Human Immunodeficiency Virus (HIV)-positive patients treated with the antiretroviral drug abacavir (ABC) may develop a potentially fatal ABC-associated hypersensitivity syndrome (ABC-HS), typically characterized by fever, malaise, rash, vomiting/diarrhoea and/or dyspnoea/cough. ABC-HS has been strongly associated with HLA-B*57:01 carriage and screening for this allele is recommended.
ObjectiveTo determine the prevalence of HLA-B*57:01 and to characterize suspected ABC-HS in the adult HIV population from our hospital during a 7-year period.
MethodsClinical data on patients under ABC treatment from January 2006 to December 2012 were analyzed to search for symptoms of ABC-HS. Reactions of suspected ABC-HS were characterized. HLA-B*57:01 and patch tests (1% and 10% ABC in petrolatum) with readings at 48h were performed in those without previous testing. From January 2008 routine HLA-B*57:01 screening was implemented.
ResultsFrom January 2006 to December 2007, 186 patients began treatment with ABC (data from 163 were available): 7 (4%) patients stopped ABC for suspected ABC-HS (71% males, median age 45 years) and the median time for onset of the reaction after starting ABC was 7 days. Four of the 7 patients had the HLA-B*57:01 allele and 2 of these 4 had positive patch tests. After HLA-B*57:01 screening implementation (January 2008), 573 patients were evaluated and 35 (6.1%) were HLA-B*57:01 positive; no suspected ABC-HS were observed since then.
ConclusionFour patients with suspected ABC-HS (of 6 screened) were HLA-B*57:01 positive. No ABC-HS occurred since January 2008, after HLA-B*57:01 screening was implemented.
The frequency of drug-related exanthemas in Human Immunodeficiency Virus (HIV)-positive patients is estimated to be 100 times higher than in the general population.1 The increase in adverse drug reactions is most likely multifactorial in origin, including immune hyperactivation and changes in cytokine profiles, oxidative stress and drug metabolism, and also a genetic predisposition.1 HIV itself may work as a danger signal, leading to the development of an immune response instead of tolerance.1
There are some obstacles to the diagnosis of drug hypersensitivity in HIV-infected patients, one of the most important concerns the multiple medication regimens used. In fact, antiretroviral agents (along with antibiotics used to treat opportunistic infections) are frequent agents of hypersensitivity reactions.2 One such agent with particular importance in that context is abacavir (ABC), a nucleoside reverse transcriptase inhibitor (NRTI) which is frequently used in treatment-naïve patients and whose hypersensitivity reactions occur at a frequency ranging from 2% to 9%; this is an intermediate frequency compared to that of adverse reactions reported to other NRTI drugs, such as emtricitabine (17%) and zidovudine (rare).3–6
The abacavir-associated hypersensitivity syndrome (ABC-HS) is a distinct and specific clinical syndrome defined by systemic involvement, and with an estimated incidence of 8% and a mortality rate of 0.03%.7,8 The ABC-HS is clinically characterized by at least 2 of the following manifestations typically occurring within 6 weeks after drug introduction – constitutional symptoms (fever, malaise, lethargy, headache, and myalgia), rash, gastrointestinal symptoms (nausea, vomiting, and diarrhoea), and/or respiratory symptoms (dyspnoea, and cough).6,9
The potential severity of ABC-HS contraindicates drug re-challenge. In this regard, patch tests with abacavir may be a useful diagnostic tool, although the procedures for patch testing with this drug are not yet standardized nor are the non-irritant drug concentrations fully established. The rationale for patch testing in patients with acute drug-induced hypersensitivity syndromes is the eventual metabolism of the parent drug to a reactive metabolite in skin and the identification of resident CD8+ cells in skin biopsies of such patients.10
Hypersensitivity reactions to ABC have been strongly associated with the HLA-B*57:01 allele.11 The prevalence of the HLA-B*57:01 seems to be variable among different populations and to be the highest in the European white population (about 7%).9,12 A large multicentric clinical trial from the PREDICT-1 study team established the effectiveness of prospective HLA-B*57:01 screening in preventing the ABC-HS, with positive and negative predictive values of 47.9% and 100%, respectively.13 This determined, since 2008, the recommendation of routine screening of the allele prior treatment with ABC, eventually limited by its availability and cost, depending on the region. On the other hand, the cost-effectiveness of the screening will lean on several estimates, namely the availability of appropriate laboratory assays, the population studied and the health care setting.13,14
To the authors knowledge no previous studies have evaluated the impact of HLA-B*57:01 routine screening in the prevalence of ABC-HS in Portuguese HIV-positive patients. Our aim was to determine the prevalence of HLA-B*57:01 carriage in the adult HIV population followed in our hospital and to characterize suspected ABC-HS during a 7-year period, before and after introduction of routine HLA-B*57:01 screening.
MethodsThe study has been conducted in a tertiary hospital, in the city of Porto, located in Northern Portugal. In our hospital, routine HLA-B*57:01 screening prior to treatment with ABC has been implemented since January 2008.
We first identified HIV-positive patients that underwent ABC treatment at some point between January 2006 and December 2007, consulting the dispensing records from the hospital pharmacy (group A). We also identified all HIV-positive patients who had an indication to start ABC therapy from January 2008 to December 2012 (group B); these patients had been previously screened for HLA-B*57:01 carriage (by real-time polymerase chain reaction).
Medical records of the patients were then reviewed. For patients from both groups, we identified those with any symptoms suggestive of ABC-HS (fever, constitutional symptoms, exanthema, gastrointestinal tract symptoms, and/or respiratory symptoms) and the characteristics of the reaction were recorded. Information regarding HLA-B*57:01 carriage status was also retrieved for patients of both groups.
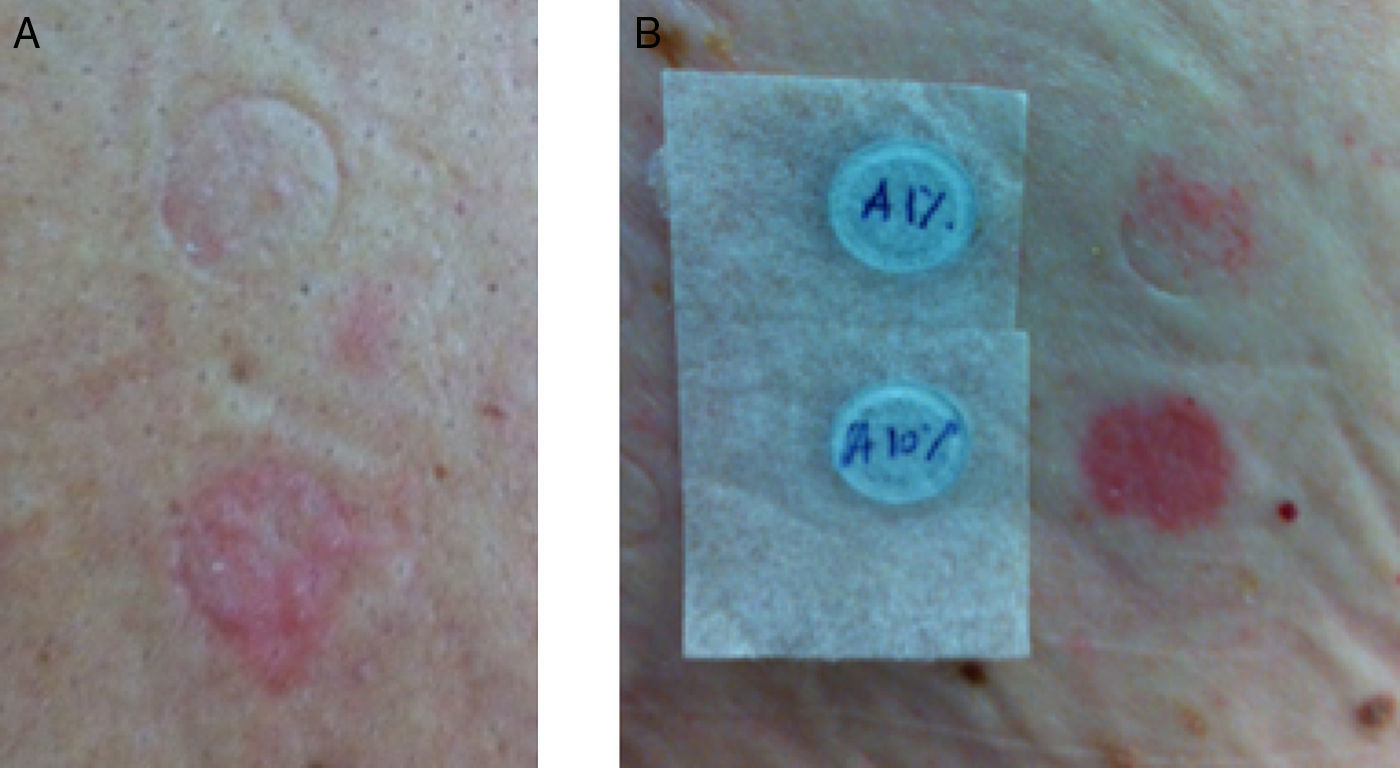
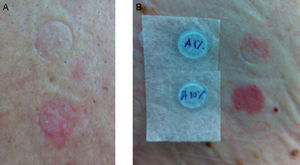
Patients with suspected ABC-HS were called to undergo patch testing with ABC. Patch tests were performed between January and July 2013, with 300mg ABC-tablets crushed in petrolatum at 1% and 10% concentrations, prepared in our Pharmacy Department, with readings at 48h and 72h.10 Positive results on patch tests were graded according to the International Contact Dermatitis Research Group: (+) mild reaction, possible erythema, infiltration and papules, (++) strong reaction, erythema, infiltration, papules and vesicles, and (+++) very strong reaction, intense erythema, infiltration and coalescing vesicles.15
This study was approved by the local ethics committee and all patients gave their informed consent for any diagnostic procedures performed.
Statistical analysisThe characteristics of the population were summarized with descriptive statistics. For qualitative data, absolute and relative frequencies were determined; for quantitative data, medians and interquartile ranges (IQR) were calculated as measures of central tendency and of dispersion, respectively. Data were analyzed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
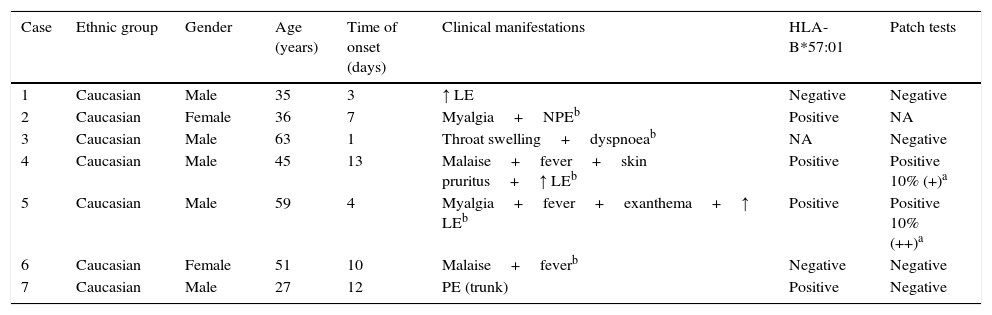
ResultsThere were 186 HIV-positive patients initiating treatment with ABC from January 2006 to December 2007 (group A). We could access the medical records of 163 of these patients, identifying 7 (4.3%) cases of suspected ABC-HS, 5 of them fulfilling the generally accepted clinical diagnostic criteria – Table 1. The patients’ median [IQR] age at the time that the reaction occurred was of 45.0 [35.0–59.0] years, and affected patients were all Caucasian and predominantly males (n=5). The median [IQR] time to symptoms onset after treatment started was 7.0 [3.0–12.0] days. Three patients had liver enzymes elevation, 3 presented with exanthema and 3 other with fever; 1 patient had respiratory involvement. The median time between the onset of symptoms and ABC treatment withdrawal was 1.0 day (range: 0–8 days). All reactions were managed on an outpatient basis. Only one patient needed additional treatment for the reaction (case 2), where antihistamine and corticosteroid were used. We were able to assess 6 patients for the presence of HLA-B*57:01 allele, and to perform patch tests with ABC in 6 (one patient was unable to return to the hospital to perform this test). Two patients had a positive patch test (Fig. 1). Four patients with suspected ABC-HS (of 6 screened) were positive to the HLA-B*57:01 allele (Table 1).
Description of the cases of suspected ABC-HS identified from group A (n=7).
| Case | Ethnic group | Gender | Age (years) | Time of onset (days) | Clinical manifestations | HLA-B*57:01 | Patch tests |
|---|---|---|---|---|---|---|---|
| 1 | Caucasian | Male | 35 | 3 | ↑ LE | Negative | Negative |
| 2 | Caucasian | Female | 36 | 7 | Myalgia+NPEb | Positive | NA |
| 3 | Caucasian | Male | 63 | 1 | Throat swelling+dyspnoeab | NA | Negative |
| 4 | Caucasian | Male | 45 | 13 | Malaise+fever+skin pruritus+↑ LEb | Positive | Positive 10% (+)a |
| 5 | Caucasian | Male | 59 | 4 | Myalgia+fever+exanthema+↑ LEb | Positive | Positive 10% (++)a |
| 6 | Caucasian | Female | 51 | 10 | Malaise+feverb | Negative | Negative |
| 7 | Caucasian | Male | 27 | 12 | PE (trunk) | Positive | Negative |
LE, liver enzymes; NPE, non-pruritic exanthema; PE, pruritic exanthema; NA, non-available.
After the implementation of routine HLA-B*57:01 testing, from January 2008 onwards, 573 patients with an indication to start treatment with ABC were screened. Thirty-five (6.1%) had the allele, contraindicating the use of ABC. In the remaining patients from group B that started treatment with this drug, no suspected ABC-HS cases were observed.
DiscussionIn this study, we report a prevalence of suspected ABC-HS of 4.3% (3.1% using strict clinical criteria) before implementation of routine HLA-B*57:01 screening, in a predominantly white population from a reference Infectiology Department in Portugal. ABC-HS has been described as a treatment-limiting event in approximately 4% of patients treated with ABC,8 which is in accordance with our findings. However, if we consider patients with confirmation of an underlying immunologic mechanism, the prevalence would be lower (1.2%).
The nonspecific symptoms that characterize ABC-HS may lead to an over-diagnosis of the syndrome (preventing patients from receiving an ABC containing regimen), a problem that may be addressed by validated patch testing procedures, assuming that a delayed immunologic mechanism underlies the ABC-HS.10,16 That fact possibly explains why in our study not all patients with suspected ABC-HS had a positive patch test. Another possible reason for the reported negative skin test results is the fact that the maximum non-irritant concentration for patch testing with ABC is not yet established and the diagnostic performance of patch tests in ABC-HS is not enough characterized. Additionally, we would have to consider the possibility that at least 3 cases had negative patch tests because no skin involvement occurred on the index reaction.17 There have been recent advances on the knowledge of the immunopathogenic mechanisms underlying ABC-HS and its relationship with HLA. By binding non-covalently to the antigen-binding cleft of HLA-B*57:01, abacavir induces changes in the shape and chemistry of this site, and as a result alters the affinity of endogenous peptide to HLA, ultimately leading to a systemic hypersensitivity reaction.18
In the present study, we were able to screen 6 patients with suspected ABC-HS for HLA-B*57:01 allele carriage and it was positive in 4. When considering only the two cases with a confirmed underlying immunologic mechanism, both carry the allele. In 2002, two studies (a prospective cohort and a retrospective one) reported on the association between a diagnosis of ABC-HS and carriage of the major histocompatibility complex class I allele HLA-B*57:01.19,20 The first study, conducted in Western Australia, found the allele in 78% of the patients with ABC hypersensitivity; in the second one, the same association was found in 46% of a North American HIV population, with a male predominance. Variations across populations in the predictive values for markers would justify the lower sensitivity of HLA-B*57:01 in predicting hypersensitivity to ABC.20 Broader data on the subject come from a systematic review and meta-analysis of the pharmacogenetics of ABC hypersensitivity.11 A significant association between HLA-B*57:01 expression and ABC hypersensitivity was found, regardless of the diagnostic criteria used, although a significantly stronger association was found for studies in which ABC-HS diagnosis was confirmed by patch tests.11 However, on comparing studies that applied the same diagnostic criteria, no significant differences were observed between ethnic groups, supporting the hypothesis that the apparent lower sensitivity of HLA-B*57:01 in some ethnic groups translates in fact a high rate of false-positive diagnoses.11 We have to admit the possibility that in our study some of the suspected cases of ABC-HS may have also been false positive diagnoses, which would increase the association between ABC-HS and HLA-B*57:01, as stated above.
We found a median time to symptoms onset after ABC treatment initiation of 1 week, slightly earlier than the median of 11 days described by Hetherington et al., but within the first 6 weeks after starting therapy, the period where 90% cases are expected to manifest.8 ABC-HS tends to become more severe and potentially life-threatening with continued dosing, and immediate and permanent discontinuation of ABC is mandatory resulting in a rapid reversal of symptoms; re-challenge with ABC is contra-indicated.13 In our series, there was an early suspicion of ABC-HS leading to a rapid treatment withdrawal (median of one day after symptoms onset) and this may have led to a more favourable evolution, with no need for hospitalization in any case.
Clinical risk factors for ABC-HS have not been consistently identified.7 A retrospective analysis conducted on 5332 ABC-treated subjects, including 197 cases of suspected ABC-HS, identified as protective factors both African ancestry and previous antiretroviral therapy (ART).21 In the present study, we did not compare subjects from different ethnic origins and we had no access to the information on the patients’ previous ART status (i.e. if they were ART-naïve by the time they started ABC), so we could not assess such risk association in our series. Another large retrospective study also found female gender to be a significant risk factor for developing a suspected ABC-HS.7 In our sample males were predominantly affected (71.4%), but the sample size does not allow other conclusions than those referred in the above mentioned study.7
Our study also has some strengths. To identify potential cases of ABC-HS we not only applied clinical diagnostic criteria, but we also performed patch tests. Additionally, we looked at what happened to ABC-treated patients after the implementation of HLA-B*57:01 screening in order to assess the clinical impact of this policy. In our study we found that, after the implementation of routine HLA-B*57:01 testing, no suspected ABC-HS cases were observed among patients that started treatment with this drug (those for whom HLA-B*57:01 allele carriage was excluded). To sum up, routine screening prevented the development of ABC-HS, reinforcing the benefit of prior screening. The results of PREDICT-1 study have shown that prospective HLA-B*57:01 screening can reduce the incidence of hypersensitivity reaction to ABC.13 Screening for this allele is currently recommended for all patients prior to beginning therapy with ABC since it virtually eliminates all immunologically confirmed ABC-HS.3
In conclusion, in our series the prevalence of ABC-HS was 4.3% using broad clinical criteria and 1.2% after patch test confirmation. No cases of ABC-HS occurred after HLA-B*57:01 routine screening implementation. This is the first report on the clinical efficacy of that screening policy in a Portuguese population. However, its cost-effectiveness in the Portuguese context remains to be established and should be addressed in future studies.
Conflicts of interestThe authors declare no conflicts of interest.