In September 2004, Alexander Babiak, an interventional pulmonologist from Ulm (Germany), used transbronchial cryobiopsy (TBCx) technique for the first time in 7 patients with interstitial lung disease (ILD).1 Since then, multiple studies have been reported with varying results on diagnostic yield and complication rates associated with this technique.
In this descriptive retrospective study, we will provide information about patient characteristics, diagnostic yield and complication rates of all TBCx performed in the first 51 patients, between April 2014 and August 2016, at the 12 de Octubre University Hospital, for the diagnosis of ILD or suspected rejection post lung transplantation (LT), approved by the Ethics Committee under number 13/202.
Patients were required to have a chest computed tomography (CT) within a month prior to the procedure, in those patients with typical features of usual interstitial pneumonia (UIP) on CT, TBCx was not performed. Exclusion criteria for TBCx were: bleeding diathesis, thrombocytopenia (<50×109/L) and severe respiratory functional impairment.2 After informed consent regarding diagnostic yield and risks of TBCx and an evaluation of the anesthesiological risk, patients scheduled for TBCx were admitted to the department the same day of TBCx and thereafter discharged in <24h if they presented with clinical and radiological stability.
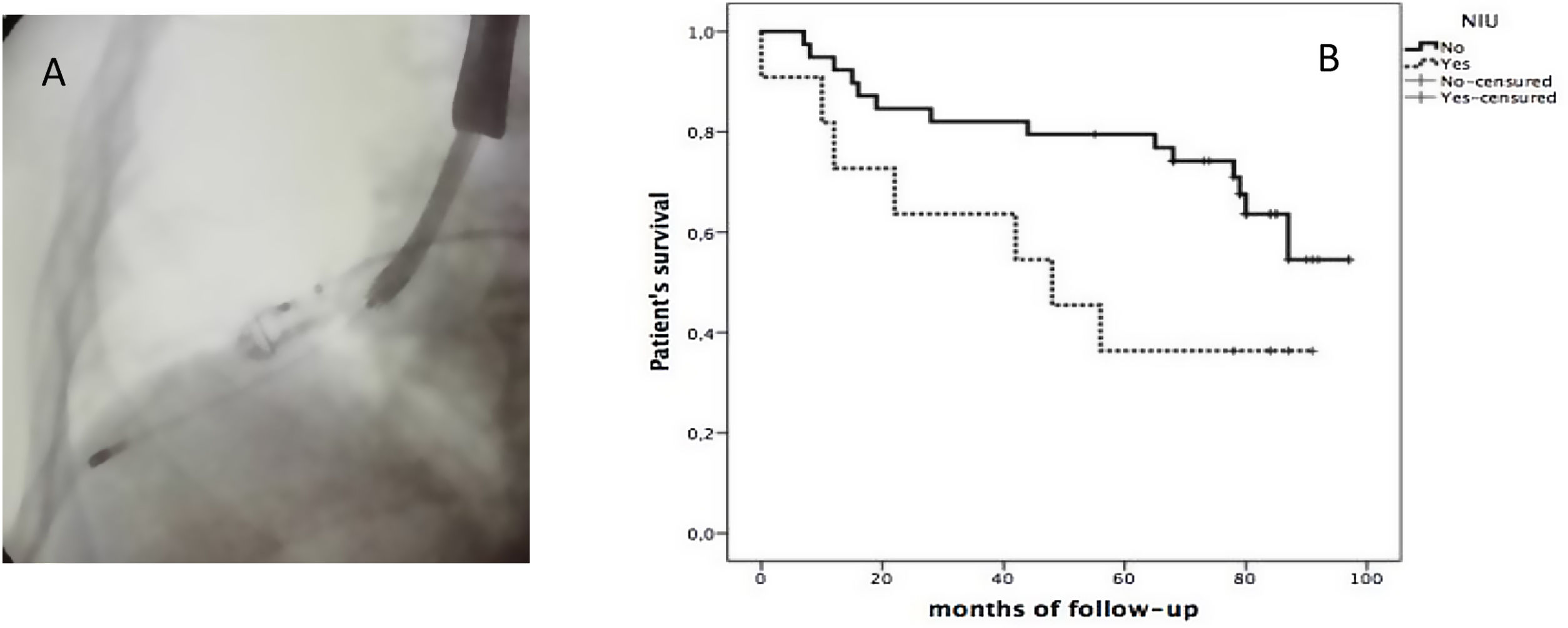
All were performed using rigid bronchoscopy (RB) by “Effer Dumon”. Following intubation, an Olympus balloon was placed in the selected bronchus (when the target was located in the upper lobes, placement was achieved with the help of a biopsy forceps). Then a cryoprobe (1.9 or 2.4mm size, depending on the preference of the endoscopist, Erbokryo® CA, 10448-000 model ERBE Elektromedizin, Tübingen, Germany) was introduced via the working channel of the therapeutic videobronchoscope (VBC) (Olympus® BF-190) and under fluoroscopic was positioned at a distance of 10–20mm from the pleura (Fig. 1A), then it was cooled for approximately 3–5s, retracted together en bloc and the balloon was inflated. Biopsies was taken from one or more of the most affected segments. It was repeated to obtain at least 3 adequate biopsies. All biopsies were evaluated by a pathologist specialized in Pulmonology.
Mild bleeding was considered if it resolved with simple aspiration, moderate if it was necessary to apply cold saline and/or chemical vasoconstrictor, or severe if selective intubation was necessary and/or admission to intensive care.
Patients with non-definite patterns or inconclusive histological results were discussed in a multidisciplinary meeting, in order to obtain consensus on diagnostic.
The statistical analysis was performed with the program SPSS v. 20.0 for Mac. Statistical significance a p value<0.05. Patient survival was drawn by the Kaplan–Meier method and different groups were compared with the Log Rank test.
A total of 51 patients (26 men (51%)), mean age 63 years (range 56–71) underwent TBCx at our institution, with median diffusing capacity for lung carbon monoxide 66% (51–76). The overall diagnostic yield was of 78.4% (40 patients). The most frequent pathology was UIP (12, 30% of total) followed by organizing pneumonia (OP) 15%, and sarcoidosis 8%. In 3 patients a repeat TBCx was performed, due to lack of conclusive diagnosis following the first sampling, being diagnostic after second one. Furthermore, TBCx were performed in 5 patients with suspected rejection post LT, rejection was confirmed in 2 patients (33%).
Of the 11 non-diagnostic samples; 4 biopsy specimens demonstrated exclusively bronchial wall histology (in 1 of them a second TBCx was performed with a diagnosis of UIP) and 7 samples showed parenchyma without pathology. Two of these underwent surgical biopsy; one turned out to be UIP and the other sample revealed emphysema. The other 8 patients were followed up clinically and radiologically to ascertain a final diagnosis.
Four (8%) developed a pneumothorax, all of them required chest drainage. One of these patients had an acute exacerbation with subsequent clinical worsening and respiratory failure, resulting in death at 13 days following TBCx (showed a histological UIP pattern, that was associated with a Sjögren's syndrome).
Mild bleeding occurred in 7 patients (14%), moderate in 3 patients (6%) and no severe.
The follow up of these 51 patients evaluated after 8 years from the beginning of using TBCx: 30 patients (59%) were alive as of June 2022, and 21 patients died. Median overall survival was 78 months (range 44–88 months). Overall survival of those with a histological UIP pattern based on TBCx sampling was 36% at 90 months, with worse prognosis compared to the 55% for those without UIP histology (Fig. 1B).
The most recent clinical practice guidelines on the diagnosis of idiopathic pulmonary fibrosis (IPF) refer to the use of TBCx and the challenges of obtaining appropriate material for the histopathological criteria for UIP. The limitations are particularly due to the subpleural predominance of pathologic changes and the potential for sampling errors resulting in less confident exclusion of features that may suggest an alternative diagnosis, compared with surgical lung biopsy (SLB).3
In fact, TBCx is more likely to demostrate a probable UIP pattern than a definite UIP pattern given the limited sampling of subpleural lung parenchyma in most cases.4
Comparing diagnostic yields between major studies: MULTICRIO compared between classic transbronchial biopsies and TBCx in 124 subjects and according to histopathology results the diagnostic yield was higher for TBCx (55%) vs 19% (p<0.0001),5 other with 77 patients randomized to TBCx or forceps biopsy, were statistically significant differences: 74% for TBCx vs 34%,6 69 patients: 76%7 and 297 patients: 86%.8
Mild to moderate bleeding is the most frequent complication, with reported rates as high as 78%.2,9 Time greater than 4s with a larger size cryoprobe was considered as a risk factor according to a recent report.10 Pneumothorax rates vary between 0% and 28%7,11–13 risk factors are the histological pattern of UIP, reticulation on CT and/or biopsies close to the pleura.2
While TBCx has yet not been established as the gold standard, cost and complications are lower than SLB, in the presence of a high concordance of the diagnostic utility.4
Authors’ contributionsAll authors have made substantial contributions.
All authors had worked to make possible this article which had supported very important aspects from the suspect of ILD or LT rejection to final diagnosis and treatment.
Informed consentThe authors confirm that the patient's written consent has been obtained.
FundingThe authors declare that they have not received any fees or funding for the development of the article.
Conflicts of interestThe authors declare that they have no known competing financial interests.