INTRODUCTION
Type 1 diabetes mellitus (DM-1) is frequently associated with loss of bone mineral density (BMD), especially when of long duration with poor metabolic control or presence of concomitant diseases such as diabetic nephropathy, retinopathy, or peripheral vascular disease1-5. Pathologic BMD values have also been detected at diagnosis of type 1 diabetes (subtype IIa) in young patients over 20 years of age with history of hyperglycemia, who had remain undiagnosed until glucose threshold was reached and symptoms associated with progressively lower C-peptide levels were observed6.
There is increasing interest in osteopenia or osteoporosis onset as a clinical expression of secondary metabolic disease and heightened recognition of the need for specific diagnostic and therapeutic protocols to be developed. The incidence of bone disorders in patients with DM-1, who share the usual causes of osteoporosis with the non-diabetic population, may be underestimated. Bone metabolic disease can remain undiagnosed until the diabetes is of longer duration in older patients with more frequent microvascular disorders7.
Treatment of diabetics with bone disease consists of an effective and intensive insulin therapy to achieve medium-term metabolic control plus an adequate intake of calcium and vitamin D8,9. Antiresorptives may be indicated in cases of poor dietary compliance or in presence of nephropathy. However, little has been published on the use of bisphosphonates to treat osteoporosis in diabetic patients, with a single report on the successful use of pamidronate in a small sample of patients with Charcot neuroarthropathy10. Pamidronate has been shown to reduce the risk of vertebral and non-vertebral fracture by increasing BMD at lumbar spine (LS) and femoral neck (FN) of patients with osteoporosis and neuroarthropathy. However, no study has been published on its effects in a population with DM-1 and associated bone metabolism disorders11.
The objective of this study was to assess the response to Risedronate, another bisphosphonate, in DM-1 patients with osteopenia or osteoporosis receiving intensive insulin treatment, considering BMD effects and any microvascular complications.
MATERIALS AND METHODS
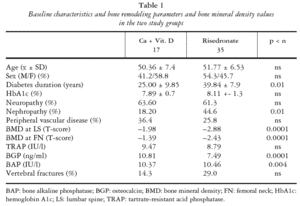
This 24-month investigation (January 2003-December 2004) included 52 patients diagnosed with DM-1 under long-term follow-up by our Endocrinology and Diabetes Unit and on whom data were available on metabolic control, diabetes duration, and severity of micro-macroangiopathy3,8. At baseline and at 6 and 12 months of a 12-month treatment program, all patients underwent systematic X-ray examination of dorsal spine and LS, and BMD was measured by DXA on a Hologic QDR 2000 scanner (Waltham, MA) using previously published methods2,3,8. Comparisons were made with reference BMD values for the Spanish population12. Data were also gathered on bone formation and resorption markers tartrate resistant acid phosphatise (TRAP), osteocalcin (BGP), and bone alkaline phosphatase (BAP), following a previously procedure8, and on diabetes markers blood glucose and hemoglobin A1c, which was measured by high-performance liquid chromatography (Bio-Red Variant; Munich, Germany) using the reference method of the Diabetes Control and Complications Trial (range: 4.8-6.7%). Urinary albumin-to-creatinine ratio was determined by immunoturbidometric method (Roche Diagnostics, Basel, Switzerland), considering microalbuminuria 30-300 mg/mg and macroalbuminuria > 300 mg/mg.
Patients were clinically examined to assess presence/progression of retinopathy, peripheral vascular disease, coronary heart disease (CHD), and diabetic neuropathy. Neuropathy was defined by a score of > 2/8 using the clinical portion of the Michigan Neuropathy Screening Instrument13.
Foot evaluation included skin, hair, musculoskeletal deformities, sensory and motor examinations, and vascular assessment including pedal pulses and Doppler.
Patients were classified with CHD if there was self-reported or hospital history of myocardial infarction, angina, coronary artery bypass, or angioplasty or if there were definitive ischemic changes in the Minnesota coding of the electrocardiogram.
At the diagnosis of their bone disease, when they were recruited to the study, all patients received a report on their metabolic control status and mineral metabolism results. They were also informed about the study protocol and the therapeutic options under investigation: conventional regimen of calcium plus vitamin D or novel regimen of calcium plus vitamin D plus risedronate (calcium 1,200 mg/day; vitamin D $ 800 IU/day, risedronate 30 mg/week). The novel regimen was indicated for all patients with osteoporosis (WHO criterion, T score > 2.5). Patients refusing this option and patients with osteopenia (WHO criterion, T score of 1.5 to 2.5) received the conventional regimen.
Both groups (conventional and novel regimen) were treated with an intensive insulin regimen of Insulin Mix 25® before breakfast and evening meal plus a dose of Humalog Regular® before lunch and afternoon snack.
All patients participated in the unit's diabetes education program and were prescribed the appropriate exercise regime and diet (1,500-2,000 kcal daily in six meals).
The study was approved by the Research Ethics Committee of our hospital.
STATISTICAL ANALYSIS
Independent means were compared using the Student's t test when population variances were equal and Welch's approximation when unequal. In cases of different variances, data were transformed using neperian logarithm or square root. For qualitative variables, contingency tables were analyzed using the Fisher's exact test.
A two-way analysis of variance with repeated measures was used to study the behavior of variables over time from observations at baseline, 6, and 12 months of treatment, analyzing effects of treatment, time, and treatment-time interaction. When differences were statistically significant, pairwise comparisons were performed among time points in each group and between groups at each time point. The Bonferroni method was used to adjust significance levels and Box-Cox transformation to correct for heterogeneous variance.
Finally, factors that might influence BMD gain at one or two sites were analyzed by bivariate analysis (scatter plot and linear correlation) and by multiple linear regression analysis in order to assess the effects of each variable on the response variable. The multivariate model included all variables. The data for one patient were excluded because of the lack of relationship between age and diabetes duration in a single observation.
SPSS-10 and STATA 7.0 statistical packages were used for the analyses.
RESULTS
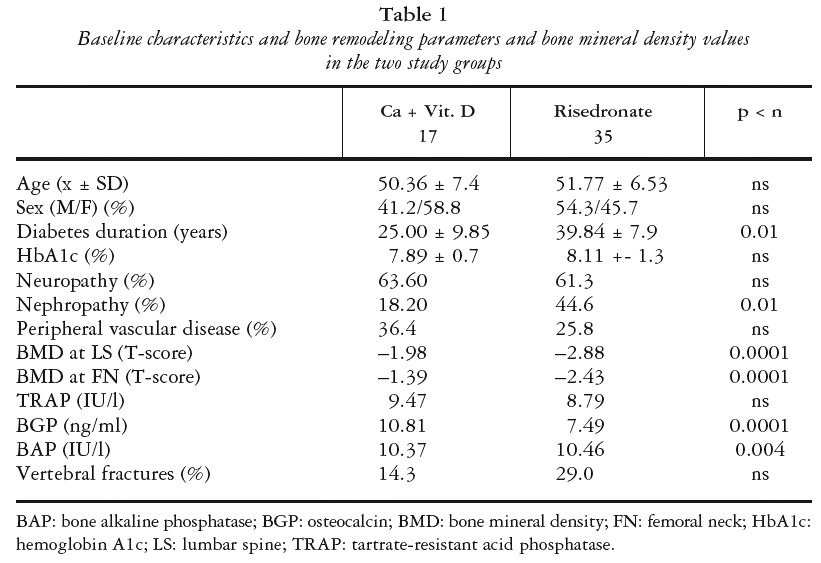
Group 1 (conventional regimen) comprised 17 DM-1 patients with mean diabetes duration of 25 years and mean age of 50.36 years with diagnosis of osteopenia or with diagnosis of osteoporosis but refusing a weekly dose of risedronate in addition to calcium + vitamin D supplementation. Group 2 comprised 35 DM-1 patients with mean diabetes duration of 39.8 years and mean age of 51.77 years with a diagnosis of osteoporosis who gave written consent to receive a weekly dose of risedronate (30 mg/7 days) and calcium + vitamin D supplementation. Both groups were followed up with clinical, laboratory, and BMD analyses at 6 and 12 months.
Table 1 shows the baseline characteristics of the patients, including bone remodeling parameters and bone density values. No patient in either group was lost to the follow-up and no change in treatment option was made during the study period.
METABOLIC CONTROL (HBA1C)
There was no difference in HbA1c values between the groups at any time. Both groups showed an improvement in these values at 6 months and 12 months versus baseline, with a value of 7.58 at 12 months (p < 0.0001) (risedronate) and 7.29 (p < 0.01) (conventional).
BONE REMODELING MARKERS
Tartrate resistant acid phosphatase
Although there was no significant difference in TRAP values between the groups at baseline, by 6 months the risedronate group showed significantly superior values compared with the conventional group (14.10 vs. 11.11 IU/l, p < 0.005), and this difference was more significant at 12 months (14.87 vs. 10.71 IU/l, p > 0.0001).
In the risedronate group, there was a significant difference in TRAP values between baseline and 6 months (p < 0.0001) but not between 6 and 12 months. The conventional group showed no significant changes in TRAP values during the study period.
Osteocalcin
At baseline, the risedronate group had a significantly lower mean BGP compared with the conventional treatment group (7.49 ng/ml vs. 10.81, p < 0.0001). At 6 months, there was no significance difference between the groups (9.35 ng/ml vs. 8.97), whereas at 12 months the risedronate group showed significantly higher values (10.91 ng/ml vs. 9.23, p < 0.001).
The risedronate group showed significant increases at both 6 (p < 0.0003) and 12 (p < 0.0001) months, whereas the conventional group showed significant decreases at the same time points (p < 0.01 and p < 0.03).
Bone alkaline phosphatase
The risedronate group had a significantly lower BAP at baseline versus the conventional treatment group (10.46 IU/l vs. 11.37, p < 0.004), whereas the BAP of the former was significantly higher at both 6 (12.7 IU/l vs. 9.83, p < 0.0001) and 12 (13.4 vs. 9.28, p < 0.0001) months. BAP values of the risedronate group were significantly increased at 6 and 12 months (p < 0.0001 at both time points vs. baseline), whereas the values of the con ventional treatment group reduced at both time points (p < 0.003 and p < 0.0001).
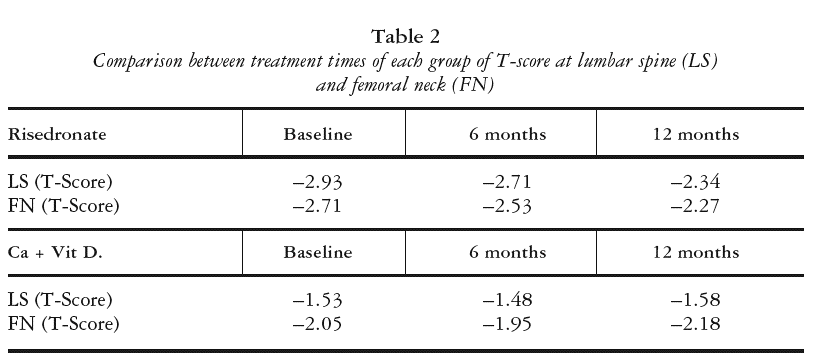
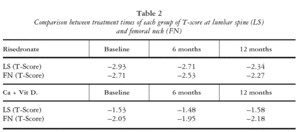
BONE MINERAL DENSITY COMPARISONS (TABLE 2)
Lumbar spine
The risedronate group had a lower baseline BMD at LS versus the conventional group and showed a significant increase in this value at 6 and 12 months (p < 0.0001 in both cases). The conventional treatment group showed no significant changes in BMD at LS during the 12-month period.
Femoral neck
The risedronate group had a lower baseline BMD at FN versus the conventional group, with no significant increase in the first 6 months but significant differences between 6 and 12 months and between 12 months and baseline (p < 0.0236 and p < 0.0001, respectively).
The conventional treatment group showed no significant changes in BMD at FN during the 12-month period.
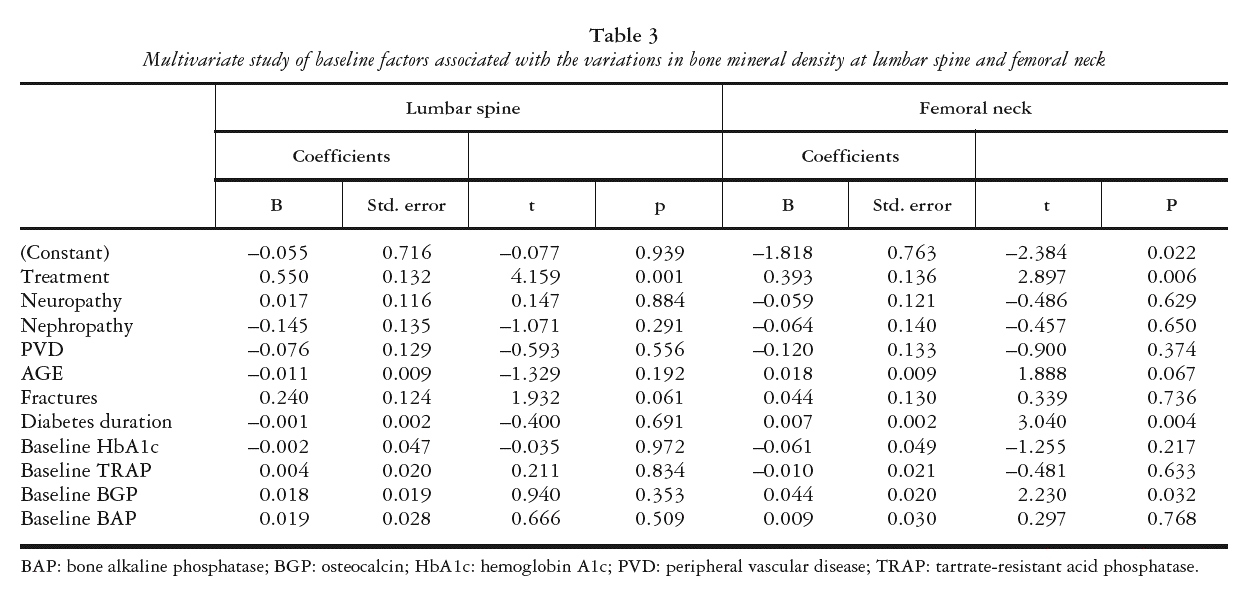
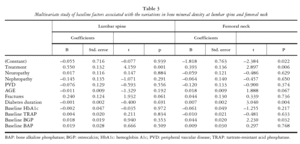
MULTIVARIATE STUDY OF BASELINE FACTORS ASSOCIATED WITH VARIATION IN BMD
BMD gain in LS was solely associated with treatment modality in the multivariate analysis, with risedronate-treated patients showing greater bone mass gain in LS at one year of treatment versus the conventionally treated group (p = 0.0001). Patients with previous vertebral fracture showed higher bone mass gain values but differences did not reach statistical significance. BMD gain in FN was associated with risedronate treatment (p < 0.006) and also with longer diabetes duration (p < 0.04) and higher baseline BGP levels (p < 0.032) (table 3).
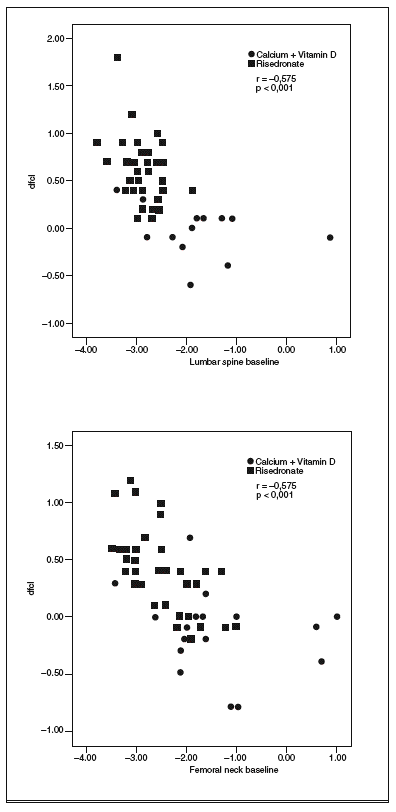
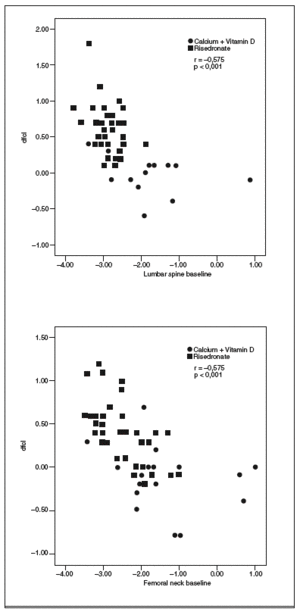
The degree of improvement in BMD at FN and LS was associated with the baseline levels of these variables. Thus, patients with lower baseline BMD values showed a greater response to risedronate treatment (LS: r = 0.575; p < 0.001; FN: r = 0.599; p < 0.001) (fig. 1).
Fig. 1. Correlation between the baseline levels of bone mineral density (BMD) at lumbar spine or femoral neck and BMD gain at the two sites.
The only complication of risedronate treatment observed in these patients was gastric discomfort, reported by 2.8% of the risedronate group. No cessation of treatment was required in any case. No patient in the series presented with a new fracture during the study period.
DISCUSSION
The presence of osteoporosis and osteopenia in patients with DM-1 has been associated with long-term poor metabolic control, diabetes duration, and presence of certain complications, including neuropathy, retinopathy, and nephropathy2,3,6,14. Presence of menopause, low calcium intake, and smoking have also been implicated, among other factors1,4,15. The present study found that a weekly dose of risedronate, a third-generation antiresorptive, produced a significant improvement in BMD at LS and FN at 6 and 12 months.
The group receiving risedronate had a longer diabetes duration and more frequent nephropathy versus the conventionally treated group but shared a similar medium metabolic control. Both groups achieved a significant decrease in HbA1c values during the study period as a result of intensive insulin therapy with reinforcement of an educational/motivational program. Although an improvement in BMD was reported after a 7-year program of intensive therapy following the Diabetes Control and Complications Trial (DCCT) model8, the gain was not independent of factors such as age, HbA1c, and type of micro-macrovascular complications, as found in the present study with the addition of risedronate. In fact, the risedronate group showed an increased gain in BMD despite their worse complications and longer duration of diabetes. Moreover, the patients with worse BMD, evaluated with T-score, were those who responded to risedronate best in the medium term.
The risedronate-treated patients showed an exceptional gain in BMD at LS and especially in FN. Although no new fractures were observed in our series during the 12 months of the study, these gains in BMD and our finding of an improvement in bone remodeling parameters are consistent with a report that risedronate prevented new vertebral fractures in post-menopausal women at high risk16,17. A study of type 2 diabetic women of older age but shorter diabetes duration than our series found that short-term treatment with alendronate did not reduce the fracture rate18. A recent meta-analysis reported that risedronate was the only agent to demonstrate non-vertebral anti-fracture efficacy in more than one trial after more than three years of treatment19.
The two groups commenced the study with different BMD levels, with mostly osteopenic patients undergoing the conventional regimen and osteoporotic patients receiving the risedronate dose. Among the former, BMD loss was arrested after 12 months but there was no significant change in values of BMD at FN or LS. In the risedronate group, there was an increase in bone formation markers (BGP, TRAP and BAP). Together with diabetes duration, these were the factors that determined a better bone mass response at both studied sites in patients with DM-1 who received risedronate for one year.
In contrast to the complication rate of 17% reported in a study of the efficacy of risedronate therapy in vertebra, a rate of only 2.8% was associated with risedronate use in the present study, in all cases gastric disorders (dyspepsia, nauseas, abdominal discomfort) that did not require cessation of risedronate treatment20.
In these patients with a long history of DM-1 and newly diagnosed bone metabolic disease, calcium and vitamin D supplementation prevented further BMD loss, but only the addition of risedronate was able to achieve BMD gain in LS and FN at 6 and 12 months of treatment, with a highly acceptable tolerability profile. The medium-term use of an antiresorptive such as risedronate can improve the BMD of patients with a long history of DM-1 and with micromacrovascular complications. The influence of this treatment was independent of the level of metabolic control.