3ª PONENCIA
LA PAREJA ESTÉRIL DEL SIGLO XXI
From natural to assisted procreation
J. Testart
INSERM Unité 355, 92 140
Clamart and Centre d''AMP
Hôpital Américain, 92 202 Neuilly (Francè)
Struggling against sterility has always been one of man''s worries, even if no really efficient means were proposed during the pre-scientific period. In parallel, it is interesting to note that, on the contrary, in the struggle against fertility solutions were offered.
1. HINDERING OR ASSISTING PROCREATION
Intrauterine devices were invented thousands of years ago in the form of a small stone inserted into a camel''s uterus by caravan camel drivers, even as coïtus interruptus is mentioned in the Bible. Castration of harem guards has been a common practice for ages and the contraceptive virtues of vaginal inserts exercing a spermicidal effect has long been recognised. The cervical cap was first developed in the seventeenth century (using half a lemon) and, at about the same period, the condom was developed, first to fulfil a prophylactic function and later a contraceptive function.
One should also remember the divers potions or toxic substances that women have long been absorbing to avoid or interrupt pregnancy.
Finally, the twentieth century has produced a variety of new means for hindering conception. However, even if these procedures resulting from scientific study have notably improved the efficacy and the feasibility of contraception, they follow the path of technical evolution rather than that of invention.
For assisted conception it is a completely different story. Even if more or less magical potions had long been proposed to stimulate masculine ardour or to promote female production, assisted conception appeared only about a quarter of a century ago with the use of gonadotropic hormones and artificial insemination.
Opposing fates thus separate the two fields of procreative mastery historically: artifices abounded for inhibiting the function whereas they remained inexistent for stimulating it. The Christian ethic, opposed to any procreation manipulation, was especially vehement in its condemnation of negative (contraceptive) manipulations. Its intervention does not, therefore, explain the absence of positive manipulations (Medically Assisted Procreation = MAP). Could it be that fatalistic tendencies or the fear of divine retribution in connection with sterility led people to renounce any effort at controlling their own destiny? With contraception turning to a variety of natural substances (from mercurial potion to beaver testicles to acacia spermicidal pessaries), it is surprising that the same inventiveness was not put to the service of sterility by, for example, taking advantage of the gonadotropic properties found in the urine of elderly women or of gravid mares.
We would all agree that it is generally easier to hinder a phenomenon than to make it function; this observation becomes all the more appropriate if the function is inaccessible, as was long the case for fertilization, hidden away inside the female organism. Under such conditions it was easy to place barriers between the gametes of the two sexes. Still, aiding the gametes to meet remained quite difficult. It required knowledge and, especially, expertise, in service of a strategy.
2. THE STRATEGY OF THE STRUGGLE AGAINST STERILITY
This strategy has consisted in trying to get around those factors, from amongst all factors naturally limiting the chances of procreation, which specifically hinder fertilization(1). Action has therefore been directed at the number of gametes, their proximity and, finally, their interaction.
2.1. Increasing the Number of Oocytes Which Can Be Fertilized
As the chances of conception are naturally limited to one sole embryo per female cycle, the first intervention into problems of sterility in the couple was to increase the number of oocytes exposed to the risk of being fertilized, whether the cause of sterility was male or female (Table 1). Artificial insemination thus habitually calls for a slight ovarian stimulation (e.g. clomiphene citrate) in order to obtain several ovulations. In the case of in vitro fertilisation and embryo transfer (IVF-ET), a stronger level of stimulation using gonadotropic hormones can be justified by the resulting collection of oocytes (mastery of the number of transferred embryos, possibility of freeze-drying any «supernumerary» embryo. It is essentially to this artifice of superovulation that IVF-ET owes its relative success. Recent progress in the efficacy of clinico-biological techniques has led to a few attempts to go back to the natural cycle. Results have remained, however, to poor for this practice to be acceptable(2). The average level of IVF-ET ovarian stimulation is 8.4 oocytes per patient(3), of which about half develop into split embryos.
Table 1 Number of gametes exposed to fertilization | |||
| Method for fertilization | Number of Oocytes | Level of Insemination | Number of Spermatozoa |
| Sexual intercourse | 1 | Vagina | 200.000.000 |
| Artificial insemination | 1-3 | Uterine cervix | 10.000.000 |
| Uterine cavity | 5.000.000 | ||
| Fallopian tube | 1.000.000 | ||
| In vitro fertilization | 5-20 | Test tube or box | 50.000 |
| Capillary tube | 5.000 | ||
| Under zona (SUZI) | 5 | ||
| In ooplasm (ICSI) | 1 | ||
2.2. Reducing the Number of Male Gametes Necessary for Fertilization
Even while ovarian production was being increased, it became possible to reach fertilization with fewer spermatozoa than the hundreds of millions normally produced upon ejaculation. As it happened, on the one hand the number was too large for IVF (risks of polyspermy) and on the other hand the scarcity of gametes in the numerous situations of male infertity is such that «classical» IVF often fails. Therefore means of fertilization with a minimum number of spermatozoa were sought (and found).
2.3. Bringing oocytes and Spermatozoa Closer Together
It was the drawing together of the gametes which resulted in this reduction of spermatozoa through the elimination of natural barriers which had heretofore rendered the meeting of gametes uncertain (genital tubes, cervical mucus, secretions, perioocyte annexes...). Table 1 shows how incredibly, from artificial insemination to ICSI, the number of gametes necessary for fertilization has been reduced. It should also be emphasised that as of fertilization the chances of procreation no longer depend at all on the number of spermatozoa but rather on that of the oocytes, which have then become embryos.
2.4. Drawing Together in Time of Mature Gametes
Still, the artificial association of gametes also means that one takes into account time factors relating to the acquired competence to reach fertilization. IVF-ET makes it possible to bring recently capacitated spermatozoa together with oocytes which have reached meiotic metaphase II a few hours earlier. The low rates of success with donor AI (approximately 10%) in spite of donor selection(4) can largely be explained by poor co-ordination between the medical acts and biological events(5).
2.5. Short-circuiting Gamete Interaction
Since the 80''s it has been possible for us to get around the outside envelopes of oocytes (cumulus oophorus and zona pellucida) by introducing a few spermatozoa directly into the perioocyte space(6). Fertilization thus became possible in spite of suspected defects in certain gamete recognition and attachment molecules(7) and despite serious oligospermia or lack of sperm mobility. Still, the SUZI (sub-zonal insertion) technique was quite inefficient and was advanced by a more invasive technique in 1992: ICSI (intra cytoplasmic sperm injection)(8) which gets around the final barrier to the meeting of gametes, the oocyte membrane. One could henceforth short-circuit overall gamete interaction and limit the number of spermatozoa necessary to fertilize each oocyte down to the single unit.
2.6. Other Actions
Now that these steps have been relatively well mastered, it is becoming possible to work on other aspects of fertilization, for example programming the moment of conception. GnRH (Gonadotropin Releasing Hormone) active ingredients are prescribed in 92% of ovarian stimulation cycles for IVFET(9). They make it possible to avoid untimely ovulation by blocking spontaneous surges of LH (Luteinizing Hormone). They have, as well, introduced the possibility of choosing the date of conception through the programming of medical treatments outside the influence of the random variables of female physiology. Embryo freeze-drying has increased this mastery of time by making pregnancy programmable once fertilization has taken place.
Moreover, births have been produced using IVF oocytes taken before maturity, with no treatment to patients, and for which meiosis is completed in vitro. These results have been produced by oocytes retrieved during menstrual cycles(10) as well as under non-physiological circumstances as oocytes gathered before maturity came from women with polycystic ovaries(11) or due to Caesareans(12). Male gametes, as well, can be injected into oocytes whether the spermatozoa come from the epididymis or the testicles(13) and if spermatids have reached differentiation or not(14).
One cannot ignore that MAP gives its best results when several embryos are present in the uterus simultaneously, either following in vivo fertilization (AI after multiple ovulation) or after in utero transfer of the embryos (IVF-ET). The consequence of these practices is the high frequency of multiple pregnancies (over 25% with IVF-ET). As long as the evolutionary potential of each embryo remains unknown, maintaining success rates will necessitate transferring embryos in excessive numbers. Identifying viable embryos therefore adds a new stake to the game and implementation of this action overlaps identification of «normal» embryos, even if the philosophies governing the two are different: in the absence of morphological or biochemical markers of viability, the latter can only be partially evaluated on the basis of genetic criteria and therefore enters into the framework of preimplantation genetic diagnosis (PGD), even if French law (1994) has not yet accepted this extension of PGD.
3. A STRATEGY HEADING TOWARDS COMPLETION
Even if a lot remains to be done in better understanding the biological mechanisms thus modified or eliminated and to improve the efficacy of biomedical acts, the MAP strategic plan has largely been completed. Indeed, the strategy of drawing gametes closer together and limiting their numbers reaches completion with ICSI. It would now seem appropriate to come back to the biological aspects of this technique which has undergone rapid expansion resulting in approximately 15,000 trials in France in 1997, that is to say almost half of the IVF-ET interventions.
3.1. ICSI: the MAP Extreme
It is now incontestable that ICSI «works» despite disrespect of biological rules once thought to be inevasible. Criticism of our Belgian colleagues, guilty of having experimented such an invasive technique on humans prior to any real trials on animals, remains historically valid. Still, the birth of thousands of children conceived by ICSI today constitutes a source of information on the risks of the technique. A few worrying studies were brought forth, but their results were never confirmed. Thus the high frequency of genetic anomalies observed in only 15 cases(15) has been contradicted by several subsequent studies, one of which was on 1,082 karyotypes(16). Moreover, the Australian analysis indicating mental deficiency in boys conceived by ICSI(17) has received methodological criticism [18] and has not been confirmed by a more extensive study(19). Such contradictions raise the question of «episcientific aspects» in assessing results(20). In France, a multi-centred study carried out by IVF biologists (BLEFCO)(21) on 2,919 ICSI pregnancies confirmed the results of the Brussels group: ICSI does not seem to create any anomalies but is obviously prone to transmit a parental anomaly, for example inventing «hereditary sterility».
As shown by the observation of children up to 2 years of age(19), the mechanical crossing of oocyte membranes and the injection of either artificial media (essentially PVP = polyvinylpyrrolidone) or normally excluded spermatic elements (plasmic membrane, acrosome and its enzyme contents) do not effect development.
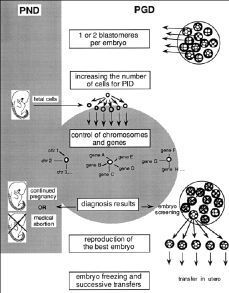
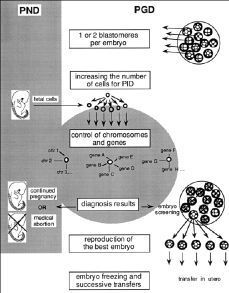
Figure 1.Comparative potentialities of prenatal diagnosis (PND) and preimplantation genetic diagnosis (PGD) to assure healthy babies.
3.2. From Random MAP to Controlled MAP
MAP strategy should henceforth be oriented towards the least efficient aspects of therapeutic acts. For example, biologists should take better advantage of the immature oocyte stocks to palliate hypo-fertility linked to female aging and to better master oocyte artificial activation conditions to favour the beginning of development. For clinicians it seems important to improve the levels of success of embryo transplantation, both as concerns the moment of the act of transfer and that of implantation (here the main effort is linguistic to avoid confusing the two steps by saying: «I''m implanting the embryos...»). All these techniques and procedures are, however, part of the same strategy aimed at permitting procreation, by a couple, of children which they would have conceived on their own had they not been hampered by sterility. These biomedical actions, overall, are therefore part of what can be termed random assisted procreation because they do not seek to influence the characteristics of the future children. Controlled procreation, on the contrary, concerns possibly fertile couples for which biomedical assistance has the exclusive or supplementary goal of leading to the birth of children meeting certain genetic requirements. This evolution corresponds to ancestral concerns and finds concrete expression in the sorting of IVF embryos(22,23). Indeed, preimplantation genetic diagnosis (PGD) cannot be considered an early prenatal diagnosis (PND) as it offers the possibility of extension to new genetic indications(24). We have shown(25) that biological research supplies tools which are capable of revolutionising the practice of MAP by developing a new procreation strategy more strongly oriented towards the genetic quality of children.
4. A FEW LESSONS FROM MAP
The first observation which can be made is that after a quarter of a century of MAP the average «performance» of human couples (20% to 30% conception per female cycle) is similar to that presently attained by sterile couples using MAP and that this level certainly does not represent a limit imposed upon the species. There is every reason to believe that it will become possible to do better with a sterile couple than nature can do with a fertile couple, and this thanks to the progressive skirting of physiological hindrances which explain the low level of fertility of our species(1). The originality of this promise should be noted since in other fields medicine has the sole ambition of restoring handicapped patients to the natural state, never of doing better.
A second observation is that the incontestable and sudden success of MAP has led to the installation of numerous specialised infrastructures (biomedical teams, trainings, specialized diploma, reviews and ad hoc conferences); it has brought forth a specific economy (team materials and consumables, patient assessments and treatments) and a corresponding social interest (patient associations, ethics and reflection committees, media space, etc.). That is to say, it has engendered de novo a complete sector of human activity, capable of propelling its own dynamic on the basis of the ever more explicit demands of the ever more numerous «patients». This situation and the competition which is developing between the teams involved should push MAP towards proposals which would have been unthinkable not long ago. The strategy of drawing the gametes closer together having reached its peak with ICSI, new proposals are beginning to shift away from the project of defeating sterility as it has been understood up to now: bring together two mature gametes to form an egg capable of developing in the framework of the couple. Already one is working on the maturity of the collected gametes (spermatids, first order oocytes), on their origin (testicles, primordial follicles), on the moment of procreation (programming of female cycles, conservation of gametes and embryos, procreation after sterilisation or menopause), on the length or functioning ex vivo of natural phenomena (oocyte maturation, spermatogenesis, blastulation, assisted opening), on the number of those involved (sperm donors, oocyte donors, embryo donors, surrogate mothers). Plus, it is becoming possible to recognise abnormal embryos (PGD). It is certainly this last register which opens the widest perspectives for a new strategy, one which will aim at assuring the «quality» of children.
The third observation is the surprising ease with which we can short-circuit natural phenomena with no apparent consequences for children. If all goes with man as with rodents (and in biology thus seems to be the case), one could even use spermatocytes(26) or freeze-dried spermatozoa(27) to fertilize oocytes or clone and then reclone females over several successive generations with their own follicle granulosa cells(28). This in retaining only the recent works of the Yanagimachi group in Honolulu.
It is incontestable that we are already led to act before understanding and it is foreseeable that this tendency will get worse. Normal procreation mechanism specialists are always those who are the most shocked by these disrespectful cuts at their discipline: they can always try to understand spermatogenesis, folliculogenesis, oocyte maturation, sperm organite functions or gamete interaction... In practice one can do without their knowledge. To the scientist who after twenty years'' research insists on the role of the spermatic centriole or the importance of cytoplasmic maturation of the egg, the «wonder-worker» sticks out his tongue, pointing to the thousands of babies conceived in disrespect of such phenomena. So, we must get used to the realities facilitated by the artifice, which is to say, for the scientist, to keep a low profile while the practitioner is all too boastful. This game between knowledge and power should not, of course, lead us to forget that new knowledge is an investment in future expertise. Still, we should not ignore that the suffering of our contemporaries can sometimes legitimate action even before science is capable of granting its guarantee.
In the case of PND, in utero extraction of foetal cells results in a diagnosis leading to either permitting or terminating pregnancy. It consists, thus, in a binary and dramatic choice.
In the case of PGD, 1 or 2 blastomeres are amputated from each in vitro embryo. This operation has no deleterious effect. These isolated cells can be cultivated to produce millions of identical cells (clones) on which as many genetic particularities could be brought to light as in the entire Human Genome programme. The results of the diagnosis would make it possible to retain only the «best» available embryos. In order to make this eugenic investment profitable by the birth of a baby (at present the rate of birth after one embryo transfer is only about 10%) it could become necessary to reproduce the chosen embryo in several examples (embryo cloning) which would be conserved by freeze-drying. In this way, the successive transfer of 1 or 2 embryos each time (identical twins) would assure the birth of the child chosen in the egg.
BIBLIOGRAFIA
1. Testart J. La procréation: une fonction naturellement inhibée. Med Sci 1995;11:447-53.
2. Claman P, Domingo M, Garner P, Leader A, Spence J. Natural cycle IVF-ET at the University of Ottawa: an inefficient therapy for tubal infertility. Fertil Steril 1993;60:298-302.
3. FIVNAT. Bilan 1995. Contracept Fertil Sex 1996;24:694-9.
4. Le Lannou D. Bilan Fédération des CECOS, 1995. Contracept Fertil Sex 1996;24:688-90.
5. Testart J, De Ziegler D. Importance to time gametes meeting in assisted procreation. in: GIFT: from basics to clinics. Serono Symp. 63. Raven Press, 1989;245-56.
6. Lassalle B, Courtot AM, Testart J. In vitro fertilization of hamster and human oocytes by microinjection of human sperm. Gamete Res 1987;16:67-76.
7. Finaz C, Martín-Ruiz C, Lefèvre A. Interaction des gamètes et protéines de reconnaissance. Med Sci 1998;14:175-82.
8. Palermo G, Joris H, Devroey P, Van Steirteghem A. Pregnancies after intracytoplasmic injection of a single spermatozoon into an oocyte. Lancet 1992;340:17-8.
9. FIVNAT. Stimulation de l''ovulation. Contracept. Fertil Sex 1996;24:710-2.
10. Russel J, Knezewich K, Fabian K, Dickson J. Unstimulated immature oocyte retrieval: early versus midfollicular endometrial priming. Fertil Steril 1997;67:616-20.
11. Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril 1994;62:353-62.
12. Hwu YH, Lee RK, Chen CP y cols. Development of hatching blastocysts from immature human oocytes following in vitro maturation and fertilization using a co-culture system. Human Reprod 1998;13:1916-21.
13. Nagy Z, Janssenwillen C, Janssens R y cols. Timing of oocyte activation, pronucleus formation and cleavage in humans after ICSI with ejaculated spermatozoa. Human Reprod 1998;13:1606-12.
14. Tesarik J, Mendoza C, Testart J. Viable embryos from injection of round spermatids into oocytes. N Engl J Med 1995;333:525.
15. In''t Veld P, Brandenburg H, Verhoeff A y cols. Sex chromosomal abnormalities and ICSI. Lancet 1995;346:773.
16. Bonduelle M, Aytoz A, Van Assche E, Devroey P, Liebaers I, Van Steirteghem A. Incidence of chromosomal aberrations in children born after assisted reproduction through ICSI. Human Reprod 1998;13:781-2.
17. Bowen J, Gibson F, Leslie G, Saunders D. Medical and developmental outcome at 1 year for children conceived by ICSI. Lancet 1998;351:1529-33.
18. Te Velde E, Van Baar A, Van Kooij R. Concerns about assisted reproduction. Lancet 1998;351:1524-5.
19. Bonduelle M, Joris H, Hofmans K, Liebaers I, Van Steirteghem A. Mental development of 201 ICSI children at 2 years of age. Lancet 1998;351:1553.
20. Testart J. Episcientific aspects of the epigenetic factors in artificial procreation. Human Reprod 1998;13:783-5.
21. BLEFCO. Pratique de l''ICSI en France: analyse de 2919 grossesses. Contracept Fertil Sex 1998;26:640.
22. Testart J. L''oeuf transparent. Flammarion 1986.
23. Testart J. Le désir du gène. François Bourin, 1992. Flammarion, 1994.
24. Testart J, Sele B. Le diagnostic préimplantatoire n''est pas un diagnostic prénatal précoce. Med Sci 1996;12:1398-401.
25. Testart J, Sele B. Le diagnostic préimplantatoire: un enjeu pour le XXIe siécle. Med Sci, 1999.
26. Kimura Y, Yanagimachi R. Development of normal mice from oocytes injected with secondary spermatocyte nuclei. Biol Reprod 1995;53:855-62.
27. Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nature Biotechnol 1998;16:639-41.
28. Wakayama T, Perry A, Zucotti M, Johnson K, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998;394:369-74.