Chronic utero-placental insufficiency may result in progressive hypoxia culminating in fetal decompensation and acidosis and this is termed ‘chronic’ or ‘long-standing’ hypoxia. It is essential to recognise the features of chronic hypoxia on the CTG trace so as to institute timely and appropriate action. The current guidelines may not capture a fetus who starts labour already compromised or limited in its ability to compensate for hypoxic or mechanical stresses during labour. The aim of this short review is to explore the CTG features that allow the clinician to recognise a fetus who may present with an antenatal insult such as chronic hypoxia, anaemia, infection, fetal arrhythmias and preexisting non-hypoxic brain injury.
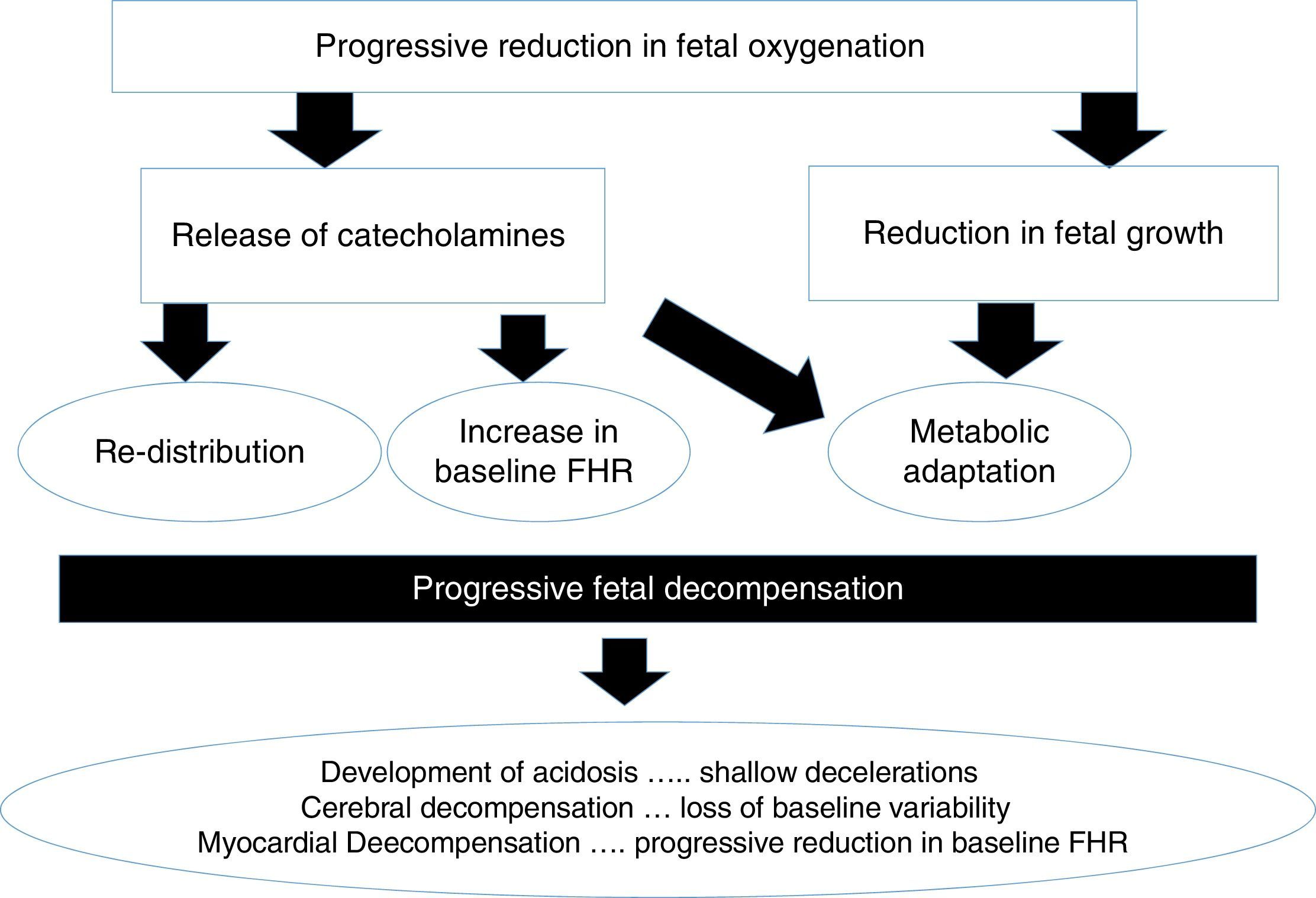
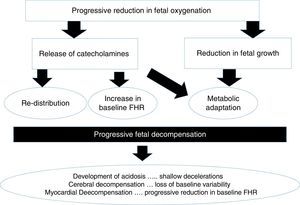
A foetus exposed to hypoxic stress in labour would be expected to demonstrate a series of physiological responses to compensate for the stress so as to avoid hypoxic-ischaemic injury. The myocardium (i.e. the pump) is protected at all cost, followed by the brain and the adrenal glands (i.e. foetal central organs) at the expense of other “non-essential” organs. CTG features which reflect this compensatory mechanisms include the onset of decelerations, absence of accelerations and progressive increase in the baseline foetal heart rate secondary to the release of catecholamines (i.e. adrenaline and noradrenaline). However, if decompensation ensues, blood supply through the carotid arteries may be reduced secondary to low perfusion pressure leading to acidosis in the brain (i.e. loss of baseline foetal heart rate variability).
Chronic utero-placental insufficiency may result in progressive hypoxia culminating in foetal decompensation and acidosis and this is termed ‘chronic or ‘long-standing’ hypoxia. It is essential to recognize the features of chronic hypoxia on the CTG trace so as to institute timely and appropriate action. This is because continuation of labour may lead to intermittent and sustained compression of the umbilical cord and progressive reduction in utero-placental circulation secondary to ongoing uterine contractions. In this situation, this may lead to rapid foetal decompensation characterized by a progressive reduction in the baseline foetal heart rate culminating in a terminal bradycardia. It is also important to recognize features of pre-existing non-hypoxic foetal brain injury on the CTG trace to optimize outcomes and to facilitate counselling of parents.
The current guidelines may not capture a foetus who starts labour already compromised or limited in its ability to compensate for hypoxic or mechanical stresses during labour. The aim of this paper is to explore the CTG features that allow the clinician to recognize a foetus who may present with an antenatal insult such as chronic hypoxia, anaemia, infection, foetal arrhythmias and pre-existing, non-hypoxic brain injury.
Understanding the foetal pathophysiology during CTG interpretationIrrespective of the guidelines used (FIGO, NICE or ACOG) there are four features that should be noted when interpreting a CTG: baseline foetal heart rate, variability, accelerations and decelerations.1–3
Baseline foetal heart rateThis is defined as the mean level of the most horizontal and less oscillatory FHR segments and is estimated in periods of 10minutes and expressed in beats per minute (bpm). FIGO considers the normal range to be between 110 and 160bpm.1 An increase in baseline rate above 160bpm for more than 10minutes is called baseline tachycardia. Common causes include maternal dehydration or pyrexia, a catecholamine response to a gradually evolving hypoxia and, more rarely, foetal tachyarrhythmias. A baseline rate of less than 110, persisting for more than 10minutes is called baseline bradycardia. In addition to an acute reduction in foetal oxygenation (i.e. acute profund hypoxia or hypotension), foetal bradycardias may occur with conduction defects in the heart (heart block) and sympatholytic drugs.
The baseline foetal heart rate is regulated by the autonomic nervous system. The parasympathetic system develops later and consequently preterm foetus tends to have higher baseline rates. Conversely, for a postdate foetus a lower baseline rate is to be expected. Rather than sticking blindly to “guidelines” it is important to consider what should be the normal, “expected” baseline rate for the foetus in question based on his/her gestational age. A foetal heart rate of 100bpm persisting for more than 10min is called a baseline bradycardia and, in the presence of normal variability, accelerations and no decelerations could be normal for a post term foetus. Conversely, a baseline rate of 160, although it is still within the “normal range” proposed by the guidelines should not be considered as normal after 40 weeks of gestation because it may be a sign of chorioamionitis or chronic hypoxia, even in the absence of other abnormal features on the CTG trace.
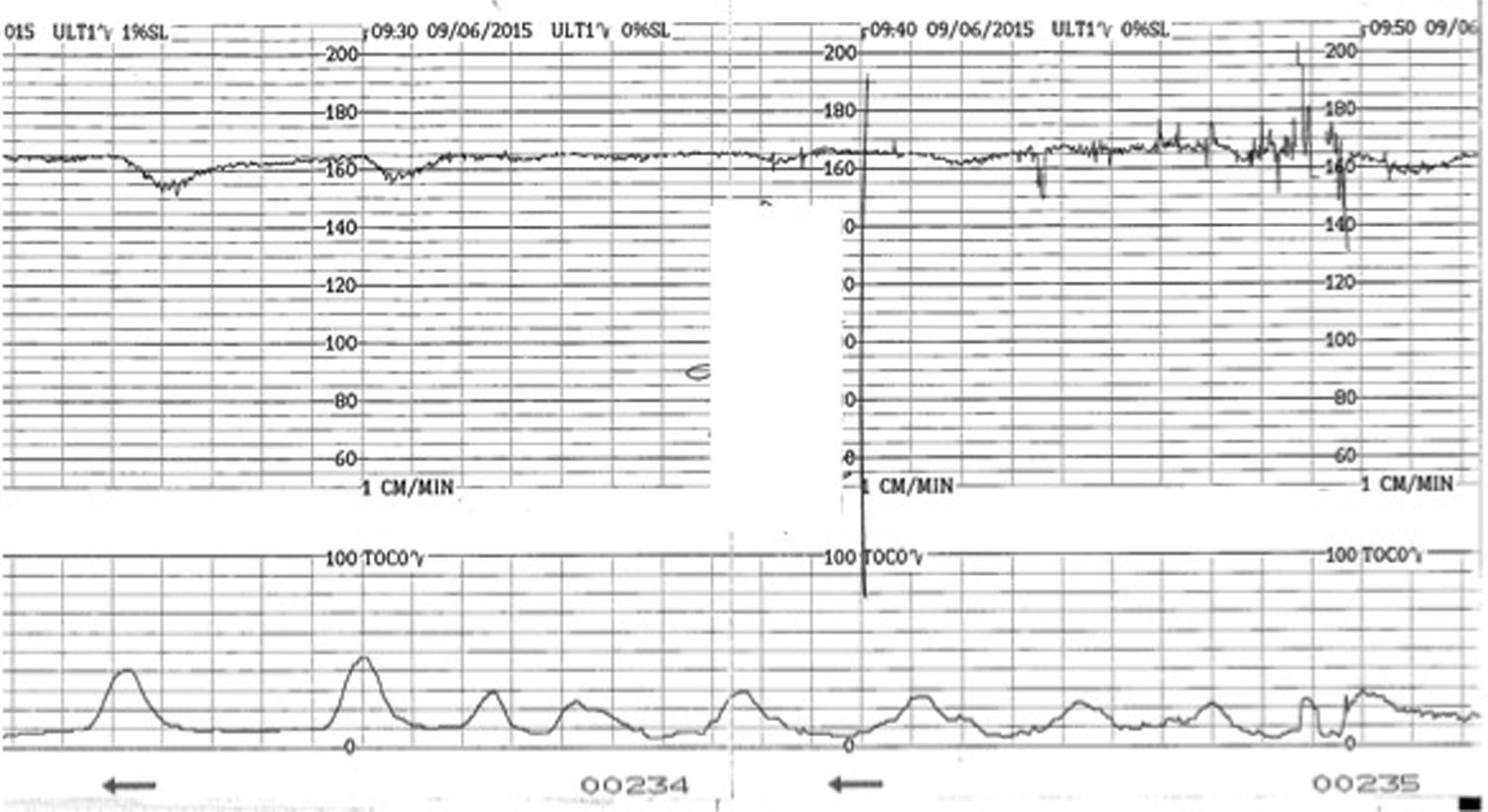
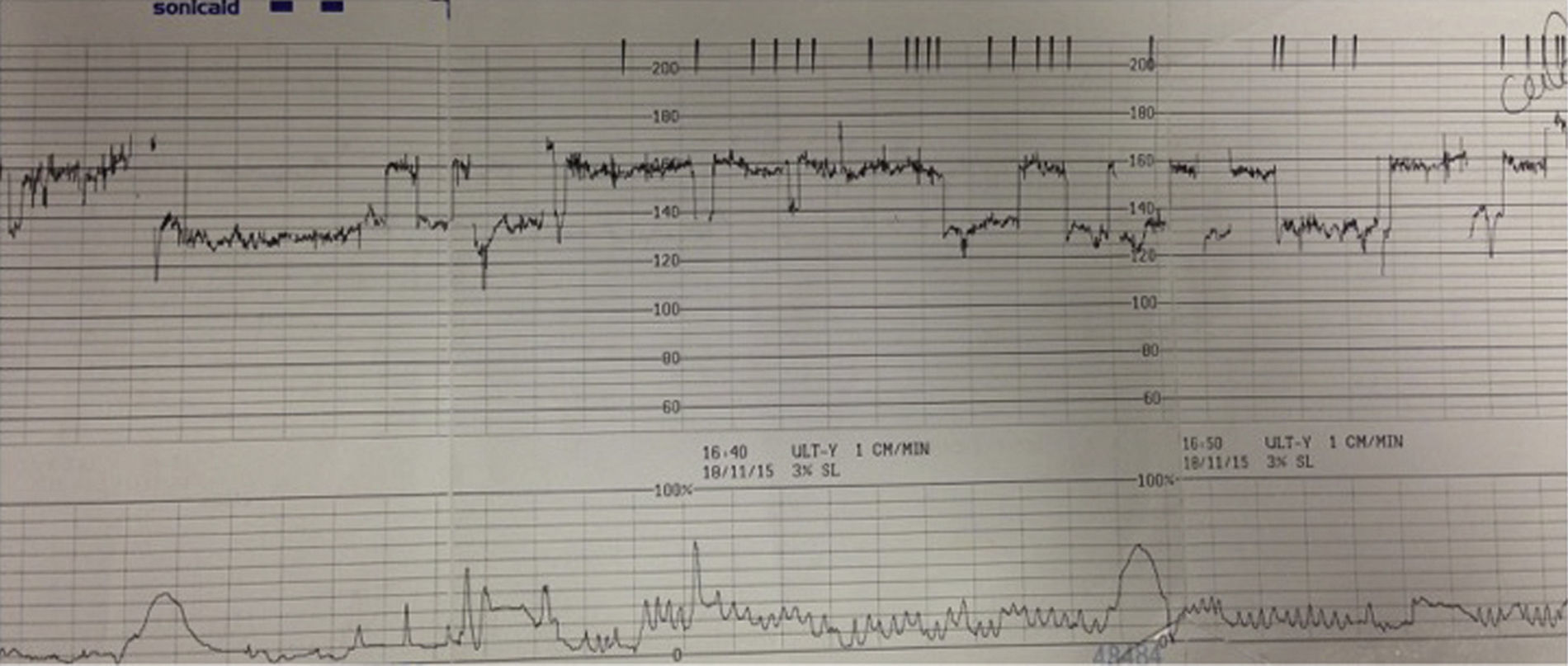
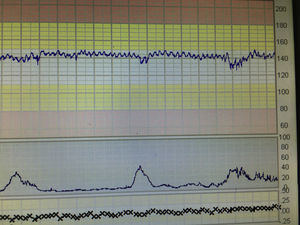
VariabilityThis refers to the “bandwidth” reflecting the oscillation of above and below the baseline and it reflects the continuous interaction between the parasympathetic and the sympathetic autonomic nervous systems. It is classified as normal (5–25bpm), reduced (<5bpm) or increased (>25bpm). The presence of normal variability gives information regarding the integrity of the autonomic nervous system. Periods of deep sleep can have reduced variability but this is unlikely to last more than 50min and will be followed by periods of normal variability. In addition, the baseline foetal heart rate would remain stable without any increase, in cases of foetal sleep. This reassuring pattern of alternating periods of reduced variability interspersed with normal variability reflects the different foetal behavioural states and is called “cycling”. Reduced variability can be associated with drugs (CNS depressants), antenatal brain injury or hypoxia leading to anaerobic metabolism and acidosis within the central nervous system. In hypoxia developing during the process of labour, reduction of baseline variability tends to be a late phenomenon and comes preceded by decelerations and increase in the baseline foetal heart rate secondary to the release of catecholamines. However, if this process had already started in the antenatal period, a higher baseline (catecholamine surge), repetitive shallow decelerations (stimulation of chemoreceptors by metabolic acids) and reduced baseline variability (i.e. acidosis within the brain), which are hallmarks of chronic hypoxia (Fig. 1) may be observed on the CTG trace.
AccelerationsThese are transient increases in the foetal heart rate of more than 15 beats from the baseline and lasting for more than 15s and are caused by the foetal somatic nervous system activity. Therefore, they are usually associated with foetal movements and are a reassuring feature. An antenatal CTG should not be considered normal without the presence of accelerations, although its absence during late labour is of “uncertain significance”. Caution should be taken not to confuse accelerations with overshoots (reflex tachycardia following a deceleration).2 Moreover, the erroneous monitoring of maternal heart rate may also present with accelerations, however, these usually have greater amplitude and coincide with uterine contractions.3 In chronic foetal hypoxaemia secondary to placental insufficiency, the reduction in the number of FHR accelerations is associated with a reduction in skeletal muscle activity.4,5
DecelerationsDecelerations are defined as a transient decrease of the FHR of more than 15bpm, lasting more than 15s. They represent a reflex response of the foetus to reduce his/her myocardial workload in response to any hypoxic or mechanical stresses and therefore, they can be secondary to cord compression, hypoxaemia, head compression or a combination of these mechanisms. Decelerations secondary to cord compression are the most commonly seen in labour, they tend to have a “V” shape with a sharp drop and sharp recovery, lasting usually less than 60s. When in response to foetal hypoxaemia, via central and peripheral chemoreceptors, the decelerations occur “late” in relation to the contraction and tend to assume a “U” shape with a delayed recovery to the baseline. Animal studies suggested that the purpose of this response is to reduce myocardial workload and oxygen demand by lowering the foetal heart rate.6 Decelerations can also occur is response to head compression, starting with the onset of uterine contractions, reaching the nadir with the peak of contraction and returning to baseline at the end of the contraction. They are benign but also rare, accounting for only about 2% of all decelerations.1 However, early decelerations occurring in early labour should be viewed with caution as foetal head compression at that stage is very unlikely and therefore, it is more likely that ‘shallow’ decelerations secondary to chronic hypoxia are being misclassified as ‘early’ decelerations.
Decelerations can also have overshoots (transient increase in foetal heart rate following the ascending limb of a deceleration) that should not be mistaken for accelerations. Overshoots may indicate ongoing foetal hypotension and hypoxia secondary to intense and prolonged compression of the umbilical cord.2
Over the years much focus has been put on the shape of decelerations and timing in relation with the contractions, which can be difficult to ascertain, and have poor correlation with neonatal outcomes. Emphasis should be placed on the foetal response and compensation mechanisms to the decelerations and here most guidelines agree that, despite the presence of decelerations, if the baseline is stable and variability normal, the risk of foetal hypoxia is low.
CTG patterns in chronic hypoxia and pre-existing foetal injury with case examplesMany foetuses sustain neurological damage leading to neonatal encephalopathy secondary to causes that operate during the antenatal period. These include hypoxic, metabolic, genetic, vascular, haematological as well as inflammatory mechanisms. Although, it may be argued that in some cases neurological damage may have already occurred, it is vital to appreciate that subjecting such a foetus to repetitive umbilical cord compression and reduced utero-placental perfusion secondary to progressively increasing frequency, strength and duration of uterine contractions in labour, may potentiate the pre-existing damage or worse, cause additional neurological damage. Therefore, the timely identification of pre-existing foetal brain injury on the CTG trace would facilitate counselling of parents regarding the guarded prognosis and is likely to optimize outcomes by avoiding the additional stress of labour to a foetus limited in his/her ability to mount a successful compensatory response to hypoxic stresses during labour. The exact contribution of antenatal factors to neonatal encephalopathy remains unclear and range from 10 to 90% in different studies.7–10 It is also important to note that not all pre-existing foetal injuries will lead to CTG abnormalities and therefore, they may never be recognized during the intrapartum period. Also, it is unclear as to the exact contribution of intrapartum hypoxic insult to the pre-existing foetal damage in the foetus in question or the magnitude of this extra injury.
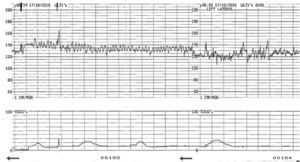
Chronic hypoxiaA foetus who is exposed to prolonged periods of hypoxia due to placental insufficiency will adapt to the suboptimal intrauterine environment by reducing growth, redistributing the oxygenated blood to vital organs (brain, heart and adrenals) and restrict, ‘non-essential’ somatic movements and would attempt to increase the heart rate to obtain more oxygenated blood from the placenta. Failure of these compensatory mechanisms may result in hypoxia and acidosis of the foetal brain (Fig. 2).
Typically, such a foetus exposed to prolonged chronic hypoxia will present with reduced foetal movements in the antenatal period or in early labour and the CTG will show a baseline rate on the upper limit of the normal range, decreased baseline variability, with no accelerations and possibly ongoing shallow decelerations (Fig. 1). In this context, decelerations may not fulfil the guidelines criteria of more than 15bpm drop for more than 15s but they are still relevant.
The reduced baseline variability and reduction in foetal movements in association with intrauterine growth restriction (IUGR) and mild hypoxaemia has been shown in various studies.4,11,12 Also, in the foetal sheep model, foetal heart rate patterns induced by chronic foetal placental embolization show a reduction in the number of FHR accelerations and both long and short-term variability. It has been speculated that the reduction in FHR variability associated with placental insufficiency in the animal model is likely the delay in the normal maturation of the autonomic control of FHR variation5 and the increased baseline due to increased circulation of catecholamine.13 In response to chronic hypoxia the foetus tries to increase the cardiac output, mainly by increasing the heart rate, to supply the vital organs.
In chronic foetal hypoxia, although some brain damage may have already occurred, the onset of uterine contractions and resultant reduction in utero-placental circulation will increase the risk of hypoxic-ischaemic encephalopathy (HIE) as well as myocardial failure leading to terminal bradycardia and potential intrapartum stillbirth. This has also been studied in the animal model where a detrimental effect of pre-existing mild hypoxia on foetal outcome following repeated umbilical cord occlusions has been shown.13
The recognition of this CTG pattern suggestive of ‘chronic hypoxia’ should prompt an immediate delivery by caesarean section, unless a spontaneous or operative vaginal delivery is imminent. In the presence of decelerations with contractions, tocolytics such as terbutaline should be considered while preparing for delivery to avoid progressive myocardial decompensation secondary to ongoing uterine contractions and resultant reduction in foetal oxygenation.
Foetal anaemiaChronic foetal anaemia is a rare phenomenon in modern obstetric practice as most foetuses with anaemia are usually identified during ultrasound scan and treated in the antenatal period. It can, however, present for the first time during the intrapartum period, either because a chronic foetal anaemia has been missed or due to a sudden foetal bleeding during labour (i.e. an acute foetal blood loss). If it is recognized and timely action is taken, the prognosis for these babies is not uniformly poor.
Since the introduction of routine anti-D prophylaxis there has been a reduction in incidence of rhesus (D) isoimmunization, but other antibodies like anti-c and anti-Kell can be also implicated in foetal haemolytic disease severe enough to cause significant foetal anaemia. Foetal infection by parvovirus B19 is also a recognized caused of chronic foetal anaemia. Foetal haemorrhage in labour is rare and can be secondary to feto-maternal haemorrhage (FMH) or bleeding from a ruptured vasa previa. FMH of >80mL and >150mL is estimated to occur in 1 in 1000 deliveries and in 1 in 5000 deliveries, respectively14 and it accounts for 3.4% of all intrauterine deaths and 0.04% of all neonatal deaths.15
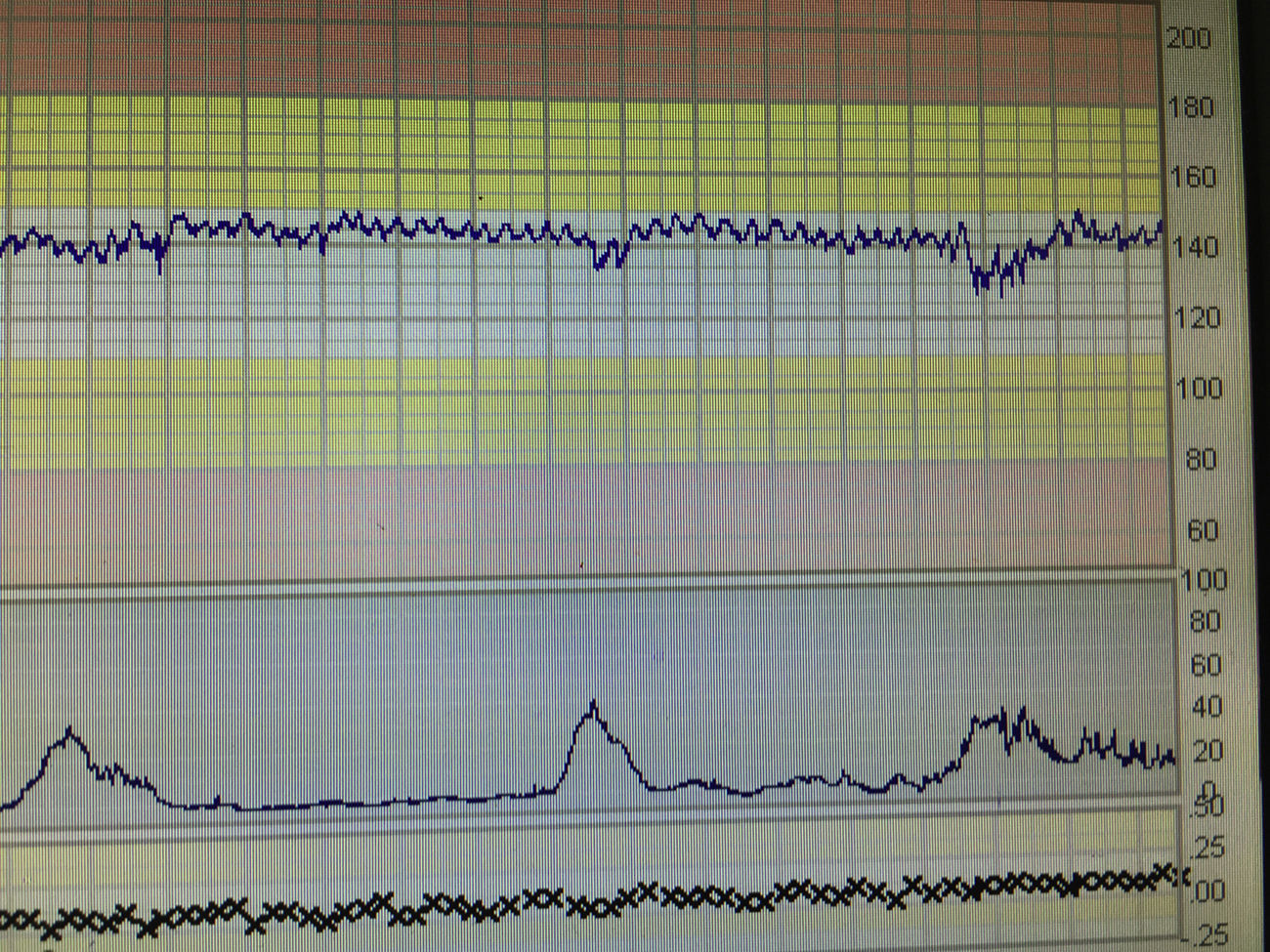
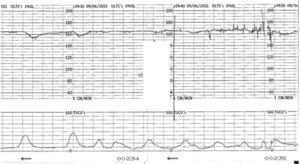
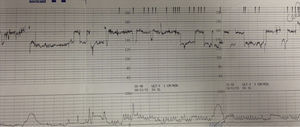
Sinusoidal foetal heart rate (SHR) pattern is defined as a regular, smooth, undulating signal with amplitude of 5–15bpm, and a frequency of 3–5 cycles per minute lasting more than 30min with absent accelerations.1 Severe foetal anaemia can present with true sinusoidal pattern on CTG. It has also been associated with late decelerations. It was first described by Manseau et al. in 1972 in severely affected, Rhesus-sensitized, anaemic and dying foetuses, and was called ‘sinusoidal’ (SHR) because of its “sine waveform”.16 In cases of acute anaemia/hypovolemia secondary to bleeding from vasa previa or feto-maternal haemorrhage the sinusoidal pattern is called “atypical” (Fig. 3) in view of the less smooth, saw-tooth form.17 This is also called the “Poole Shark Teeth” pattern.2,18
The aetiology of this rare FHR pattern is still poorly understood but it is likely to represent the absence of central nervous system control over the heart. In animal studies, chemical or surgical vagotomy and arginine vasopressin infusion produced SHR pattern, thus confirming the role of autonomic nervous system dysfunction. Also a rise in arginine vasopressin levels in the blood of post haemorrhagic/anaemic foetal lamb was documented.19
True sinusoidal pattern is typically associated with situations that cause chronic foetal anaemia or hypovolemia: iso-immunization, massive feto-maternal haemorrhage, twin-to-twin transfusion syndrome, bleeding vasa previa and foetal intracranial haemorrhage.20 It has also been documented in several high risk obstetric conditions such as diabetes, preeclampsia, amnionitis and some foetal malformations (gastroschisis, hydrocephalus, cardiac malformations).2
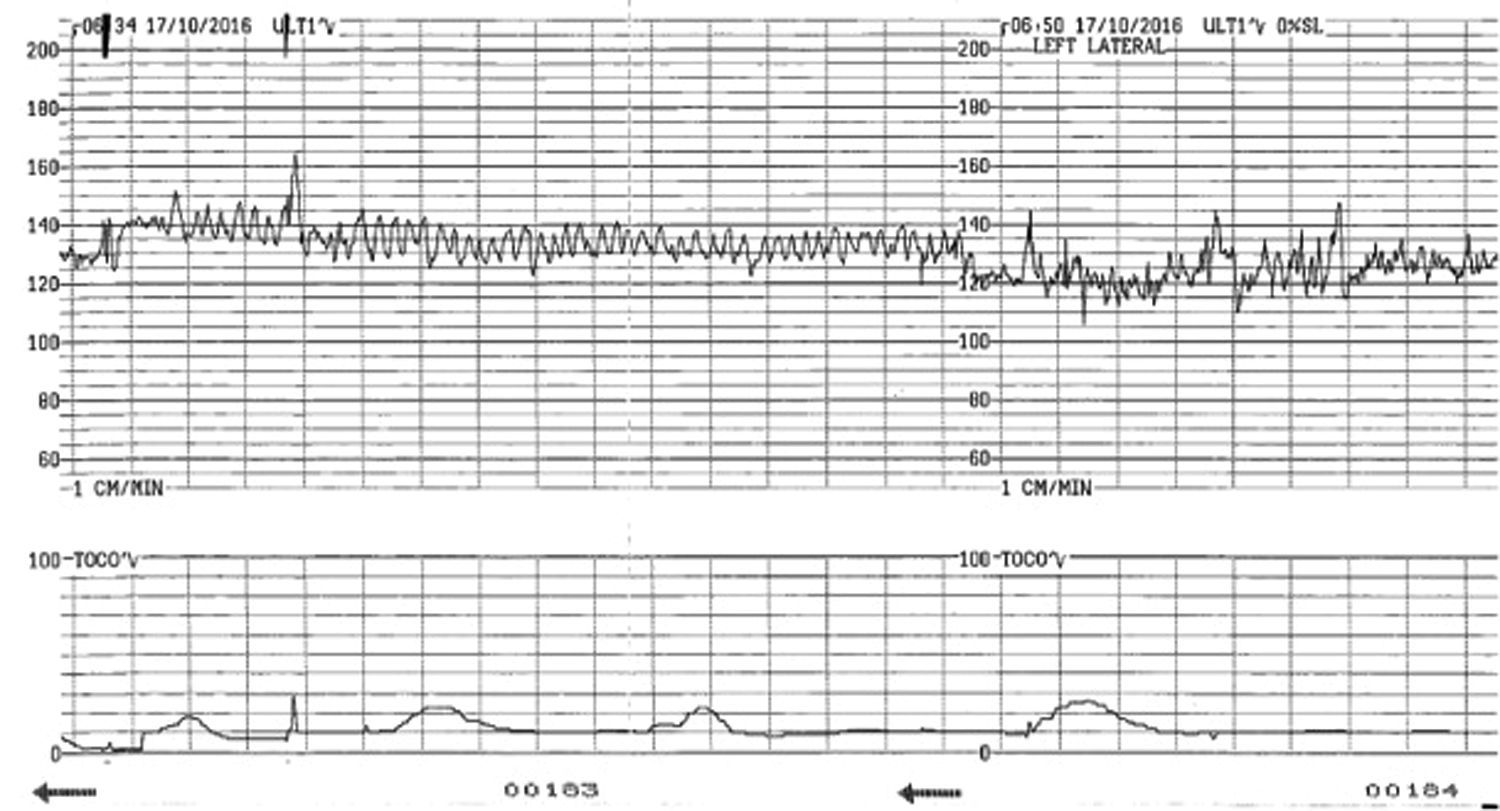
Sinusoidal pattern is a sign of foetal compromise and urgent delivery or, when possible and indicated intrauterine transfusion needs to be organized. It is however important to distinguish true sinusoidal pattern from pseudo-sinusoidal heart rate patterns. These are patterns in which undulatory waveforms alternate with episodes of normal baseline variability or reactivity (Fig. 4). This pattern is not typically associated with foetal compromise and do not need any intervention. It can be seen in physiological conditions like rhythmic movements of the mouth such as thumb sucking or with the use of narcotic analgesics.
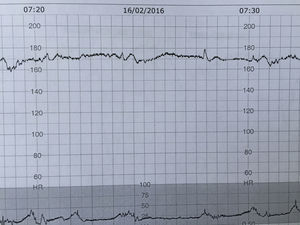
Foetal anomaliesThe foetal heart rate is determined by the interaction between the central nervous system, the vagus nerve, and the heart. Foetal anomalies involving the brain or the heart may alter the foetal heart patterns without correlating with a hypoxic or acidotic state. The few retrospective studies evaluating the use of CTG in foetuses with congenital structural heart disease in labour did not shown any characteristic foetal heart rate pattern related to specific heart defects. In case of significant foetal arrhythmias the CTG can show very specific patterns (Fig. 5). Foetuses with arrhythmias may not be able to further increase their heart rate to compensate for the hypoxic or mechanical stress of labour as any further ‘catecholamine-mediated’ increase in the heart rate may lead to rapid myocardial decompensation. Rarely, the CTG cannot be interpreted to exclude ongoing intrapartum hypoxia. In this scenario, delivery by caesarean section is recommended if the foetal cardiac rhythm abnormalities are not amenable for medical treatment.
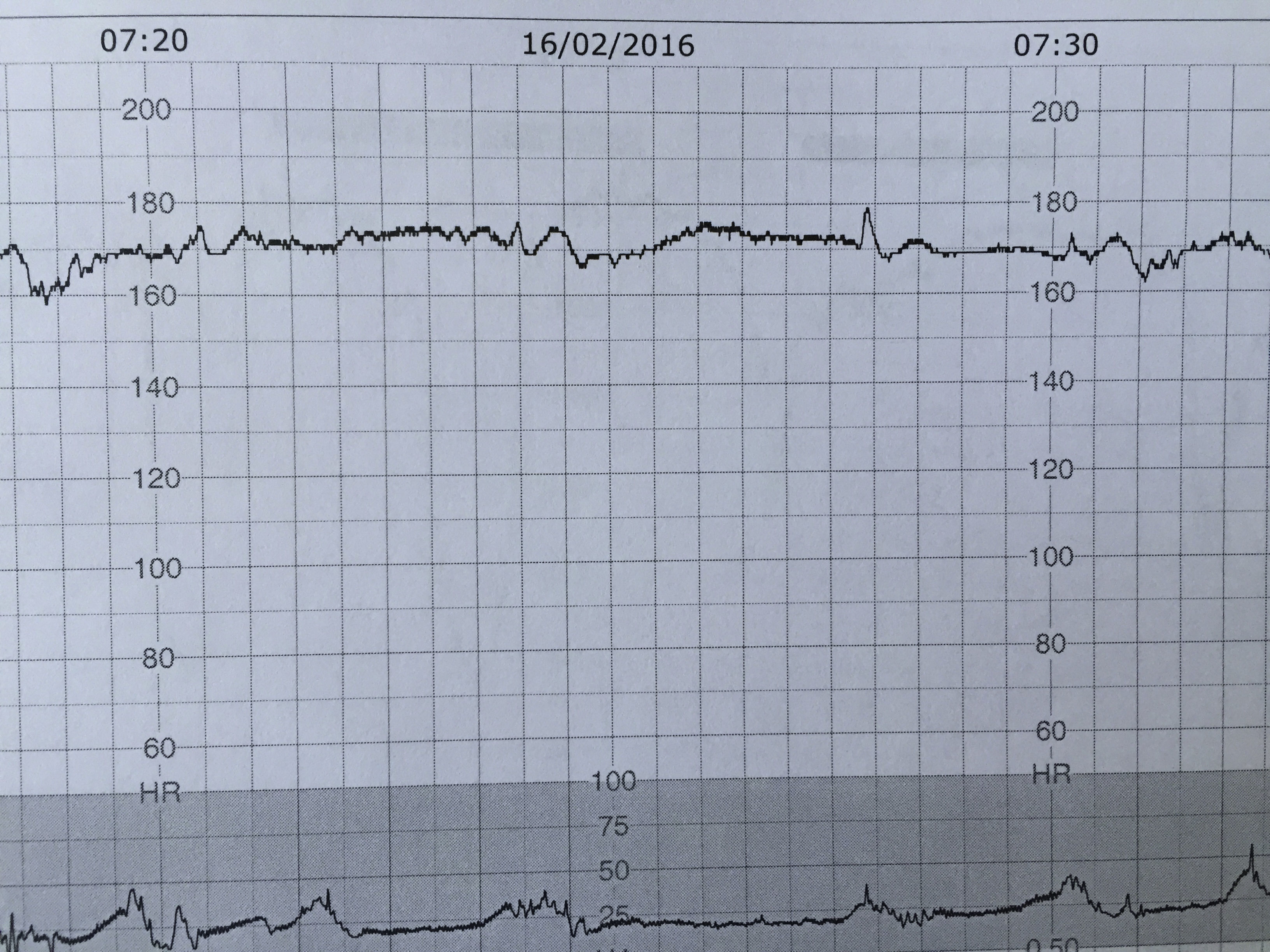
Foetal brain injury can present with a CTG pattern similar to chronic hypoxia with reduced variability as a key marker (Fig. 6). Sinusoidal pattern has also been described in foetus with hydrocephalus and brain haemorrhage.20
Foetuses with gastroschisis, an abdominal wall defect typically located on the right side of a normally inserted umbilical cord with bowel protruding through the defect, have an increased risk (10–15%) of intrauterine foetal death (IUD). Some authors have proposed weekly CTG monitoring from 33 weeks to reduce the risk of stillbirth.21 The typical pathological finding of the CTG is reduced variability. It has been debated whether factors other than hypoxia, for example fluid and protein loss or pressure on the bowel, could contribute to the development of pathological CTG.
Foetal intestinal volvulus is a rare life-threatening condition, associated with foetal compromise and reduced baseline variability on the CTG.22 Most reported cases presented in the early third trimester with an average of 32.5±2.6 weeks.23 Typical prenatal signs of in utero volvulus are reduced foetal movements, static abdominal mass with dilated bowel loops and reduced foetal heart rate variability with no accelerations on CTG. It has been speculated if parasympathetic overactivity from the volvulus or foetal pain contributed to the CTG abnormalities.24 Delivery by caesarean section and surgical treatment should be arranged. Foetal volvulus can be complicated by bowel perforation, bowel necrosis and/or bowel atresia. Late diagnosis of volvulus contributes to high rate of morbidity and mortality.
InfectionInflammation secondary to infection can cause direct neurological injury and also acts synergistically with hypoxia to increase the risk of encephalopathy. Cardiotocography was not designed to detect infection and only a minority of cases (8–12%) of chorioamnionitis will present with maternal pyrexia or tachycardia. There are, however, some CTG patterns that should raise the suspicion of underlying infection. An increase on foetal heart rate without preceding decelerations cannot be attributed to compensatory mechanism of evolving hypoxia and causes such as maternal dehydration, pyrexia secondary to epidural or infection should be excluded. Again, instead of blindly applying the ‘arbitrary’ limits of normality defined by guidelines, one needs to individualize the baseline HR for the given foetus and use the same foetus as its own control.25 Comparison of the CTG trace with a previous trace may help to assess the normal baseline for the foetus in question. Also lack of cycling has been recently shown to be associated with foetal infection26 and no guidelines to date take into account this important parameter of foetal wellbeing.
Maternal pyrexia with suspected infection should be managed with paracetamol, hydration and intravenous antibiotics. Infection alone can cause neurological damage and if delivery is not eminent, if oxytocin is required to achieve progress in labour or if there are additional risk factors such as meconium or growth restriction delivery by caesarean section is recommended. This is because foetal inflammation significantly potentiates the risk of foetal neurological injury by up to 78 fold.8
ConclusionCurrent guidelines on intrapartum foetal monitoring stipulate arbitrary cut offs for baseline foetal heart rate for all babies. This may lead to midwives and obstetricians missing on going pre-existing hypoxic or non-hypoxic and inflammatory injury in a foetus after 40 weeks of gestation. This is because due to the progressive maturation of the parasympathetic nervous system, the normal baseline foetal heart rate of a foetus at 41 weeks may be 110bpm (i.e. at the lower limit of the normal) Hence, if this foetus is exposed to a pre-existing hypoxia or an inflammation such as chorioamnionitis, the baseline foetal heart rate may increase to 150bpm and will not cross the upper limit of ‘160bpm’ proposed by guidelines. It is vital to remember that “one guideline box does not fit all babies” and we have proposed a ‘Foetal Monitoring Checklist’ (Table 1) to be performed at the beginning of every CTG recording to avoid the pitfalls of missing a pre-existing foetal injury.
Suggested ‘Foetal Monitoring Checklist’: to be used at the beginning of every CTG trace.
| 1 | Baseline foetal heart rate appropriate for gestation |
| 2 | Confirm normal variability and cycling |
| 3 | Confirm presence of accelerations (if not in labour/early labour) |
| 4 | Exclude shallow/late decelerations |
| 5 | Consider the wider clinical picture: meconium, temperature, foetal growth, chorioamnionitis |
The authors declare no conflicts of interest.