As a result of the SARS-CoV-2 (COVID-19) pandemic, cases of acute invasive pulmonary aspergillosis (AIPA) have been detected in patients admitted to intensive care units (ICU) with severe acute respiratory distress syndrome (ARDS) secondary to SARS-CoV-2 pneumonia who were intubated to invasive mechanical ventilation and who received systemic immunosuppressive therapy (glucocorticotherapy, tocilizumab),1–4 leading to high lethality.1,2 This association of AIPA and COVID-19 is already recognized.5–7 SARS-CoV-2 pneumonia causes damage to the respiratory epithelium, decreased mucociliary clearance and immune dysfunction/dysregulation, which increase the risk that aspergillary hyphae may infiltrate lung tissue leading to AIPA.2–5,7,8 However, until now we had practically no known cases of subacute invasive pulmonary aspergillosis (SAIPA, or semiinvasive),9,10 and even less in patients who had not been intubated in the ICU.10
We present a case of a 69-year-old man with a history of systemic arterial hypertension, B chronic lymphatic leukaemia (B-CLL) in Binet-Rai stage A1 (stable, expectant management), ex-smoking, mild sleep apnoea-hypopnoea syndrome, chronic obstructive pulmonary disease (COPD) in GOLD stage B2 (mMRC 2 dyspnoea, non-exacerbator emphysematous phenotype, FEV1=65.7%). He was admitted to hospital on day 7 of symptoms (fever, asthenia, hyporexia, anosmia, dysgeusia, dry cough, pleuritic chest pain, dyspnoea on minimal efforts) in a monographic COVID-19 hospital in Madrid (Spain) with a diagnosis of SARS-CoV-2 pneumonia with acute hypoxemic respiratory failure (baseline PaO2=53.6mmHg) that was initially controlled with conventional nasal cannula (NC) at a flow of 4l/min (maintaining SpO2 of 95%). Two days later, he progressed to severe ARDS (PaO2/FiO2=75) and was escalated in oxygen therapy to a mask-reservoir at a flow of 15l/min (maintaining SpO2 of 92% and tachypnoea of 24breaths/min with use of respiratory accessory muscles and dyspnoea when speaking) and was admitted to the intermediate respiratory care unit (IRCU). Analytically, an inflammatory hyperresponse was found, the C-reactive protein (CRP) increased to 112.0mg/l.
At IRCU, high-flow nasal cannula (HFNC) alternated with continuous positive airway pressure (CPAP) were installed. As immunomodulatory treatment, dexamethasone was administered intravenously (20mg/day for the first 5 days, followed by 10mg/day for the next 5 days, and then 6mg/day). No other immunomodulatory therapies were used. Thromboprophylaxis was performed with compression stockings and enoxaparin subcutaneously. On day 7 of admission (14 of symptoms), due to an elevation of D-dimer (66210ng/ml) added to respiratory deterioration, a thoracic computed tomography (CT) angiography was performed, which revealed a diagnosis of intermediate-low risk acute pulmonary embolism (PE), whose distribution was in lobar, segmental and subsegmental arterial branches of the left lower lobe (LLL) (Fig. 1A). Consequently, enoxaparin was increased at therapeutic doses. The evolution of the patient's respiratory situation was favorable, being able to withdraw CPAP after 32 days and de-escalate from HFNC to NC at 6l/min after 45 days, maintaining SpO2 of 97–98% and adequate respiratory mechanics.
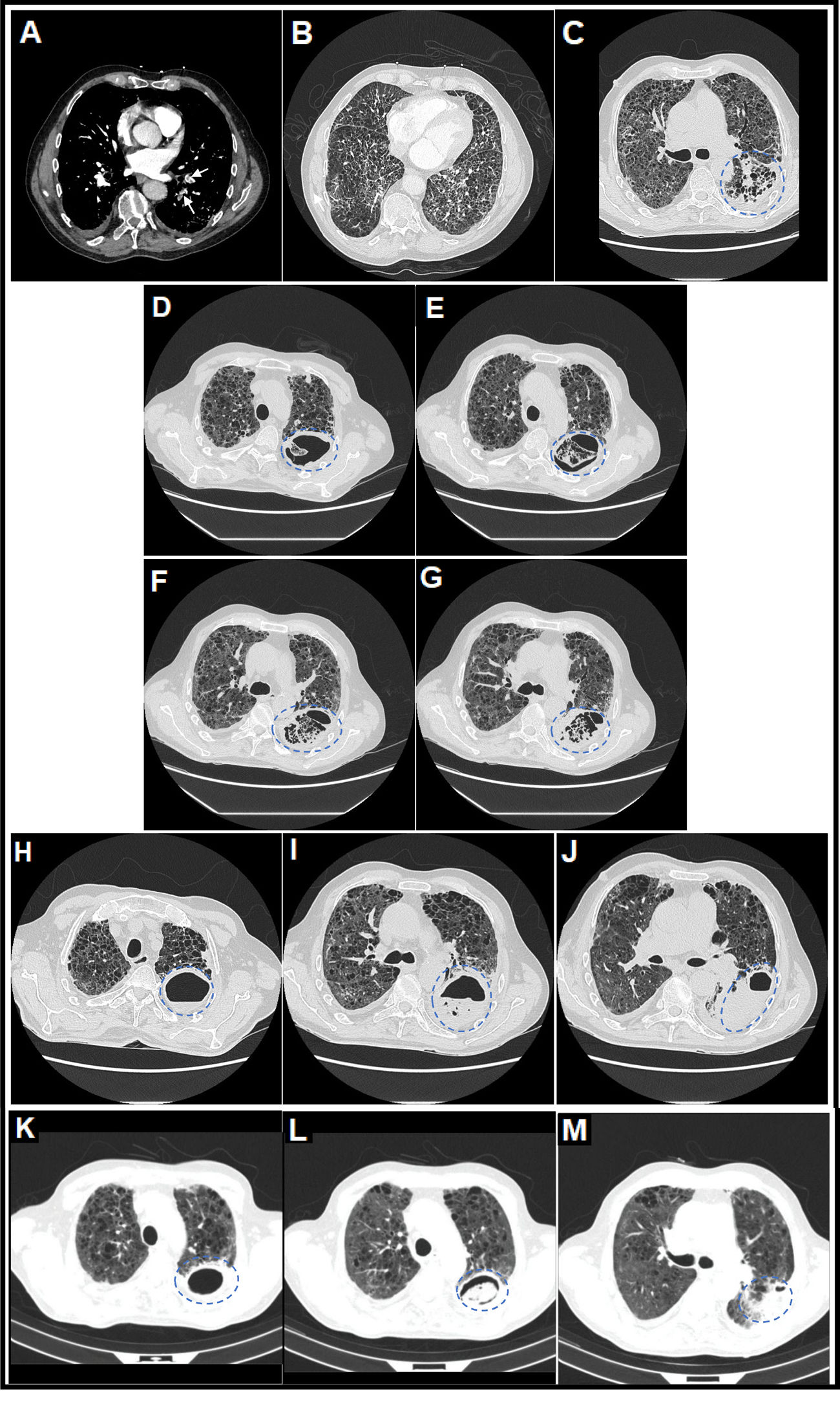
(A) Thoracic computed tomography (CT) angiography image (mediastinal window) on day 7 of admission in which central filling defects were observed in lobar, segmental and subsegmental pulmonary arterial branches of the left lower lobe (LLL), compatible with a diagnosis of acute pulmonary embolism (PE) (white arrows). (B) Thoracic CT angiography image (pulmonary parenchyma window) on day 7 of admission in which a severe extensive diffuse bilateral multilobar pulmonary parenchymal involvement of the reticular interstitial type was observed, characterized by increased attenuation in ground glass and thickening of interlobular and intralobular septa, compatible with a diagnosis of SARS-CoV-2 pneumonia, associated with extensive generalized vacuolization of the lung parenchyma compatible with marked centrilobular and paraseptal pulmonary emphysema. (C) Thoracic CT image (lung parenchyma window) on day 32 of admission in which it was observed that, on the previous reticular interstitial lung pattern, a consolidative area appeared with incipient cavitations with a tendency to coalescence in the upper segment (S6) of the LLL (surrounded by a discontinuous blue circumference), related to acute pulmonary infarction (secondary to acute PE) and incipient signs of subacute invasive pulmonary aspergillosis (SAIPA). (D–G) Thoracic CT images (lung parenchyma window) on day 47 of admission in which it was observed that a true large cavitated infiltrate had been established in the LLL (12.5cm in maximum diameter approximately) with thick walls and with reticular content inside compatible with an aspergillary fungal lung abscess (SAIPA) (surrounded by a broken blue ellipse) with possible super-added bacterial superinfection (possible bacterial lung abscess) on a bed of acute pulmonary infarction due to acute PE, of bilateral pneumonic affectation by SARS-CoV-2 and of emphysematous lung parenchyma. Adjacent, a predominantly left basal bilateral pleural thickening reaction was observed in the context of SAIPA. (H–J) Thoracic CT images (lung parenchyma window) on day 74 of admission in which it was observed that the cavitated infiltrate located in the S6 of LLL, of similar size (surrounded by a dashed blue ellipse), had been partially filled with liquid content forming an air-fluid level. (K–M) Thoracic CT images (lung parenchyma window) on day 104 from the date of hospital admission and on day 26 from the date of discharge from the hospital, after two months with antifungal therapy with posaconazole, in which it was observed that the cavitated infiltrate located in the S6 of the LLL (surrounded by a dashed blue ellipse) had decreased considerably in size (from 12.5 to 6.1cm in larger diameter) and whose content had been more structured adopting a spherical morphology constituting a “fungal ball” and thus resembling more of a chronic cavitary pulmonary aspergillosis (CCPA), to which SAIPA had evolved in this patient.12,13
However, coinciding with the withdrawal of CPAP, the patient began with a fever of 38.1°C, hyporexia, asthenia, weight loss, very thick and yellow bronchorrhea (without haemoptysis), pleuritic left rib pain and increased dyspnoea, all of this added to a rebound in CRP (increased to 233.5mg/l). Thoracic CT was performed in successive evolutionary phases (days 32, 47 and 74 of hospital admission) (Fig. 1C–J), and superadded to a marked bilateral reticular interstitial lung pattern (SARS-CoV-2 pneumonia), and emphysematous (COPD), a large, thick-walled, new-appearing cavitated infiltrate (12.5cm in diameter) located in the upper segment (S6) of the LLL with a reticulated material inside was evidenced, which was associated with thickening of the adjacent basal pleura. Sputum were cultured on different days and the fungus Aspergillus fumigatus grew, which was sensitive to triazoles in fungigram. A. fumigatus IgG serology was positive (185mg/l, the upper limit of normality – ULN – was 30mg/l), not the IgE (0kU/l), nor the galactomannan index in blood (0.08, the ULN was 0.50). Sputum cultures for mycobacteria were negative. And sputum culture for bacteria resulted in usual saprophytic flora. The diagnosis was consistent with SAIPA. It was not an AIPA, given the time of evolution (≥21 days) and since no serious acute worsening of the patient's respiratory situation was found; in fact, it was possible to gradually de-escalate oxygen therapy requirements. Nor was it initially a chronic cavitary pulmonary aspergillosis (CCPA) since the time of evolution was <3 months. Specifically, the evolution time until the appearance of the aspergillary lung abscess was 32 days from hospital admission and 39 days from the date of onset of COVID-19 symptoms.
Three conditions were fundamentally identified that predisposed the patient to develop SAIPA. The first was the formation of a previous cavity in LLL (the patient had an acute pulmonary infarction in LLL secondary to acute PE) on which the Aspergillus fungus later settled, as described in chronic pulmonary aspergillosis.11–13 The previous marked emphysematous lung pattern and SARS-CoV-2 pneumonia itself also facilitated the settlement of Aspergillus.11–13 The second condition was the patient's immunosuppression situation, mainly due to the systemic glucocorticotherapy received (which he maintained during his almost three months of hospitalization due to persistent pulmonary inflammation secondary to SARS-CoV-2 pneumonia with lasting hypoxemic respiratory failure), to which advanced age and previous diagnoses of B-CLL and emphysematous COPD also contributed.11,12 And the third condition could be HFNC and CPAP therapies, whose flows and pressures could facilitate the introduction of aspergillary hyphae from the environment into the patient's respiratory tract.
The patient was prescribed antifungal treatment with posaconazole oral tablets at a dose of 300mg/day (after a loading dose of 300mg twice on the first day) for a minimum of six months. Among the triazoles, posaconazole was chosen due to its lower profile of adverse effects, including its lower hepatotoxicity, given the alteration of the liver profile present by COVID-19. Tolerance to posaconazole was good. In addition, to cover the possibility of nosocomial bacterial superinfection on the aspergillary lung abscess, a 21-day antibiotic cycle of meropenem and linezolid was indicated. Subsequently, the fever subsided, and the productive cough, left rib pain, dyspnoea, asthenia, hyporexia, and CRP (45.6mg/l) decreased.
As another problem, 9 days after CPAP was removed, the patient developed a secondary spontaneous left partial pneumothorax,14,15 which was treated by placing a left chest drainage tube (CDT) with aspiration, leading to a rapid resolution of pneumothorax. The CDT could be removed three days later. This pneumothorax, being an air cavity, although located in the pleural space instead of in the lung parenchyma, could also be a predisposing condition for SAIPA.11–13 On lungs with diffuse alveolar damage due to bilateral SARS-CoV-2 pneumonia and associated ARDS, to which were added in our case marked bilateral pulmonary emphysema and an aspergillary abscess in S6 of LLL, repeated episodes of severe cough (frequent symptom in COVID-19), as well as abnormal respiratory mechanics, can cause a sudden increase in pressure in the distal airway and cause an alveolar rupture and secondary air leak to the peribronchovascular pulmonary interstitium, and from there the air can dissect proximally and centripetally until reaching the mediastinum (pneumomediastinum) and the subcutaneous cellular tissue of the thoracic wall and cervical region (subcutaneous emphysema), as well as the pleural space (pneumothorax). This is what is known as the Macklin effect,15 responsible in our case for the left partial pneumothorax, which would be of a secondary spontaneous type. The barotraumatic mechanism of the pneumothorax was ruled out since it appeared 9 days after the CPAP was removed.
Finally, given the improvement in the respiratory situation, the patient could be discharged home with oxygen therapy using NC at 1l/min, maintaining SpO2 of 95% and dyspnoea mMRC 3. At the outpatient check-up, after two months of treatment with posaconazole, a control thoracic CT showed a considerable decrease in the size of the cavitated infiltrate (from 12.5 to 6.1cm in diameter) (Fig. 1K–M) and of the productive cough. The content of the infiltrate adopted a spherical morphology, constituting what is known as a “fungal ball” and now resembling a CCPA, to which SAIPA had evolved in this patient.12,13
In summary, we want to show with this scientific letter that severe SARS-CoV-2 pneumonia can be associated with pulmonary aspergillosis.5–7 Although the vast majority of cases are AIPA in intubated ICU patients,1–4 other forms of pulmonary aspergillosis, SAIPA and chronic,9,10 can also be found, even in patients who had not been intubated at any time,10 especially if they meet certain predisposing conditions: immunosuppression situations (e.g., glucocorticotherapy), the formation of previous pulmonary cavities and the use of non-invasive respiratory support.11–13
Authors’ contributionAll the authors of the article has contributed substantially to the elaboration of the manuscript.
FundingThe authors declare that no funding was received for this article.
Patient consentInformed consent was obtained from the patient for publication of the clinical data and images present in this manuscript. It was collected in the electronic medical record.
Conflict of interestThe authors declare that they have no conflict of interest directly or indirectly related to the contents of this manuscript.