Introduction
A recent study confirms the impact of the association between asthma and allergic rhinitis on the quality of life; which requires adequate treatment that will reduce the morbidity of asthma.1 In order to reach an adequate and efficient treatment, an etiologic diagnosis is needed to identify the cause; this is basically identified by skin tests. For achieving reliable skin tests results is necessary to use high quality extracts produced in laboratories that fulfill the proper conditions for human use. The quality of the extracts can be measured, using different methods including specific IgE determination of allergens and skin reaction to inoculation of standarized products, according to the method used by each laboratory. The comparison of allergenic extracts potency from different manufacturers done by in vitro techniques showed less potency of Dermatophagoides pteronyssinus (Dp) in Mexico and Europe than those produced in the United States.2 On the other hand, patients allergic to Dp with specific IgE >0.70 KU/L, revealed significant differences when compared by skin prick tests using extracts from Stallergenes (Antony, France), Lofarma Allergeni (Milan, Italy), and ALK- Abelló (Hørsholm, Denmark).3 Efforts have been made in Cuba at attempting to define an optimal concentration of mite allergenic extracts, taking into account that at greater allergen concentration there is greater sensitivity but less specificity.4,5 This study proposes that since domestic mite allergens are the leading cause of sensitization in patients with asthma and allergic rhinitis, a comparison must be made using allergenic extract products from laboratories in Spain, Argentina and Cuba by means of the skin prick tests to measure allergenic potency for Dp, Dermatophagoides farinae (Df), Dermatophagoides siboney (Ds) and Blomia tropicalis (Bt).
Methods
Skin prick tests were performed in 56 patients between 2 to 15 years of age with allergic asthma and/or rhinitis; previously assessed by a family physician and otolaryngologist then transferred to the allergy service of the Previsora Policlinic in Camagüey City from January to April 2011.
Skin tests were carried out using Dp, Ds and Bt, produced and standardized by the Centro Nacional de Biopreparados de Cuba (BIOCEN) in dilutions of 4,000 UB/mL, 20,000 UB/mL, and 100,000 UB/mL. Extracts of Dp, Df and Bt produced and standardized by DIATER laboratories in Argentina were also used at 50 000 UB/mL. Extracts of the same species produced by ALK- Abelló from Spain were also used. These were standardized in HEB (1 HEB: 1000 UB). The extracts of Dp and Df were 30 HEP/mL and Bt at 10 HEP/mL. DIATER PRICK lancets sterilized by gamma radiation were used in all patients.
Statistical analysis was performed using the statistical functions and tools of Microsoft Excel.
Results
The study group included 44 female patients (78.5%); of these, 35 presented rhinitis (62.5%); 15 had rhinitis and asthma, and 6 only had asthma. As for the patients' age the mode was 5 and the arithmetic mean was 9.5. Of the patients suffering only from rhinitis, in 78% this was moderate persistent and the rest was intermittent. Of the patients only with asthma or associated with rhinitis: 10 were mild, 8 moderate, and 3 severe. In 84.6% of the study group their symptoms were related to changes in season and house dust exposure.
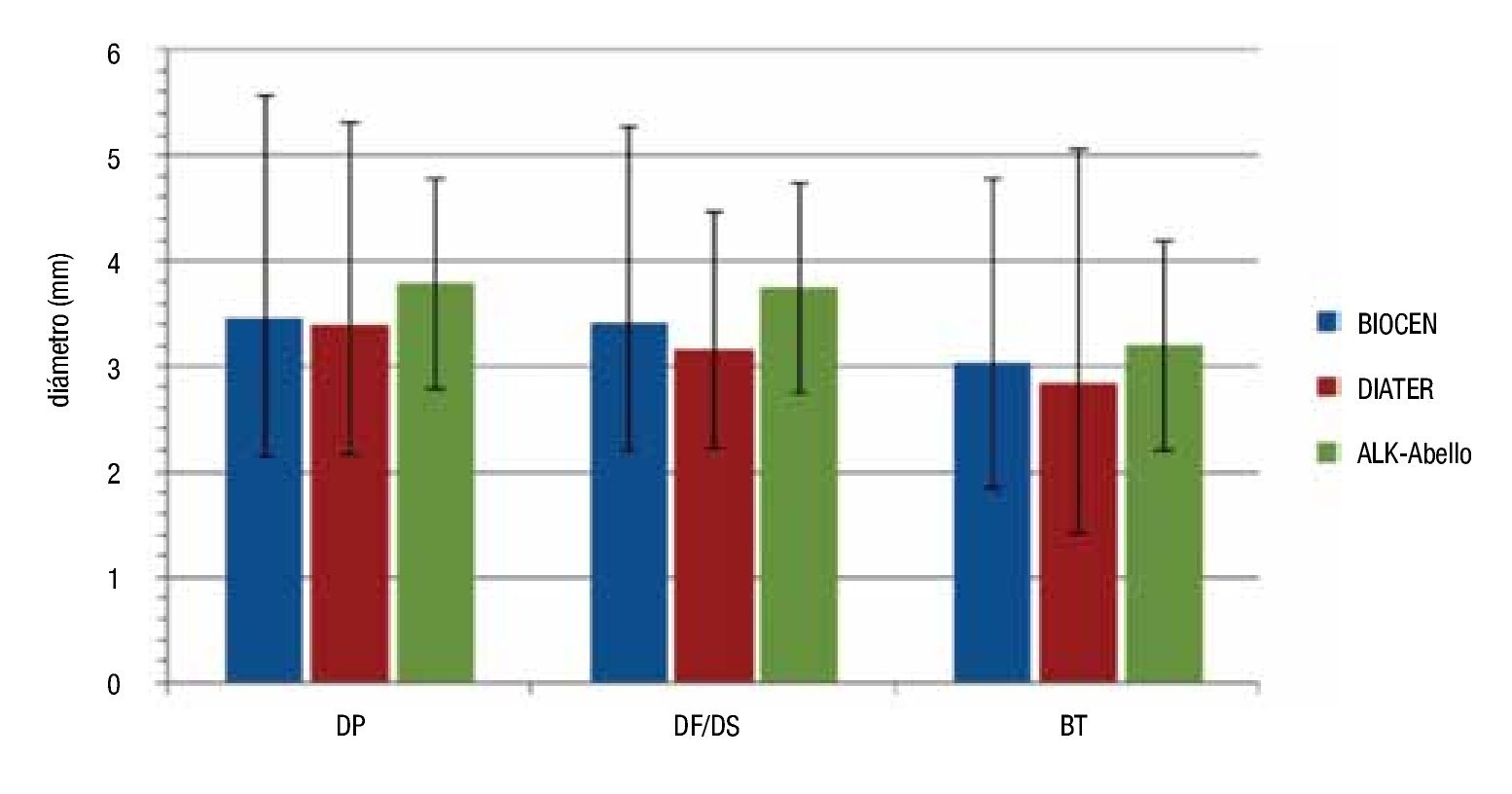
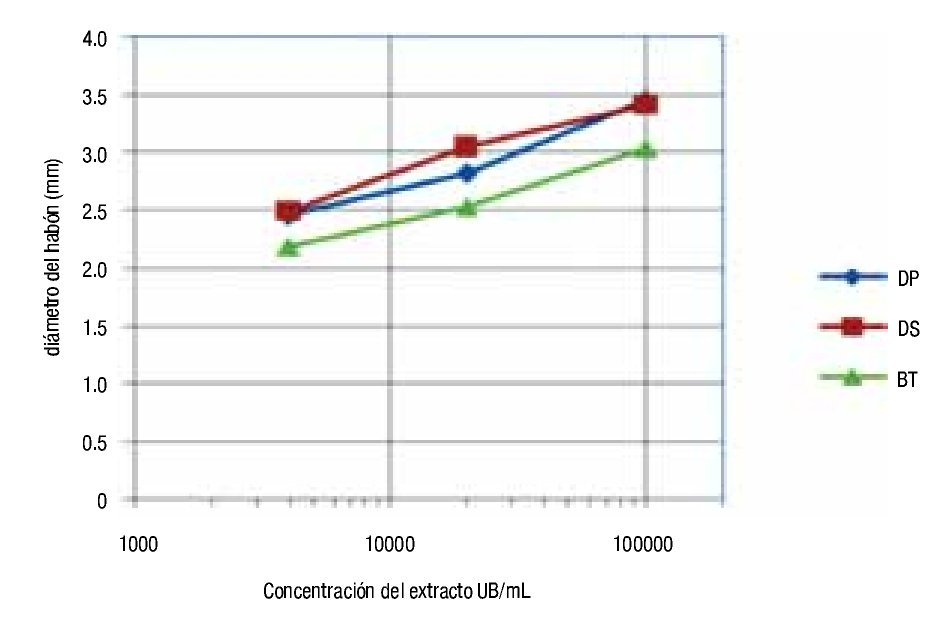
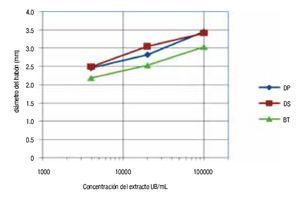
With respect to the wheal diameter, the size reactions were similar among all the extracts; somewhat greater for ALK-Abelló, but without reaching significant difference in any case (ANOVA, p >0.05) (Figure 1). Concerning the diameter of wheals and the allergen concentration used in the skin prick tests BIOCEN products showed linear dependence between diameter average and the logarithm of allergen concentration, when using concentrations between 4000 to 100 000 UB/mL (Figure 2).
◊ Figure 1. Average diameter of the wheals using different products (BIOCEN, DIATER, ALK); Vertical bars show 95% confidence interval.
◊ Figure 2. Association between wheal diameter and allergen concentration used in skin prick tests with BIOCEN products.
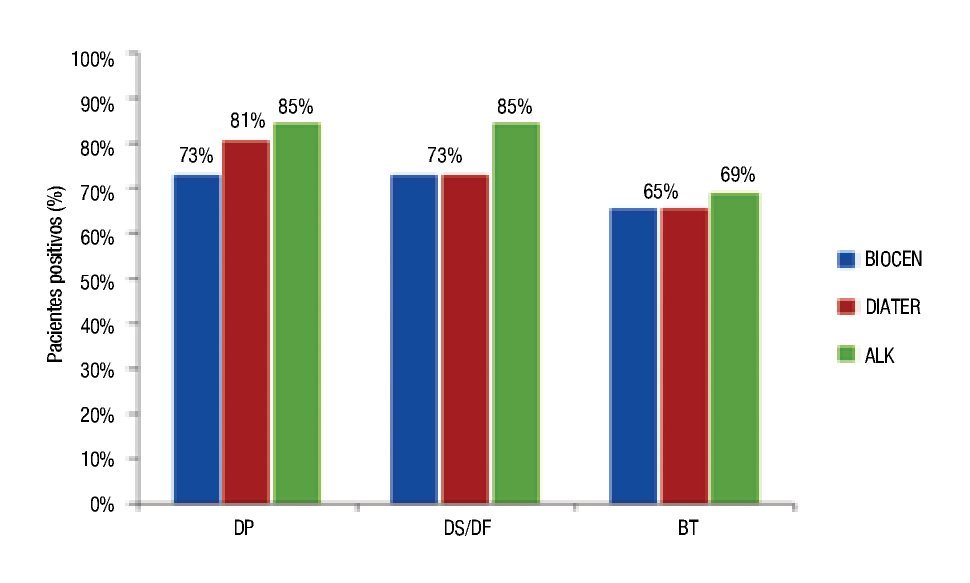
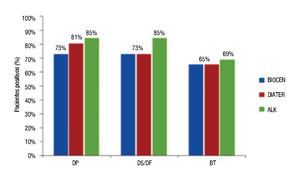
Regarding the behavior of patients with positive skin prick tests (diameter ≥3 mm), a slightly higher proportion showed response for ALK-Abelló Dp and Df amounting to 85%, in contrast to BIOCEN and DIATER products (Figure 3).
◊ Figure 3. APatients with positive skin prick tests (diameter ≥3 mm) with the three extracts.
Discussion
The average wheal diameter was similar among the different extracts which indicate a biological similarity; however, different results were obtained by foreign authors.2,3 The precision of the in vitro method does not show the assumed biological activity differences in such a limited study group. Furthermore, the in vitro method is dependent on the degree of allergic sensitization of a given population and the patient selection. Therefore, activity values determined in vitro with different geographic populations cannot be completely reproduced. BIOCEN products allowed for a linear dependence between wheal diameter average and the logarithm of allergen concentration when using concentrations of 4000 to 100 000 UB/mL. As expected there was similar dependency among the three products. These results match a study done in Cuba, with the same products.
Slightly larger wheals were observed for Dp, Ds and Df than for Bt, matching the findings of other Cuban studies; this suggests a minor degree of sensitization to this mite in Cuban territory.5-8 Authors in different latitudes found a significant degree of association between wheal diameter and specific IgE values for the mite.10
The ratio of positive patients was slightly larger for ALK- Abelló and fairly similar for BIOCEN and DIATER, with exclusion of Dp. The comparison made among products demonstrates a larger proportion of patients are positive to Dp or Ds than to Bt; showing similar behavior among the three manufacturers. Allergenic extracts are complex mixtures of protein; these products are made by different manufacturers and are not identical. Differences can be appreciated if extracts are made from whole culture (which contain more fecal matter and Group 1: Der p 1, Der f 1, Der s 1 allergens) or from isolated mite bodies (which contain more Group 2 allergens, also important allergens). Differences found in the study might be somewhat explained by this distinction since BIOCEN and DIATER are both whole culture extracts but ALK products are made from isolated mite bodies.
Although there was high similarity and cross reactivity between Df and Ds,11 there are small differences that might have a diagnostic consequence on some patients.
There still is not a full spectrum of allergens for Bt and there might be geographical differences between species and cultures from different countries; this might have had some effect on the results.
Another factor that might have influenced the results is that the diagnosis is based fundamentally on clinical features. It has been observed that allergic rhinitis (AR) and nonallergic rhinitis (NAR) share some immunopathological characteristics. Patients with AR have specific IgE to allergens that can be found systemically, positive skin tests, and local IgE produced in the nasal mucosa. Studies in patients with NAR using nasal provocation with allergen have revealed positive nasal provocation results with negative skin test results.12
Reaching a diagnosis of allergic rhinitis or asthma is difficult in children without objective proof of allergenic indicators, which might show that some infants and children who begin to walk have nasal allergies. In addition some infants and children occasionally have episodes of wheezing associated with viral infection; most are not diagnosed with asthma in growth.13
Conclusion
The relative potency of mite allergenic extracts produced and standardized by DIATER (Argentina), BIOCEN (Cuba), and ALK- Abelló (Spain) is similar in all three products and provide an appropriate result for a specific diagnosis.
Corresponding author: Olimpio Rodríguez Santos.
Heredia e/ Bembeta y Lugareño Edificio. D. Apartamento 2 Camagüey Cuba.
C.P. 70100. Phone: (53 32) 255065, (53 32) 291046, ext. 142. Cell phone: (53) 53650473.
E-mail: olimpiors@finlay.cmw.sld.cu