Molina Gil-Bermejo, Manuela Aguilar-Guisado, Manuel Poyato, Julia Praena-Segovia, Elisa Cordero, Candela Caballero-Eraso, Luis Jara-Palomares.
All authors declare their approval for the current version of the manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestL.J.-P. has served as an advisor or consultant for Actelion Pharmaceuticals, Bayer HealthCare Pharmaceuticals, Leo Pharma, Menarini, Pfizer, and ROVI. C.C.-E. has served consultant for Antares consulting. No other conflict of interest declared.
Members of CortiCOVID-19 Team: See Appendix.