The patient experience is defined as all the interactions that occur between patients and the healthcare system. The experience of patients with respiratory disease with home respiratory treatments (HRT) is not captured in currently available Patient-Reported Outcome Measures (PROM). We present the psychometric validation of the Patient-Reported Experience Measure (PREM) ‘HowRwe’ in Spanish and for respiratory patients with HRT.
Material and methodsAfter translation following ISPOR guidelines (International Society for Pharmacoeconomics and Outcomes Research), the questionnaire was administered to adult respiratory patients who were receiving treatment at Hospital Universitario de La Princesa. The administration was done in two stages with 6 months of difference between the pre- and post-test.
ResultsWe studied 228 respiratory patients, with a mean (SD) age of 64.1 (13.2) years, 52.2% were men, 68.0% were married or coupled, and 56.6% were retired. Reliability coefficients of the scale were adequate, with α=.921 and Ω=.929 for pre-test, and α=.940 and Ω=.958 for post. The confirmatory factor analysis tested for pre- and post-intervention, showed an excellent overall fit: χ2(2)=49.380 (p<.001), CFI=.941 and SRMR=.072; and χ2(2)=37.579 (p<.001), CFI=.982 and SRMR=.046, respectively. No statistically significant associations were observed for neither age, adherence nor quality of life, except between HowRwe post-test and quality of life pre-test (r=.14 [.01,.26]; p=.035). No significant differences were found in sociodemographic variables. No differences in pre-test or post-test were found in effect of HRT. 85.6% of patients found the content of HowRwe “Useful”, and the preferred channel to respond it were paper, app and email.
ConclusionsThe Spanish version of the ‘HowRwe’ questionnaire to measure the experience in respiratory patients with home respiratory treatments (HRT), has adequate psychometric properties and conceptual and semantic equivalence with the original English version.
La experiencia del paciente se define como todas las interacciones que ocurren entre los pacientes y el sistema de salud. La experiencia de los pacientes con enfermedades respiratorias con terapias respiratorias domiciliarios (TRD) no se refleja en las Medidas de resultados informados por el paciente (PROM) disponibles actualmente. Presentamos la validación psicométrica de la Medida de Experiencia Reportada por el Paciente (PREM por sus siglas en inglés) ‘HowRwe’ en español y para pacientes respiratorios con TRD.
Material y métodosDespués de la traducción siguiendo las pautas de ISPOR (Sociedad Internacional de Farmacoeconomía e Investigación de Resultados), el cuestionario se administró a pacientes respiratorios adultos que estaban recibiendo tratamiento en el Hospital Universitario de La Princesa. La administración se realizó en dos etapas con 6 meses de diferencia entre el pre y post test.
ResultadosSe estudiaron 228 pacientes respiratorios, con una edad media (DE) de 64,1±13,2 años, el 52,2% eran hombres, el 68,0% estaban casados o en pareja y el 56,6% eran jubilados. Los coeficientes de confiabilidad de la escala fueron adecuados, con α=.921 y Ω=.929 para el pretest, y α=.940 y Ω=.958 para el post. El análisis factorial confirmatorio testado para pre y postintervención, mostró un ajuste global excelente: χ2(2)=49.380 (p<.001), CFI=.941 y SRMR=.072; y χ2(2)=37,579 (p<.001), CFI=.982 y SRMR=.046, respectivamente. No se observaron asociaciones estadísticamente significativas ni para la edad, la adherencia ni para la calidad de vida, excepto entre HowRwe postest y calidad de vida pretest (r=.14 [.01,.26];p=.035). No se encontraron diferencias significativas en las variables sociodemográficas. No se encontraron diferencias en el efecto de la TRH en el pretest o postest. El 85,6% de los pacientes encontró “útil” el contenido de HowRwe y el canal preferido para responder fue el papel, la aplicación y el correo electrónico.
ConclusionesLa versión española del cuestionario ‘HowRwe’ para medir la experiencia en pacientes respiratorios con tratamientos respiratorios domiciliarios (TRH), tiene adecuadas propiedades psicométricas y equivalencia conceptual y semántica con la versión original en inglés.
Evaluation of healthcare is an evolving concept, and the patient's perspective is increasingly sought to provide a more patient-centered service. Methods such as surveys address the needs of policy makers for accountability and transparency,1 but these have been criticized for a number of reasons, including excessive survey length, infrequent sampling frequency, slow feedback and failure to use results to improve care. Furthermore, these normally do not have objective validity, so their interpretation is subjective.2 Self-reported questionnaires are being used to gather information about patients’ health-related quality of life, health outcomes that matter most to patients with, experience of treatments, and perceptions on the care delivered by the healthcare team. Any initiative to improve the quality of care for patients requires robust instruments to capture patients’ perceptions of the healthcare that they receive. The experience of respiratory patients receiving home respiratory treatments, and their point of view on the quality of healthcare received, are not captured in currently available Patient-Reported Outcome Measures (PROM).3 Quality of care has been a priority for health professionals, but recent evidence identifies the patient experience as one of the pillars of quality care.4 Furthermore, patient experience is a key element for improving health care in the context of person-centered care.5 The patient experience is defined as all the interactions that occur between the patient and the healthcare system,6 within the framework of a specific organizational culture that influences the perception of the person served.7 A Patient-Reported Experience Measures (PREM) is a measure of a patient's perception of their personal experience of the healthcare they have received. PREM instruments should focus on the aspects of care that matter to the patient.8 PREM results can be used to improve services and provide a patient's view on these improvements that moves away from the technological or economic model that is often employed in service design. The evaluation that patients make of their experience using PREM focuses on the humanity of care9 and its value.4 Unlike satisfaction, the characteristics/events of patients interacting with the healthcare system are objectively assessed. Patient experience is more objective than patient satisfaction.8 Therefore, PREMs are tools that kind of photograph “what” happened in a patient-health system interaction and “how” it was from the patient's perspective.10 Moreover, PREMs have demonstrated positive associations with health outcomes.4 In the implementation of a PREM, it is also necessary to keep in mind that the ability to express emotions, opinions and facts is not distributed uniformly throughout the population.11
The patient experience has three dimensions: relational, functional and integrative. The relational experience refers to the interaction between the healthcare professional and the patient.12 This is the focus of most efforts to improve patient engagement and patient-centered care. Functional experience, or satisfaction with the result, refers to the immediate benefits perceived by patients at that moment.13 This does not cover medium and long-term benefits or patient outcomes. Finally, integration experience, or service integration, relates to the patient care experience, which crosses traditional silos.14 This is the approach of system-wide approaches.
Some PREMs have been developed focused on specific respiratory diseases such as chronic obstructive pulmonary disease (COPD),15,16 bronchiectasis,16 idiopathic pulmonary fibrosis (IPF),17 obstructive sleep apnea (OSA),18 or pediatric asthma.19 Some PREMs have also been developed for respiratory patients receiving home respiratory therapies.20 Although they are very useful instruments, their use does not allow cross-reference data within the same organization. However, to date there is no PREM that is specific to all respiratory disorders, with or without home respiratory therapy.
‘HowRwe’ is a generic, brief and simple PREM developed in 2009, following the criteria required for patient-reported quality of life measures.21,22 HowRwe is considered clear, brief, generic, suitable for frequent use, compatible with multiple forms of data collection, responsive and with good psychometric properties. Its first validation was published in 2014.23 The core premise of HowRwe is that all patients want high quality service both from staff and from the organization as a whole. Patient experience can be classified in terms of relationships with staff and system function.24
We aimed to determine the psychometric validation of the PREM ‘HowRwe’ in Spanish and for respiratory patients receiving home respiratory therapy.
Material and methodsDesign, setting, and participantsThe validation study was conducted in a sample of 228 adult patients diagnosed with respiratory disease, COPD, interstitial lung disease (ILD), OSA, cystic fibrosis (CF), or other conditions with respiratory involvement such as neuromuscular disease (NMD), receiving or not home respiratory therapy. These patients were treated in the Pulmonology Department of the La Princesa University Hospital (Madrid, Spain). The questionnaire was administered during a follow-up visit with the doctor, the nurse or the person responsible for monitoring home respiratory therapy. The inclusion criteria were: patients 18 years and older, with a respiratory condition under treatment/monitoring by the Pulmonology Department. The exclusion criteria was psychophysical inability to complete questionnaires or refusal to answer them. For the sample size calculation, we took into account the recommendation of including a minimum sample size of 100–200 patients.25–27 The measurement of the questionnaires was carried out twice: pre-test and post-test 6 months later.
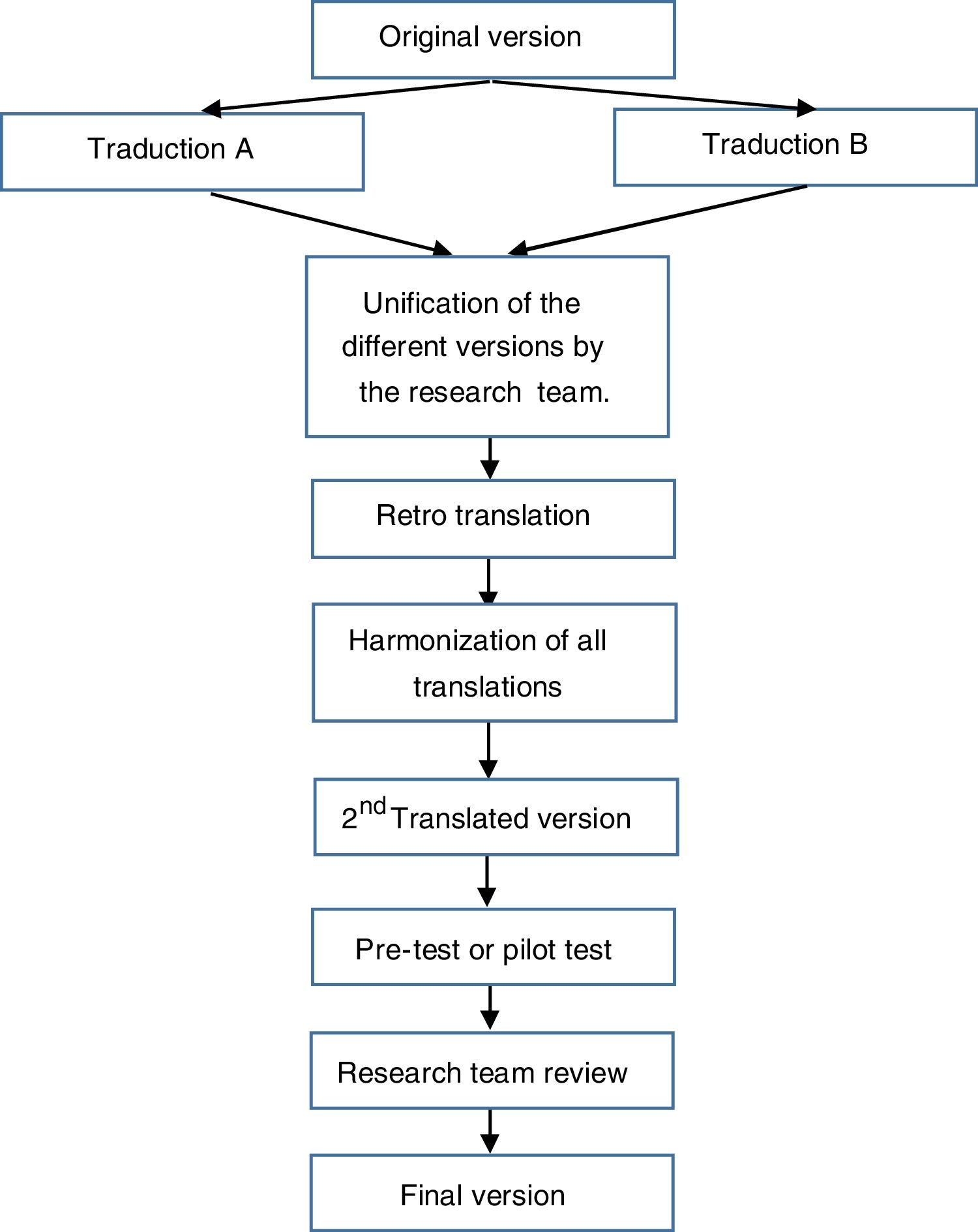
Questionnaire translation and descriptionFor the translation of the questionnaire, the principles of good practices for the translation and cultural adaptation process made by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR)28 Working Group were taken into account. The stages for the translation were (see Fig. 1):
- (a)
Initial preparation and obtaining the authors’ permission.
- (b)
Translation of the original questionnaire into the target language by two independent translators working in the field of health and research (psychologists and nurse).
- (c)
Unification of the different versions by the research team.
- (d)
Translation of this version back to the original language by two native English translators.
- (e)
Comparison and review of the different versions with the original questionnaire by the research team.
- (f)
Harmonization of all translations in order to guarantee conceptual equivalence.
- (g)
Pilot test: the translated questionnaire was administered to 10 patients for their evaluation. A support text was included in order to collect any doubts that could be raised with any question regarding comprehension and writing, and to prove the global assessment of the questionnaire by patients.
- (h)
The results of the pilot test were analyzed and the translation was finalized by the research team.
- (i)
Editing the questionnaire.
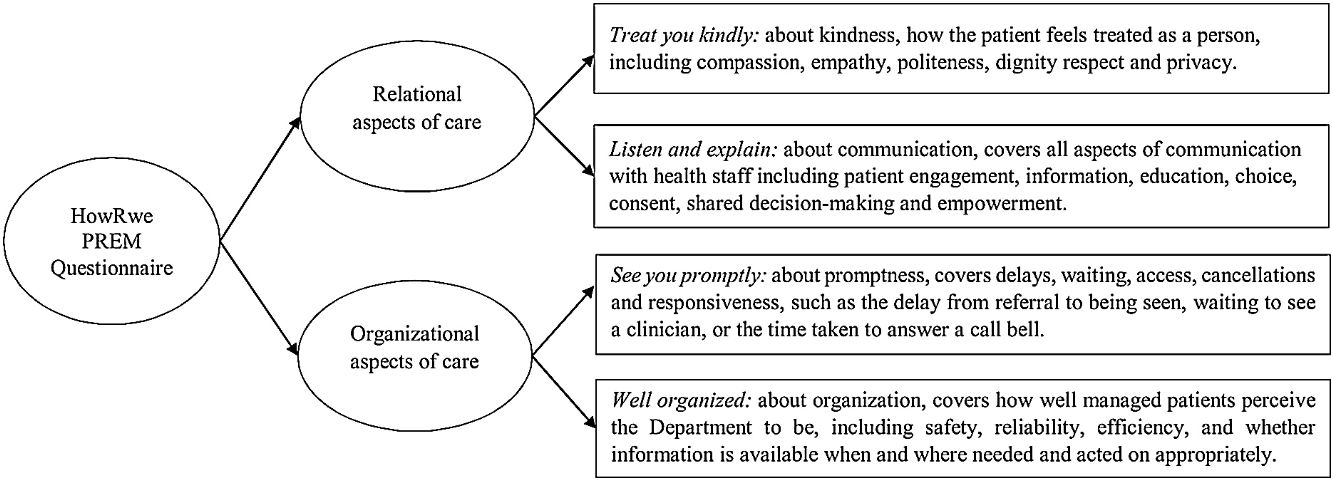
The HowRwe questionnaire has a core question (How are we doing) and four items. The items are short and inclusive, rather than restrictive. The items cover four aspects of experience:
- -
Item 1: Treat you kindly (kindness) covers how you are treated as a person including compassion, empathy, emotional support, politeness, dignity, respect and privacy.
- -
Item 2: Listen and explain (communication) covers all aspects of communication with health staff including patient engagement, information, education, choice, consent, shared decision-making and empowerment.
- -
Item 3: See you promptly (promptness) covers delays, waiting, access, cancelations and responsiveness, such as the delay from referral to being seen, waiting to see a clinician, or the time taken to answer a call bell.
- -
Item 4: Well organized (organization) covers how well managed patients perceive the unit to be, including safety, reliability, efficiency, and whether information is available when and where needed and acted on appropriately.
The response alternative is a 4-point Likert scale: Excellent (3), Good (2), Fair (1) and Poor (0). For analysis and reporting, each response level for each item is allocated a score on a 0–3 scale. The summary HowRwe score is calculated for individual respondents by adding the scores for each item, giving a scale with 13 possible values from the minimum, 0 (4×poor) to the maximum, 12 (4×excellent).
The evaluation protocol was approved by the Ethics Committee (CEICm) at the Hospital Universitario La Princesa (Registration No. 5210/23).
Data collectionSeveral sociodemographic and clinical data were collected (Table 1).
Measured variables.
| Socio-demographic variables |
| Age |
| Sex |
| Civil status |
| Employment situation |
| Clinical variables |
| Principal diagnostic |
| Dyspnea at the time of the visit (measured with mMRC) |
| Last lung function in stable phase (last 3 months) |
| FVC, absolute percent predicted |
| FEV1, the percentage predicted |
| FEV/FVC, in percentage % |
| DLCO, in percentage % |
| IAH |
| Oxygen saturation at the time of the visit (baseline) |
| BMI, weight and height |
| Other |
| Year in which the diagnosis was made |
| Years of follow-up in the Pulmonology External Consultation |
| Treatment variables (home respiratory therapy) |
| Type of home respiratory therapy prescribed |
| CPAP |
| Oxygen therapy (concentrator, portable, both) |
| Mechanical ventilation |
| Aerosol therapy |
| Adherence |
| Experience with home respiratory therapy |
| Other |
| Perception of the adequacy of the questionnaire |
| Preferred channels to answer these questions |
FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; FEV/FVC: ratio of the forced expiratory volume in the first one second; DLCO: diffusing capacity carbon monoxide; AHI: Apnea-Hypopnea Index; BMI: body mass index; CPAP: continuous positive airway pressure.
In addition to the HowRwe questionnaire, quality of life was measured, using the Euroqol-5D-5L.29
Patients who attended the external consultations at the Pulmonology Department of the La Princesa University Hospital, after their consultation, were invited to participate in the study. Once they signed the informed consent, patients responded to the questionnaires. At the 6-month follow-up visit, patients again answered the HowRwe and Euroqol-5D-5L questionnaires. In this way we obtained the psychometric properties of the questionnaire in two stages (pre- and post-test). Although a specific intervention was not planned, the treatment received by each patient was recorded.
Data analysisFirst, descriptive statistics were calculated. Then, descriptive and reliability statistics for single items and the total score of the Spanish version of the HowRwe scale in pre- and post-test were calculated. These included mean, standard deviation, minimum and maximum scores, and internal consistency estimates for individual items (homogeneity and alpha if item deleted) and the scale (Cronbach's alpha and McDonald's omega).
For the study of the internal structure, a confirmatory factor analysis was hypothesized, estimated and tested in data from pre-test and post-test. The model hypothesized a one factor of general health-related quality of life and patient experience which explained the four items of the scale. To assess the model fit, several criteria were used: the chi-square statistic, the comparative fit index (CFI), and the standardized root mean square residual (SRMR). The root mean square error of approximation (RMSEA) was not used, as it has shown poor performance in structural models with few degrees of freedom. CFI values above .90 (better over .95) and SRMR values below .08 (better under .06) are considered indicative of good fit.30,31
Finally, we studied the relations between pre- and post-intervention scores in the Spanish version of the HowRwe scale and sociodemographic clinical variables. To test the relation of pre- and post-test scores with age, adherence and quality of life, Pearson's correlations were used. To test the relation with categorical variables, which included sex, marital status, diagnosis and therapy, analysis of variance (ANOVA) was calculated.
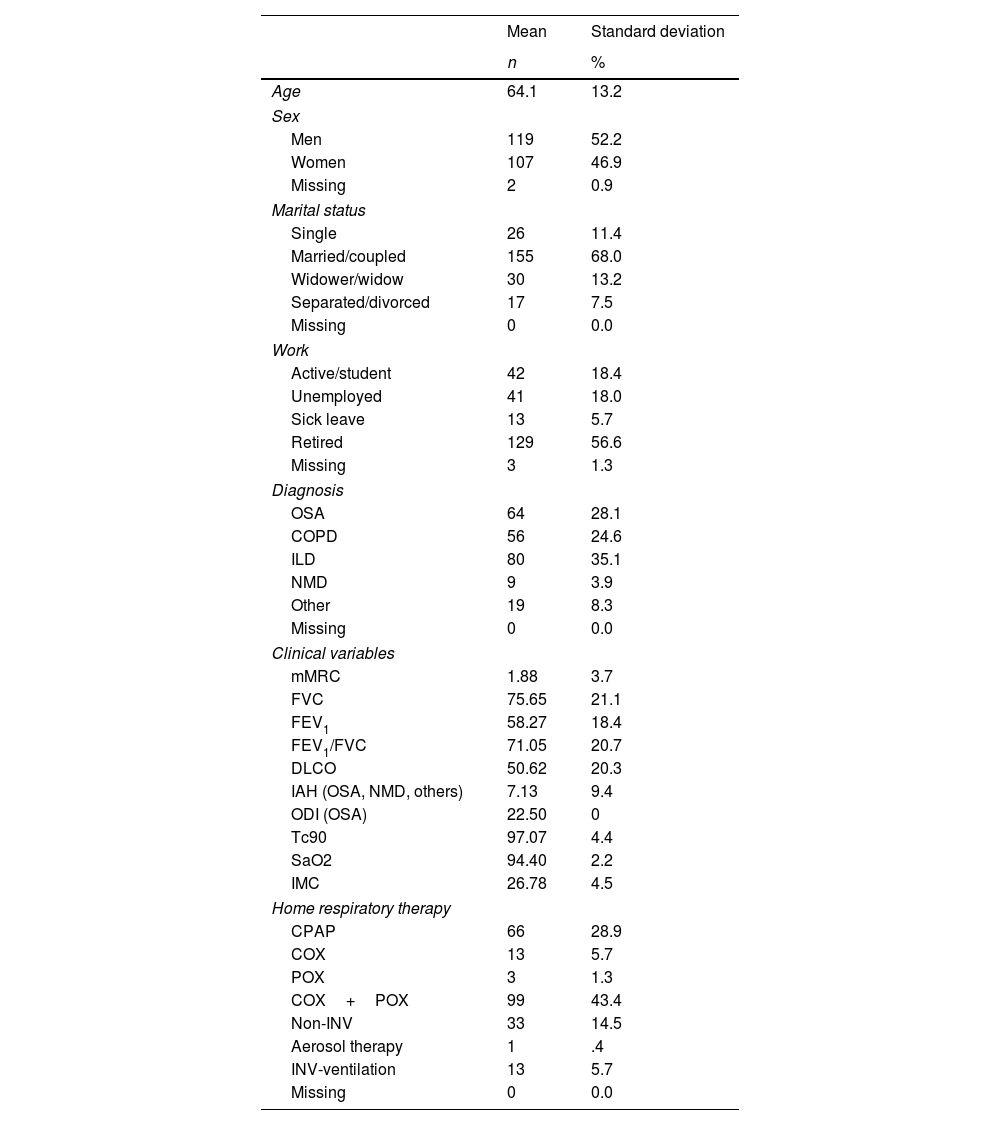
ResultsTwo hundred and twenty-eight participants were included in the study. 52.2% (n=109) were men. Mean age was 64.07 years (SD=13.2). Most of the participants were married or coupled (68.0%, n=155) and retired (56.6%, n=129). Regarding the diagnosis, 35.1% (n=80) were diagnosed with ILD, 28.1% OSA (n=64), 24.6% (n=56) COPD, 3.9% (n=9) NMD, and 8.3% (n=9) had other conditions, including CF, bronchiectasis and asthma. Regarding therapies, patients mostly used COX (oxygen therapy concentrator) and POX (oxygen therapy portable concentrator) (43.4%, n=99), CPAP (continuous positive airway pressure) (28.9%, n=66) and non-invasive ventilation (14.5%, n=33). More details are presented in Table 2.
Demographic and clinical characteristics of patients.
| Mean | Standard deviation | |
|---|---|---|
| n | % | |
| Age | 64.1 | 13.2 |
| Sex | ||
| Men | 119 | 52.2 |
| Women | 107 | 46.9 |
| Missing | 2 | 0.9 |
| Marital status | ||
| Single | 26 | 11.4 |
| Married/coupled | 155 | 68.0 |
| Widower/widow | 30 | 13.2 |
| Separated/divorced | 17 | 7.5 |
| Missing | 0 | 0.0 |
| Work | ||
| Active/student | 42 | 18.4 |
| Unemployed | 41 | 18.0 |
| Sick leave | 13 | 5.7 |
| Retired | 129 | 56.6 |
| Missing | 3 | 1.3 |
| Diagnosis | ||
| OSA | 64 | 28.1 |
| COPD | 56 | 24.6 |
| ILD | 80 | 35.1 |
| NMD | 9 | 3.9 |
| Other | 19 | 8.3 |
| Missing | 0 | 0.0 |
| Clinical variables | ||
| mMRC | 1.88 | 3.7 |
| FVC | 75.65 | 21.1 |
| FEV1 | 58.27 | 18.4 |
| FEV1/FVC | 71.05 | 20.7 |
| DLCO | 50.62 | 20.3 |
| IAH (OSA, NMD, others) | 7.13 | 9.4 |
| ODI (OSA) | 22.50 | 0 |
| Tc90 | 97.07 | 4.4 |
| SaO2 | 94.40 | 2.2 |
| IMC | 26.78 | 4.5 |
| Home respiratory therapy | ||
| CPAP | 66 | 28.9 |
| COX | 13 | 5.7 |
| POX | 3 | 1.3 |
| COX+POX | 99 | 43.4 |
| Non-INV | 33 | 14.5 |
| Aerosol therapy | 1 | .4 |
| INV-ventilation | 13 | 5.7 |
| Missing | 0 | 0.0 |
OSA: obstructive sleep apnea; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; NMD: neuromuscular; CPAP: continuous positive airway pressure; COX: oxygen therapy concentrator; POX: oxygen therapy portable concentrator; Non-INV: non-invasive ventilation; INV-ventilation: invasive ventilation.
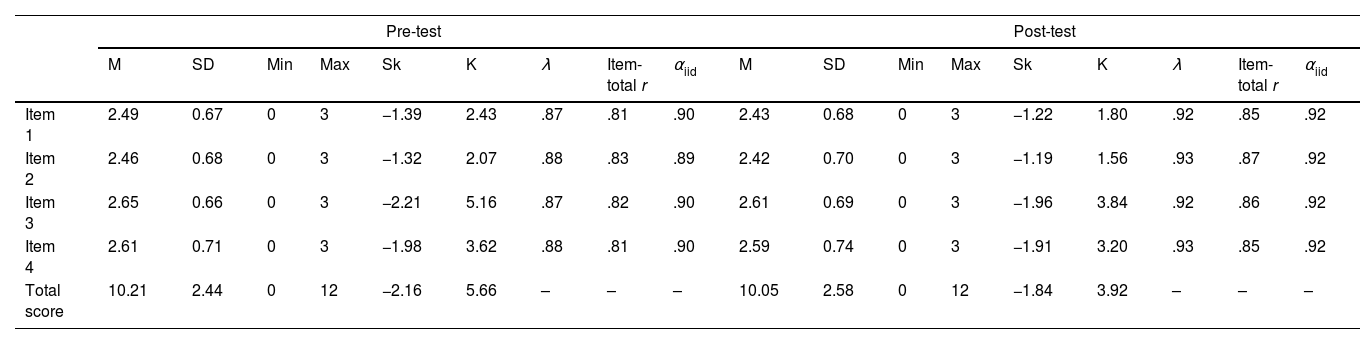
Descriptive statistics of the Spanish version of the HowRwe scale, for both pre- and post-intervention, can be consulted in Table 3. All the values for univariate skewness and kurtosis for all the variables analyzed were satisfactorily within conventional criteria for normality (−3 to 3 for skewness and −10 to 10 for kurtosis), according to the guidelines suggested by Kline.26 Higher scores were found in item 3 and item 4, both in pre- and post-intervention, whereas lower values were presented in items 1 and 2. All of them were around 2.5, meaning patients’ experience was rated between good and excellent.
Descriptive statistics and reliability estimates for the Spanish version of the HowRwe scale.
| Pre-test | Post-test | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | Sk | K | λ | Item-total r | αiid | M | SD | Min | Max | Sk | K | λ | Item-total r | αiid | |
| Item 1 | 2.49 | 0.67 | 0 | 3 | −1.39 | 2.43 | .87 | .81 | .90 | 2.43 | 0.68 | 0 | 3 | −1.22 | 1.80 | .92 | .85 | .92 |
| Item 2 | 2.46 | 0.68 | 0 | 3 | −1.32 | 2.07 | .88 | .83 | .89 | 2.42 | 0.70 | 0 | 3 | −1.19 | 1.56 | .93 | .87 | .92 |
| Item 3 | 2.65 | 0.66 | 0 | 3 | −2.21 | 5.16 | .87 | .82 | .90 | 2.61 | 0.69 | 0 | 3 | −1.96 | 3.84 | .92 | .86 | .92 |
| Item 4 | 2.61 | 0.71 | 0 | 3 | −1.98 | 3.62 | .88 | .81 | .90 | 2.59 | 0.74 | 0 | 3 | −1.91 | 3.20 | .93 | .85 | .92 |
| Total score | 10.21 | 2.44 | 0 | 12 | −2.16 | 5.66 | – | – | – | 10.05 | 2.58 | 0 | 12 | −1.84 | 3.92 | – | – | – |
M: mean; SD: standard error; Min: minimum score; Max: maximum score; Sk: skewness; K: kurtosis; αiid: alpha if item deleted.
Reliability coefficients of the scale were adequate, with α=.921 and Ω=.929 for pre-test, and α=.940 and Ω=.958 for post. Items’ evidence of reliability showed adequate estimates of item-total correlations and reliability: item-total correlations were in the range of .81 to .83 in pre-intervention, and .85 to .87 in post-intervention, well above the acceptable minimum of .3032 and the removal of any of the items supposed a decrease in the reliability estimate of the scale (see Table 3).
The confirmatory factor analysis tested for both times, pre- and post-intervention, showed an excellent overall fit: χ2(2)=49.380 (p<.001), CFI=.941 and SRMR=.072; and χ2(2)=37.579 (p<.001), CFI=.982 and SRMR=.046, respectively. Regarding the analytical fit, models were also excellent: statistically significant factor loadings were found (p<.001), ranging from .87 to .88, and .92 to .93, in pre- and post-intervention times, respectively (see Table 3). In sum, the model with one factor was considered an adequate representation of the data.
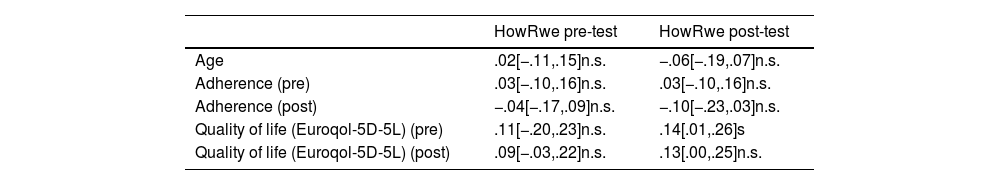
Finally, we studied associations between pre- and post-test scores in the Spanish version of the HowRwe scale with sociodemographic and clinical variables. As shown in Table 4, no statistically significant relations were found between them and age, adherence or quality of life, except between HowRwe post-test and quality of life pre-test (r=.14 [.01,.26]; p=.035).
Correlation between scores in the Spanish version of the HowRwe scale, age, quality of life and adherence, both pre-test and post-test.
| HowRwe pre-test | HowRwe post-test | |
|---|---|---|
| Age | .02[−.11,.15]n.s. | −.06[−.19,.07]n.s. |
| Adherence (pre) | .03[−.10,.16]n.s. | .03[−.10,.16]n.s. |
| Adherence (post) | −.04[−.17,.09]n.s. | −.10[−.23,.03]n.s. |
| Quality of life (Euroqol-5D-5L) (pre) | .11[−.20,.23]n.s. | .14[.01,.26]s |
| Quality of life (Euroqol-5D-5L) (post) | .09[−.03,.22]n.s. | .13[.00,.25]n.s. |
n.s.: not statistically significant; s: statistically significant.
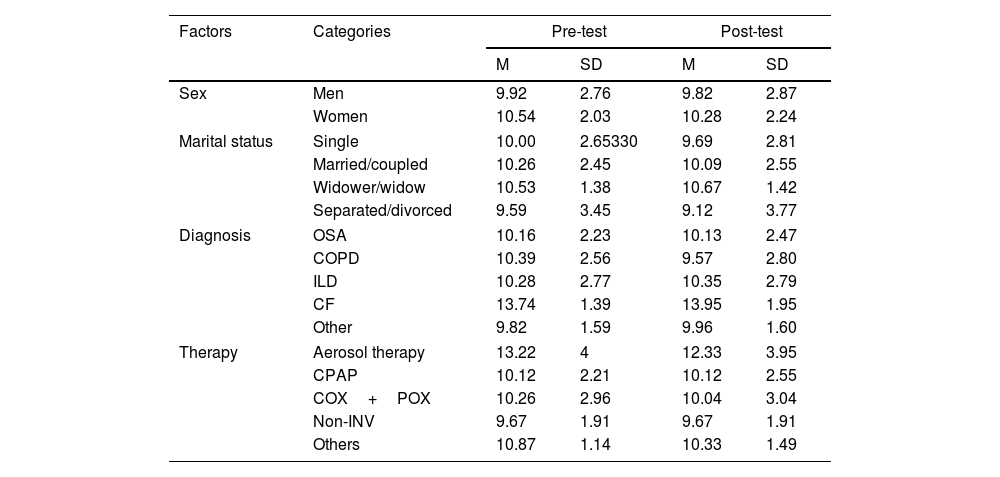
Regarding sex differences, no significant differences were found for pre-test (F(1, 224)=3.607; p=.059; η2=.016) or post-test (F(1, 224)=1.755; p=.187; η2=.008). Descriptive statistics on HowRwe scores for men and women are presented in Table 5. Regarding marital status, no differences were found, neither in pre-test (F(1, 224)=0.622; p=.601; η2=.008) nor in post-test (F(1, 224)=1.495; p=.217; η2=.020).
Descriptive statistics in the Spanish version of the HowRwe scale for sociodemographic and clinical groups, both pre-test and after it.
| Factors | Categories | Pre-test | Post-test | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Sex | Men | 9.92 | 2.76 | 9.82 | 2.87 |
| Women | 10.54 | 2.03 | 10.28 | 2.24 | |
| Marital status | Single | 10.00 | 2.65330 | 9.69 | 2.81 |
| Married/coupled | 10.26 | 2.45 | 10.09 | 2.55 | |
| Widower/widow | 10.53 | 1.38 | 10.67 | 1.42 | |
| Separated/divorced | 9.59 | 3.45 | 9.12 | 3.77 | |
| Diagnosis | OSA | 10.16 | 2.23 | 10.13 | 2.47 |
| COPD | 10.39 | 2.56 | 9.57 | 2.80 | |
| ILD | 10.28 | 2.77 | 10.35 | 2.79 | |
| CF | 13.74 | 1.39 | 13.95 | 1.95 | |
| Other | 9.82 | 1.59 | 9.96 | 1.60 | |
| Therapy | Aerosol therapy | 13.22 | 4 | 12.33 | 3.95 |
| CPAP | 10.12 | 2.21 | 10.12 | 2.55 | |
| COX+POX | 10.26 | 2.96 | 10.04 | 3.04 | |
| Non-INV | 9.67 | 1.91 | 9.67 | 1.91 | |
| Others | 10.87 | 1.14 | 10.33 | 1.49 | |
OSA: obstructive sleep apnea; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; CF: cystic fibrosis; NMD: neuromuscular; CPAP: continuous positive airway pressure; COX: oxygen therapy concentrator; POX: oxygen therapy portable concentrator; Non-INV: non-invasive ventilation; INV-ventilation: invasive ventilation.
Because of the small sample size of NMD group (n=9), for the study of the relation between HowRwe scores and diagnosis categories were recoded. NMD patients were added to the “Other” category. With the recoded variable, an analysis of variance was conducted, pointing no statistically significant differences in pre-test (F(1, 224)=0.366; p=.778; η2=.005) and post-test (F(1, 224)=1.028; p=.381; η2=.014).
The effect of therapy on HowRwe scores was also studied. Again, because of the small sample size of some of the groups, we recoded COX, POX, Aerosol therapy and invasive ventilation into the new category of “Other”. When differences among therapies (including CPAP (n=66), COX+POX (n=99), non-invasive ventilation (n=33) and Other (n=30)) were studied, no differences in pre-test (F(1, 224)=1.314; p=.270; η2=.017) or post-test (F(1, 224)=0.376; p=.770; η2=.005) were found. Descriptive statistics for the different groups are shown in Table 5.
Finally, regarding the evaluation that patients made of the questionnaire, 85.6% found the content of the questionnaire “Very useful” or “Quite useful.” 98.6% rated the questionnaire as “Very easy to understand.” Regarding the length of the questionnaire, 98.7% found it “Very adequate” or “Quite adequate” because it is a short questionnaire. Regarding the time spent, 95.6% of the patients assessed that they needed “Very little time” or “Little time” to respond. Finally, when asked what would be the best way to respond to the questionnaire (channel), by main diagnosis, patients diagnosed with OSA responded that their preference was paper (37.5%) and app (31.5%). Patients with COPD prefer paper (71.4%), patients with ILD both paper (43.7%) and app (41.2%), patients with CF and bronchiectasis prefer email (42.1%) and patients diagnosed with NMD diseases prefer the app (44.4%), followed by email (22.2%) and SMS (22.2%).
DiscussionThe Spanish version of the HowRwe questionnaire to measure the experience of respiratory patients receiving or not home respiratory therapies, presents adequate psychometric properties and conceptual and semantic equivalence with the original English version. This study is the first approach to assess the self-perceived experience in respiratory patients in Spain with a specific questionnaire that includes multiple areas beyond satisfaction.
Among the benefits found when measuring the patient experience, we find the ability to improve the care circuit, ensuring that it is more effective and efficient. Including the patient perspective in healthcare systems is proven to be a crucial enabler for change, transformation and sustainability.33
It has been observed that health-related quality of life is related to the gap between our expectations of health and our experience of it.34 The perception of quality of life varies between individuals and is dynamic, and the experience with care influences our perception of our quality of life.35 In this validation study, a statistically significant correlation was found between the post-test experience and the pre-test quality of life. The intervention between the two measurement times (pre and post) has been the standard of care. Possibly this relationship found is explained, given a better state of quality of life, the experience with the service is better, due to the connection between the care plan and the quality of life. Although we have not found a relationship between bad experience and poor quality of life, this does not mean that patients can make negative attributions about their quality of life because their experience with care (not feeling heard, not being cared for when it is considered necessary) is bad.
Although some attempts have been made to measure the experience in respiratory patients,20,36 this is a PREM that can be used transversally to all respiratory conditions either with or without HRT. This allows comparison between the different consultations of the Pulmonology Department, and helps decision-making in order to improve the care plan individually (monographic consultation) and generally (the entire service). We are therefore faced with the first generic PREM validated for respiratory patients, with good psychometric properties and rated by patients themselves as simple and easy to apply. Its application allows it to be adapted to the patient's preferred channel so that it can be answered and thus facilitate its completion.
The measurement of the patient's experience with health care includes multiple aspects that may escape a questionnaire. The experience measurement process must include mechanisms that help understand the responses, and that allow the agreed actions to improve the experience to take into account the appropriate aspects. Although HowRwe is a questionnaire that covers three important areas (relational and organizational), there may be other factors that might escape patient follow-up.
Some limitations of our research might be considered. It has been validated in a single hospital, with a specific organization and a specific care circuit. It might be of interest to explore if the questionnaire is sensitive to different organizations, so it would demonstrate greater usefulness for comparing care models.
Although the aim of this study was to determine the psychometric validation of the HowRwe questionnaire in Spanish and respiratory patients, it would be necessary to know what occurs with each item evaluated in the real-world, in order to know and establish a guide for the use of this PREM in the improvement of services. In this way, we will be able to impact the quality of life of patients by providing care through a care plan that covers not only the patient's expectations, but also those of the service itself and the health professionals.
ConclusionsThe PREM ‘HowRwe’ presents adequate psychometric properties in patients with respiratory disease with home respiratory therapies, with good reliability indices, both in the exploratory (Cronbach's alpha) and in the confirmatory (CRI). It is a questionnaire with a 4-point Likert scale, which the patient can complete quickly and which has shown good reliability and validity scores in psychometric tests. Acceptance by patients has been very positive, so it is expected that its implementation in the care routine will provide useful information to improve the patient care experience, since the questionnaire allows it to be used transversally among all monographic units of the Department of Pulmonology.
Authors’ contributionsJulio Ancochea, David Rudilla and Pedro Landete have defined the study. David Rudilla and Pedro Landete have been in charge of the request for ethical approval of the study by the CEICm. Tamara Alonso, Elena García, Patricia Pérez, Claudia Valenzuela, Rosa Girón, Enrique Zamora and Pedro Landete have been in charge of presenting the study to the patients and inviting them to participate, as well as coding each response notebook. These authors have also participated in the revision of the first manuscript, as well as the necessary corrections in successive versions. The statistical analyzes have been carried out by David Rudilla and Laura Galiana reviewed by Julio Ancochea and Pedro Landete. The final version of the manuscript has been finished by Joan Soriano and David Rudilla.
FundingThe authors declare that no funding has been received for the completion of this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.