Flexible bronchoscopy (FB) is a common method used for diagnostic sampling of the thorax. Although the safety and efficacy of FB in the general population are well-established, data on the elderly population are limited. This study aimed to determine the safety and efficacy of FB in elderly people aged ≥80 years.
Materials and MethodsWe retrospectively studied elderly patients aged ≥80 years who underwent FB at our hospital between April 2021 and March 2022. Outcomes, such as indications, sampling methods, diagnostic results, and complications, were compared with those of a control group of patients aged 18–79 years.
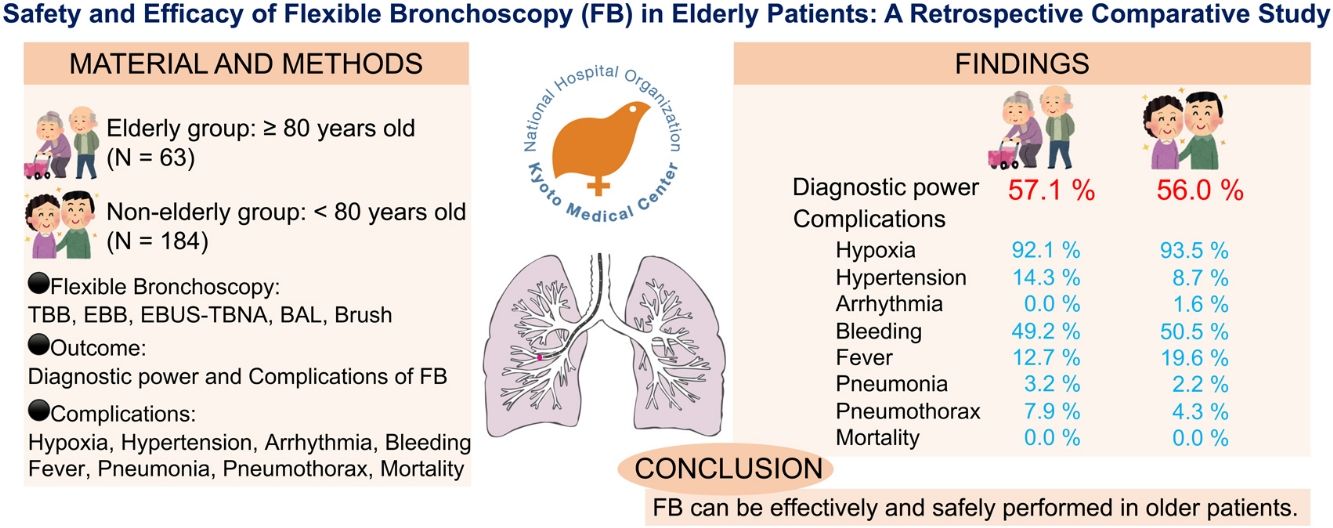
ResultsIn total, 247 patients (154 men) were included in the study, with 65 and 182 patients in the elderly and non-elderly groups, respectively. The most frequent indication for FB was the presence of a mass or nodule, with transbronchial lung biopsy, transbronchial biopsy, and endobronchial ultrasound-guided transbronchial needle aspiration performed in 162 (65.6%), 29 (11.7%), and 36 (15.6%) patients, respectively. FB led to a diagnosis in 36 (57.1%) and 103 (56%) patients in the elderly and non-elderly groups, respectively, with no significant difference observed. There were no patient deaths or significant differences in the incidence of hemorrhage, hypoxia, pneumothorax, fever, or pneumonia between the two groups.
ConclusionsThe findings of this study indicate that FB can be performed effectively and safely in elderly patients aged ≥80 years, similar to non-elderly patients.
La broncoscopia flexible (FB) es un método común utilizado para el muestreo diagnóstico del tórax. Aunque la seguridad y eficacia de la FB en la población general están bien establecidas, los datos sobre la población anciana son limitados. El objetivo de este estudio es determinar la seguridad y eficacia de la FB en ancianos ≥80 años.
Materiales y métodosSe estudiaron retrospectivamente los pacientes ancianos de ≥80 años sometidos a FB en nuestro hospital entre abril de 2021 y marzo de 2022. Los resultados, como las indicaciones, los métodos de muestreo, los resultados diagnósticos y las complicaciones, se compararon con los de un grupo control de pacientes de 18-79 años.
ResultadosEn total, 247 pacientes (154 hombres) fueron incluidos en el estudio, con 65 y 182 pacientes en los grupos de ancianos y no ancianos, respectivamente. La indicación más frecuente fue la presencia de una masa o nódulo, realizándose biopsia pulmonar transbronquial, biopsia transbronquial y aspiración transbronquial con aguja guiada por ecografía endobronquial en 162 (65,6%), 29 (11,7%) y 36 (15,6%) pacientes, respectivamente.
ConclusionesLos resultados de este estudio indican que la FB se puede Los resultados de este estudio indican que la FB puede realizarse de forma eficaz y segura en pacientes de edad ≥80 años, de forma similar a los pacientes no ancianos.
The world population is aging, with approximately 143 million people aged≥80 years in 2019, increasing three-fold since 1990.1 Flexible bronchoscopy (FB) is the primary modality used for airway assessment and diagnostic sampling of the trachea, bronchus, lung parenchyma, and hilar/mediastinal lymph nodes. The safety and effectiveness of FB in the general population is well established.2 The British Thoracic Society guidelines on the diagnostic use of FB do not present age as a hindrance to its application.3 However, this statement relies primarily on research conducted in the 1980s.4,5 As the elderly population increases, the demand for FB among elderly patients also increases. The elderly population often presents with comorbidities, such as pulmonary and cardiac diseases, necessitating cautious consideration of the benefits and risks of FB. A previous study on FB in elderly patients indicated a correlation between age and the incidence of pneumothorax,6 whereas other studies suggested that FB was well-tolerated in elderly patients with no notable increase in complications.7,8 Moreover, most previous studies included only a few octogenarians. Therefore, evidence of the effectiveness and safety of FB in elderly patients is insufficient and controversial. This study aimed to determine whether FB is safe and effective in elderly patients aged≥80 years.
Material and methodsDesign and patientsThis study retrospectively examined the medical records of patients who underwent FB at the National Hospital Organization, Kyoto Medical Center, between April 2021 and March 2022. Outpatients, patients in intensive care units, and patients receiving mechanical ventilation or bedside bronchoscopy were excluded. Additionally, patients who underwent chest drainage, received home oxygen therapy before FB, or had incomplete clinical data were also excluded from the study.
The study group consisted of patients aged≥80 years, whereas the control group included patients aged 18–79 years. Patient characteristics, including various baseline clinical data, such as age, sex, body mass index (BMI), Eastern Cooperative Oncology Group Performance Status (ECOG-PS),9 use of antithrombotic drugs, vital signs, comorbidities, and blood and serum sample test results, were collected from the electronic medical records.
Bronchoscopy procedure records included the examination time, procedural sedation, sampling methods, diagnosis, and postprocedural complications. Indications for FB were categorized as the presence of nodules/masses, granular shadow, infiltration, and ground-glass opacities according to computed tomography findings by more than two pulmonologists. The diagnoses were categorized as malignancy, infection, interstitial lung disease, and others.
The study protocol was approved by the Ethics Committee and Institutional Review Board of the National Hospital Organization Kyoto Medical Center (approval number: 22-072). The study adhered to the principles of the World Medical Association Declaration of Helsinki and reported the results in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Bronchoscopy proceduresAll bronchoscopy procedures were performed at our hospital. Patients were discharged on the day after the examination if there were no anticoagulant adjustments or complications associated with the FB. Electrocardiograms were only monitored during FB. Bronchoscopy and diagnostic techniques were performed using modified methods recommended by the British Thoracic Society and American College of Chest Physicians.3,10 Informed consent was obtained from all the patients who underwent FB, with the patients fasting for a minimum of 6h before the procedure. In accordance with the BTS and ACCP guidelines, anticoagulants and P2Y12 receptor antagonists were discontinued before the procedure; however, aspirin was not discontinued. Bronchoscopy was performed using flexible BF-260, BF-P290, and BF-UC290F bronchoscopes (Olympus Corporation, Tokyo, Japan). The larynx was anesthetized using 8% lidocaine, and midazolam (1mg/mL) was used for sedation and administered repeatedly until sedation was achieved.
The bronchoscope was inserted orally, and sampling methods, such as endobronchial biopsy, transbronchial biopsy/transbronchial lung biopsy (TBLB), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), bronchoalveolar lavage (BAL), and protective specimen brushing, were performed at the bronchoscopist's discretion. Electromagnetic navigation and cryobiopsy are not performed at our institution, and radial endobronchial ultrasound or virtual bronchoscopic navigation is performed at the clinician's discretion. The patients’ vital signs, including their electrocardiograms, pulse, and blood pressure, were closely monitored throughout the examination. In the event of significant desaturation (saturation of percutaneous oxygen (SpO2)<90%), oxygen was administered to maintain the SpO2 level at 90%. Nicardipine was administered to patients with hypertension as needed at the discretion of the bronchoscopist. Chest radiographs were obtained at the end of the procedure to confirm the development of pneumothorax, and flumazenil was administered for arousal. All bronchoscopy procedures were performed under the supervision of a specialist physician, and patients were observed for adverse events for approximately 24h after FB.
Efficacy and complicationsThe diagnostic power and complications of FB were compared between the elderly and non-elderly groups. Complications were defined as any procedural events that occurred during or after FB, regardless of the apparent association with FB. Adverse events were recorded for hypoxia (SpO2<90% and oxygen supply), hypertension (use of nicardipine), arrhythmia, bleeding (requiring intratracheal administration of adrenaline or thrombin), fever (axillary body temperature>37.5°C), pneumonia (use of antibiotics), pneumothorax, and mortality.
Statistical analysisContinuous variables pertaining to background factors and baseline laboratory data are presented as medians and interquartile ranges. The Mann–Whitney U test was used to compare continuous variables between the groups, whereas the Chi-square or Fisher's exact tests were used to compare categorical variables between the groups, as deemed appropriate. All statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). A p-value<0.05 was considered statistically significant.
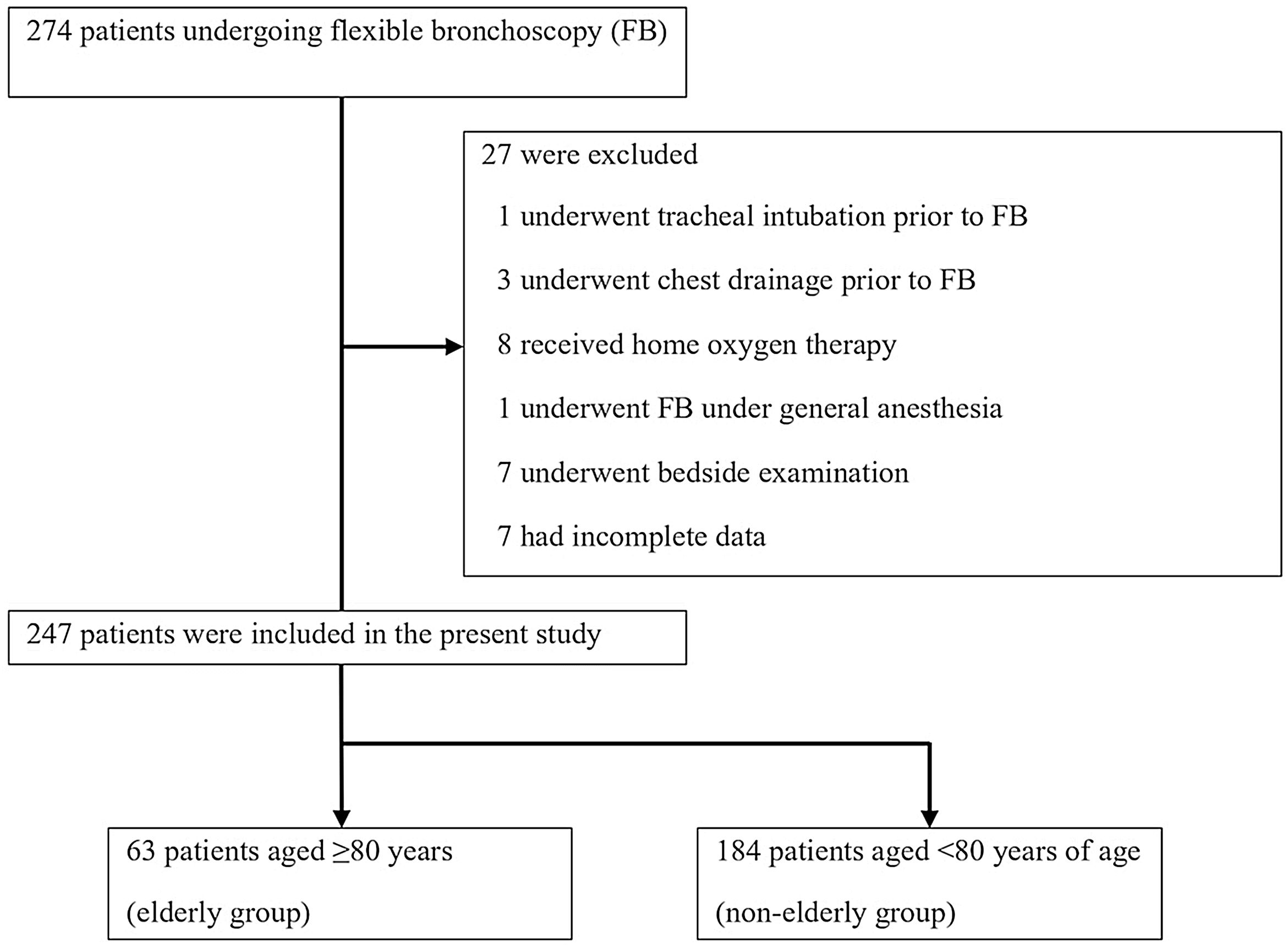
ResultsBetween April 2021 and March 2022, 274 patients underwent FB. Among them, one, three, and eight patients who underwent tracheal intubation, chest drainage, and received home oxygen therapy, respectively, prior to bronchoscopy were excluded. Additionally, 15 patients, including one who underwent FB under general anesthesia, seven who underwent bedside examination, and seven with incomplete data, were excluded. Finally, we analyzed the data of 247 patients (Fig. 1).
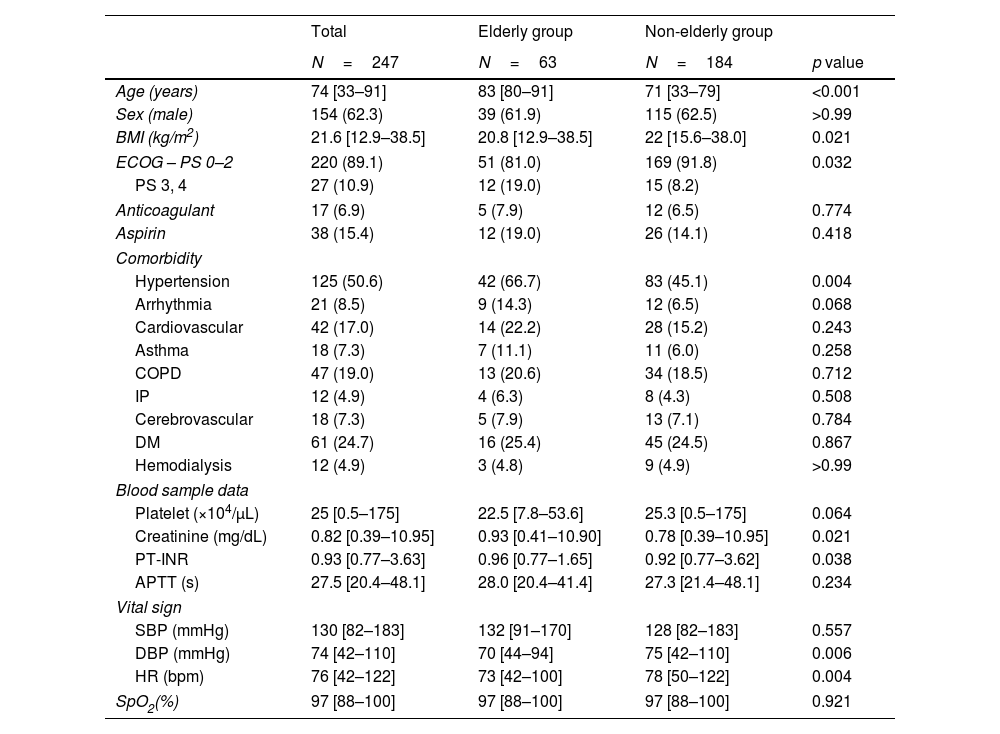
Patients characteristicsThe patient characteristics are shown in Table 1. The elderly and non-elderly groups included 63 and 184 patients, respectively. The median ages of the elderly and non-elderly groups were 83 years and 71 years, respectively. The elderly group had significantly lower BMI (20.8kg/m2 vs. 22.0kg/m2, p=0.021) and a higher proportion of ECOG-PS of 3 or 4 (12 [19.0%] vs. 15 [8.2%], p<0.032). The presence of hypertension was more common in the elderly group than the non-elderly group (42 [66.7%] vs. 83 [45.1%], p=0.004), whereas the presence of other comorbidities did not differ between the two groups. The numbers of patients receiving anticoagulants and aspirin were similar in both groups. Blood tests revealed elevated creatinine levels in the elderly group (0.93mg/dL vs. 0.78mg/dL, p=0.021). Pretest vital signs showed lower diastolic blood pressure and pulse rate in the elderly group (70mmHg vs. 75mmHg, p=0.006 and 73bpm vs. 78bpm, p=0.004, respectively).
Baseline characteristics of patients who underwent flexible bronchoscopy.
| Total | Elderly group | Non-elderly group | ||
|---|---|---|---|---|
| N=247 | N=63 | N=184 | p value | |
| Age (years) | 74 [33–91] | 83 [80–91] | 71 [33–79] | <0.001 |
| Sex (male) | 154 (62.3) | 39 (61.9) | 115 (62.5) | >0.99 |
| BMI (kg/m2) | 21.6 [12.9–38.5] | 20.8 [12.9–38.5] | 22 [15.6–38.0] | 0.021 |
| ECOG – PS 0–2 | 220 (89.1) | 51 (81.0) | 169 (91.8) | 0.032 |
| PS 3, 4 | 27 (10.9) | 12 (19.0) | 15 (8.2) | |
| Anticoagulant | 17 (6.9) | 5 (7.9) | 12 (6.5) | 0.774 |
| Aspirin | 38 (15.4) | 12 (19.0) | 26 (14.1) | 0.418 |
| Comorbidity | ||||
| Hypertension | 125 (50.6) | 42 (66.7) | 83 (45.1) | 0.004 |
| Arrhythmia | 21 (8.5) | 9 (14.3) | 12 (6.5) | 0.068 |
| Cardiovascular | 42 (17.0) | 14 (22.2) | 28 (15.2) | 0.243 |
| Asthma | 18 (7.3) | 7 (11.1) | 11 (6.0) | 0.258 |
| COPD | 47 (19.0) | 13 (20.6) | 34 (18.5) | 0.712 |
| IP | 12 (4.9) | 4 (6.3) | 8 (4.3) | 0.508 |
| Cerebrovascular | 18 (7.3) | 5 (7.9) | 13 (7.1) | 0.784 |
| DM | 61 (24.7) | 16 (25.4) | 45 (24.5) | 0.867 |
| Hemodialysis | 12 (4.9) | 3 (4.8) | 9 (4.9) | >0.99 |
| Blood sample data | ||||
| Platelet (×104/μL) | 25 [0.5–175] | 22.5 [7.8–53.6] | 25.3 [0.5–175] | 0.064 |
| Creatinine (mg/dL) | 0.82 [0.39–10.95] | 0.93 [0.41–10.90] | 0.78 [0.39–10.95] | 0.021 |
| PT-INR | 0.93 [0.77–3.63] | 0.96 [0.77–1.65] | 0.92 [0.77–3.62] | 0.038 |
| APTT (s) | 27.5 [20.4–48.1] | 28.0 [20.4–41.4] | 27.3 [21.4–48.1] | 0.234 |
| Vital sign | ||||
| SBP (mmHg) | 130 [82–183] | 132 [91–170] | 128 [82–183] | 0.557 |
| DBP (mmHg) | 74 [42–110] | 70 [44–94] | 75 [42–110] | 0.006 |
| HR (bpm) | 76 [42–122] | 73 [42–100] | 78 [50–122] | 0.004 |
| SpO2(%) | 97 [88–100] | 97 [88–100] | 97 [88–100] | 0.921 |
Data are shown with median and [range] or number and (percentage). p-Values were estimated by Mann–Whitney U tests for continuous variables and Fisher's exact tests for categorical variables.
Abbreviations: BMI, body mass index; ECOG-PS, Eastern Cooperative Oncology Group performance status; COPD, chronic obstructive pulmonary disease; IP, interstitial pneumonia; DM, diabetes mellitus; PT-INR, prothrombin time-international normalized ratio; APTT, activated partial thromboplastin time; %VC, vital capacity percentage; FEV1, forced expiratory volume in one second; FEV1%, forced expiratory volume % in one second; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SpO2, saturation of percutaneous oxygen.
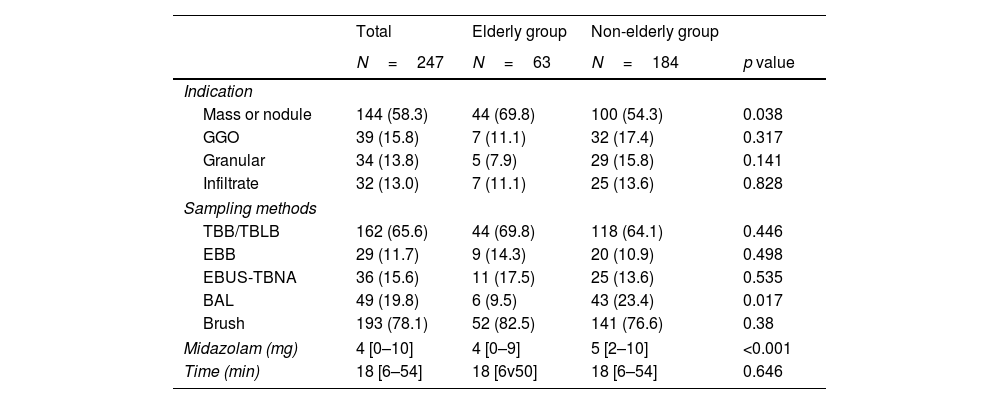
Table 2 shows the primary clinical indications for FB, sampling techniques, midazolam administration volumes, and sedation times. The most frequent clinical indication for FB was the presence of masses/nodules, and it was more common in the elderly cohort (44 [69.6%] vs. 100 [54.3%], p=0.038). BAL was performed more frequently in the non-elderly group, whereas there was no significant difference in the other examination procedures between the two groups. The median midazolam dose was 4mg in the elderly cohort and 5mg in the non-elderly cohort (p<0.001). The duration of the procedure was 18min in both groups (p=0.646).
Indications for flexible bronchoscopy, sampling techniques, and sedation.
| Total | Elderly group | Non-elderly group | ||
|---|---|---|---|---|
| N=247 | N=63 | N=184 | p value | |
| Indication | ||||
| Mass or nodule | 144 (58.3) | 44 (69.8) | 100 (54.3) | 0.038 |
| GGO | 39 (15.8) | 7 (11.1) | 32 (17.4) | 0.317 |
| Granular | 34 (13.8) | 5 (7.9) | 29 (15.8) | 0.141 |
| Infiltrate | 32 (13.0) | 7 (11.1) | 25 (13.6) | 0.828 |
| Sampling methods | ||||
| TBB/TBLB | 162 (65.6) | 44 (69.8) | 118 (64.1) | 0.446 |
| EBB | 29 (11.7) | 9 (14.3) | 20 (10.9) | 0.498 |
| EBUS-TBNA | 36 (15.6) | 11 (17.5) | 25 (13.6) | 0.535 |
| BAL | 49 (19.8) | 6 (9.5) | 43 (23.4) | 0.017 |
| Brush | 193 (78.1) | 52 (82.5) | 141 (76.6) | 0.38 |
| Midazolam (mg) | 4 [0–10] | 4 [0–9] | 5 [2–10] | <0.001 |
| Time (min) | 18 [6–54] | 18 [6v50] | 18 [6–54] | 0.646 |
Data are shown with median and [range] or number and (percentage).
Abbreviations: GGO, ground-glass opacity; TBB, transbronchial biopsy; TBLB, transbronchial lung biopsy; EBB, endobronchial biopsy; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; BAL, bronchoalveolar lavage.
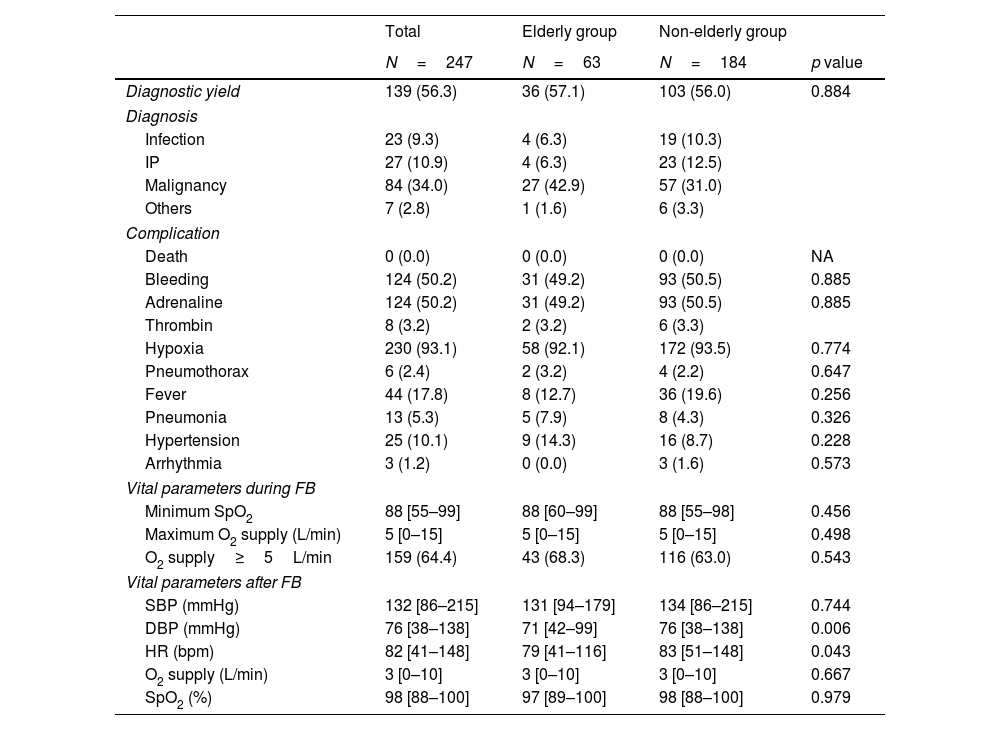
Table 3 shows the diagnostic complications and vital parameters of FB. The rate of diagnosis confirmed by FB was similar between the two groups (36 [57.1%] vs. 103 [56.0%], p=0.884). The proportion of malignancies at diagnosis was 11.9% higher in the elderly group; however, there was no significant difference (27 [42.9%] vs. 57 [31.0%]; p=0.092) between the two groups.
Diagnosis, complications and vital parameters by flexible bronchoscopy.
| Total | Elderly group | Non-elderly group | ||
|---|---|---|---|---|
| N=247 | N=63 | N=184 | p value | |
| Diagnostic yield | 139 (56.3) | 36 (57.1) | 103 (56.0) | 0.884 |
| Diagnosis | ||||
| Infection | 23 (9.3) | 4 (6.3) | 19 (10.3) | |
| IP | 27 (10.9) | 4 (6.3) | 23 (12.5) | |
| Malignancy | 84 (34.0) | 27 (42.9) | 57 (31.0) | |
| Others | 7 (2.8) | 1 (1.6) | 6 (3.3) | |
| Complication | ||||
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Bleeding | 124 (50.2) | 31 (49.2) | 93 (50.5) | 0.885 |
| Adrenaline | 124 (50.2) | 31 (49.2) | 93 (50.5) | 0.885 |
| Thrombin | 8 (3.2) | 2 (3.2) | 6 (3.3) | |
| Hypoxia | 230 (93.1) | 58 (92.1) | 172 (93.5) | 0.774 |
| Pneumothorax | 6 (2.4) | 2 (3.2) | 4 (2.2) | 0.647 |
| Fever | 44 (17.8) | 8 (12.7) | 36 (19.6) | 0.256 |
| Pneumonia | 13 (5.3) | 5 (7.9) | 8 (4.3) | 0.326 |
| Hypertension | 25 (10.1) | 9 (14.3) | 16 (8.7) | 0.228 |
| Arrhythmia | 3 (1.2) | 0 (0.0) | 3 (1.6) | 0.573 |
| Vital parameters during FB | ||||
| Minimum SpO2 | 88 [55–99] | 88 [60–99] | 88 [55–98] | 0.456 |
| Maximum O2 supply (L/min) | 5 [0–15] | 5 [0–15] | 5 [0–15] | 0.498 |
| O2 supply≥5L/min | 159 (64.4) | 43 (68.3) | 116 (63.0) | 0.543 |
| Vital parameters after FB | ||||
| SBP (mmHg) | 132 [86–215] | 131 [94–179] | 134 [86–215] | 0.744 |
| DBP (mmHg) | 76 [38–138] | 71 [42–99] | 76 [38–138] | 0.006 |
| HR (bpm) | 82 [41–148] | 79 [41–116] | 83 [51–148] | 0.043 |
| O2 supply (L/min) | 3 [0–10] | 3 [0–10] | 3 [0–10] | 0.667 |
| SpO2 (%) | 98 [88–100] | 97 [89–100] | 98 [88–100] | 0.979 |
Data are shown with median and [range] or number and (percentage).
Abbreviations: IP, interstitial pneumonitis; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SpO2, saturation of percutaneous oxygen.
None of the patients who underwent FB died during the study period. Bleeding occurred in 31 (46.2%) and 93 (50.5%) patients in the elderly and non-elderly groups, respectively (p=0.885). Two (3.2%) and six (3.3%) patients in the elderly and non-elderly groups (p>0.99), respectively, required the intratracheal administration of thrombin.
Pneumothorax occurred in two patients in the elderly group and four patients in the non-elderly group (2 [3.2%] vs. 4 [2.2%], p=0.647). Among them, one patient in the elderly group and three in the non-elderly group required thoracic drainage. All patients required prolonged hospitalization; however, they improved with thoracic drainage, obviating any surgical intervention.
More than 90% of patients in both groups required oxygenation, and a maximum oxygen flow of 5L/min or more was required in more than 60% of all patients. Oxygen flow was reduced to 3L/min after FB, and oxygen supply was not required the next day; therefore, none of the patients had their discharge date postponed due to these factors. Fever was present in 44 (17.8%) patients, and there was no significant difference between the two groups (8 [12.7%] vs. 36 [19.6%]). Among them, 13 (5.3%) developed pneumonia and were treated with antimicrobials, which was more common in the elderly group (5 [7.9%] vs. 8 [4.3%], p=0.326).
DiscussionThis retrospective study examined the complications and diagnostic power in elderly patients undergoing FB by comparing them with those of non-elderly patients. The results showed no significant differences between the elderly and non-elderly groups, demonstrating the efficacy and safety of FB in elderly patients.
Elderly patients were defined as those aged≥80 years in this study. The population aged≥80 years is expected to increase by three-fold from the current number to 426 million over the next 30 years. Furthermore, the average life expectancy, as of 2016, is 80.8 years in high-income countries. Therefore, a cut-off age of 80 years was used in this study in view of these demographic characteristics.1,11 The older the population, the greater the number of elderly patients with lung cancer. The emergence of immunotherapy, particularly for the treatment of lung cancer, implies that even older adults can be treated safely.12,13 With demographic and treatment changes, it is necessary to investigate the efficacy and safety of bronchoscopy, leading to appropriate treatment in the current setting.
ECOG-PS was used to evaluate the general condition of the patients in this study. ECOG-PS was originally developed for the evaluation of patients with cancer. Activities of daily living (ADL) in older people are often measured using scales such as the Katz and Barthel indices. The use of such indicators may help clarify the differences in patient background between the groups. However, in general, older people have lower ADL than non-elderly people, which was similar in the present study. In this context, our results showed no difference in the efficacy and safety of flexible bronchoscopy between the two groups, suggesting that the ADL indicators would not influence the present results. ECOG-PS is a simple scale that is easy to assess and more familiar to pulmonologists, unlike the Katz and Barthel indices, which are complex. Furthermore, a previous report on the use of flexible bronchoscopy in the elderly evaluated the patients using ECOG-PS.14 Therefore, ECOG-PS was used to evaluate the general condition of the patients in our study.
Regarding the efficacy of FB for the elderly, the reported diagnostic rate of FB ranges from 43.8% to 63.0%.15,16 In our study, 103 (56.0%) patients in the non-elderly group and 36 (57.1%) patients in the elderly group were diagnosed using FB, with no significant differences between the two groups. FB was as effective in the elderly group when compared with the non-elderly group.
As for safety, previous studies have investigated the safety of FB in patients aged≥80 years.4,13,15–17 They indicated that elderly patients had a higher frequency of complications, such as bleeding, pneumothorax or pneumonia, and mortality associated with FB, than that in non-elderly patients,14,15 whereas some studies suggested that the frequency of complications was the same in elderly and non-elderly patients.4,17,18 Nevertheless, many previous studies did not investigate procedures such as TBLB or BAL, which could potentially impede the accurate evaluation of associated complications. In this study, a sampling method for FB was thoroughly investigated. The frequency of needle biopsies and TBLB were similar in both groups. However, BAL was performed approximately 10% more frequently in the non-elderly group. BAL is known to increase the frequency of fever and hypoxemia,19–21 and it is possible that the difference in the frequency of BAL led to a higher frequency of fever after FB in non-elderly patients in this study. Regarding hypoxia, most patients received oxygen during FB in this study, although FB-induced hypoxia has been reported to range from 14.4% to 75.4%.22–25 A previous study reported that the frequency of oxygen requirement was similar between elderly and non-elderly patients,17 which was confirmed in this study. Since the oxygen demand promptly decreased after FB and its administration was temporary, it is possible that oxygen is readily administered at our institution. However, even after taking this into account, the results confirm that FB can be safely performed in both elderly and non-elderly patients.
The incidence of bleeding in elderly patients as a result of FB is reported to be 3.3–10.6%8,15,25; however, in this study, its frequency was 50.2%. TBLB is known to increase the incidence of bleeding and pneumothorax26–30 and TBLB was performed in 65.6% of patients. The frequency of TBLB was higher than that previously reported,16,31 which may explain the increased frequency of bleeding. However, the frequency of bleeding was comparable with that in the non-elderly group, indicating that FB can be safely performed in elderly patients.
The incidence of pneumothorax during FB in elderly patients aged≥80 years has been reported to range from 1.2% to 3.3% in previous studies.6,14,15,17 However, the frequency of TBLB was not investigated in these studies. To the best of our knowledge, this study is the first to show a correlation between pneumothorax and TBLB in individuals aged>80 years. Despite the higher frequency of TBLB, our findings were consistent with those of previous reports on the incidence of pneumothorax, indicating that FB may have been safely performed.
This study had some limitations. First, it was conducted at a single institution. Bronchoscopy methods can vary among hospitals. Since the diagnostic rate and frequency of complications did not deviate from previous reports, we hypothesized that the results are standard; however, multicenter studies are necessary to extend these findings to other contexts. Second, this was a retrospective study, and the decision to perform bronchoscopy depended on the individual physician. This could have led to selection bias, and patients who underwent FB may have had fewer comorbidities and better performance statuses. Prospective research is needed to generalize our results. However, given that a higher proportion of patients with ECOG-PS 3–4 was observed in the elderly group, we believe that the study was conducted in a real clinical setting.
The findings of this investigation suggest that elderly individuals aged>80 years with indications for FB can achieve diagnostic rates comparable with those of their younger counterparts and undergo invasive diagnostic procedures without significant complications. These outcomes may alleviate concerns regarding bronchoscopy even among older individuals. Although age and performance status may affect the decision to perform an invasive procedure, the results of this study imply that FB can be effectively and safely performed in older patients.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsZentaro Saito: Conceptualization, Methodology, Formal analysis, Writing-original draft, Reviewing and editing, Projection administration.
Issei Oi: Conceptualization, Methodology, Reviewing and editing, Projection administration.
Takanori Ito, Takuma Imakita, Osamu Kanai, Kohei Fujita, and Hiromasa Tachibana: Writing-review and editing, Investigation.
Tadashi Mio: Funding acquisition, Supervision.
Conflicts of interestThe authors declare that there are no conflicts of interest.
We would like to thank Editage (www.editage.jp) for English language editing.