The actinobacterium Arthrobacter sp. UMCV2 promotes plant growth through the emission of N,N-dimethylhexadecilamine (DMHDA). The Medicago–Sinorhizobium nodulation has been employed to study symbiotic nitrogen fixation by rhizobia in nodulating Fabaceae. Herein, we isolated three Sinorhizobium medicae strains that were used to induce nodules in Medicago truncatula. The co-inoculation of M. truncatula with Arthrobacter sp. strain UMCV2 produced a higher number of effective nodules than inoculation with only Sinorhizobium strains. Similarly, the exposure of inoculated M. truncatula to DMHDA produced a greater number of effective nodules compared to non-exposed plants. Thus, we conclude that Arthrobacter sp. UMCV2 promotes nodulation, and propose that this effect is produced, at least partly, via DMHDA emission.
La actinobacteria Arthrobacter sp. UMCV2 promueve el crecimiento vegetal a través de la emisión de N, N,-dimetilhexadecilamina (DMHDA). La nodulación Medicago-Sinorhizobium ha sido empleada como sistema para estudiar la fijación de nitrógeno por rizobios en leguminosas. En este trabajo, aislamos tres cepas de Sinorhizobium medicae y las empleamos para inducir nódulos en Medicago truncatula. La inoculación de M. truncatula con Arthrobacter sp. UMCV2 produjo un mayor número de nódulos efectivos que la inoculación solo con cepas de Sinorhizobium. Igualmente, la exposición de plantas de M. truncatula a DMHDA incrementó el número de nódulos efectivos comparado con el de plantas no expuestas. De este modo, concluimos que Arthrobacter sp. UMCV2 incrementa la nodulación en M. truncatula y proponemos que este efecto se debe, al menos parcialmente, a la emisión de DMHDA.
One of the most studied plant–bacteria relationships is that of symbiotic nitrogen fixation by rhizobia while nodulating Fabaceae9. For effective atmospheric nitrogen fixation, rhizobia-colonizing nodules require leghemoglobin to lower the intranodular oxygen concentration, conferring a pink color to functional nodules8. In addition to the rhizobia symbiont, other bacteria can improve the nodulation and plant nitrogen fixation rate through diverse mechanisms; these bacteria have been named rhizobia-helper-bacteria (RHB)15.
Arthrobacter sp. UMCV2 is an actinobacterium that endophytically colonizes the model legume Medicago truncatula1,2 and promotes plant growth through the emission of the volatile organic compound N,N-dimethylhexadecilamine (DMHDA)14. This compound modulates plant growth and root organogenesis through crosstalk with jasmonic acid and cytokinin signaling pathways12. Additionally, DMHDA triggers plant iron-uptake mechanisms, even when plants are cultured in iron-sufficiency conditions5. In this study, we investigated the nodule promoting effect of Arthrobacter sp. UMCV2 and DMHDA on M. truncatula as a contribution to the understanding of RHB action mechanisms.
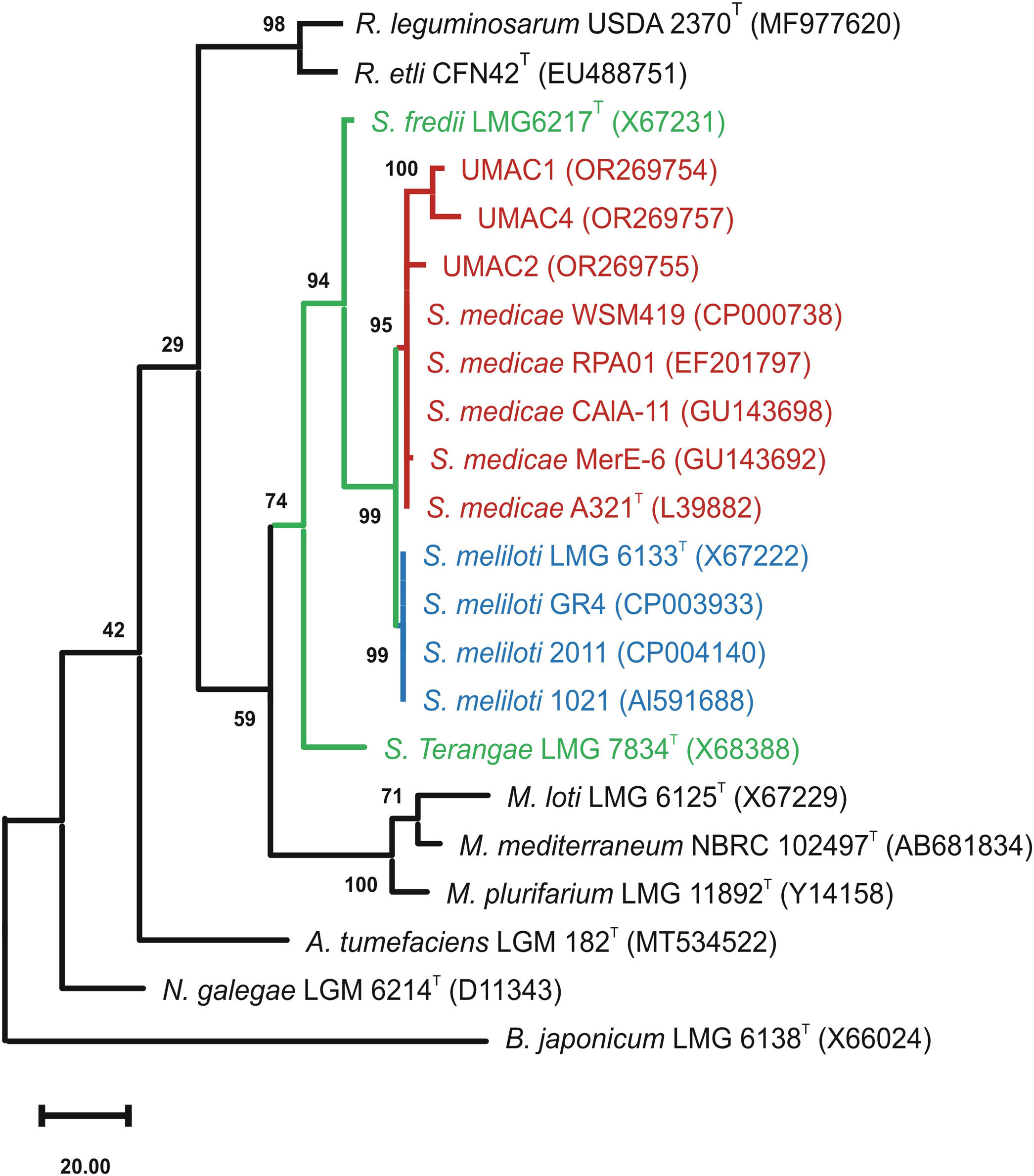
First, we isolated native rhizobia from nodules of M. polymorpha L. wildly growing in the central campus of the Michoacan University of Saint Nicolas of Hidalgo (UMSNH), México (19°41′14.05″N latitude and 101°12′15.78″O longitude). Plants were morphologically identified and deposited in the Herbarium of the Faculty of Biology-UMSNH (EBUM) under accession number EBUM-3095. Five nodules were sequentially immersed and vortexed in 70% ethanol (5min), sterile water (30s), and a 20% solution of commercial bleach (8min), followed by five washes with sterile distilled water. Nodules were crushed using a mortar and pestle with the addition of 500μl of distilled sterile water. Thereafter, 100μl aliquots were plated in PY medium (3.0g/l yeast extract, 3.0g/l peptone, 1.1g/l CaCl2, 1.5% bacteriological agar) and cultured at 30°C for 72h. Colonies were selected and examined for purity by streaking onto PY plates. Three isolated colonies were selected and denominated as UMAC1, UMAC2, and UMAC4. Bacterial DNA was extracted according to the method described by Mahuku (2004)4. Next, 16S ribosomal genes were amplified as previously described3, and sequenced (Macrogen-Korea, Seoul, South Korea). Three sequences of at least 1339 base pairs corresponding to UMAC strains were obtained and employed to perform a phylogenetic tree using the MEGA XI software10 and then deposited in the GenBank database (https://www.ncbi.nlm.nih.gov/genbank) under accession numbers OR269754, OR269755 and OR269757. The phylogenetic tree showed that UMAC1, UMAC2, and UMAC4 clustered with S. medicae type strain, suggesting their allocation to this species (Fig. 1).
Phylogenetic tree showing the taxonomic position of UMAC strains among the Sinorhizobium genus. The evolutionary history was inferred based on 16S rRNA gene sequences of the analyzed strains aligned by the MUSCLE algorithm. The phylogenetic tree was constructed using the Maximun Parsimony method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches. There were a total of 1339 positions in the final dataset. Type strains are marked with T symbols. GenBank accession numbers of the sequences are shown in parentheses. Bradyrhizobium japonicum sequence was employed as an external group to root the phylogenetic tree. Genera are abbreviated as follows: Sinorhizobium, S; Mezorhizobium, M; Rhizobium, R; Agrobacterium, A; Neorhizobium, N; and Bradyrhizobium, B.
Next, we induced nodulation in M. truncatula accession Jemalong A17. Seeds from M. truncatula were scarified and disinfected, as previously described5, and germinated in modified Fåhraeus6 plates in darkness. After 72h of germination, seedlings were transferred to pots filled with 100g of Peat moss (Premier Horticulture LTD, Quebec, Canadá, G5R). Seedlings were separately inoculated with the collection strain S. meliloti 10217, and S. medicae strains UMAC1, UMAC2, and UMAC4; and co-inoculated or not with Arthrobacter sp. UMCV2. Seedlings were inoculated employing 100μl of bacterial suspension (0.05 OD595) according to a factorial statistical design of two factors (factor 1: inoculation or co-inoculation; factor 2: Sinorhizobia strain: none, 1021, UMAC1, UMAC2, or UMAC4). Plants were cultivated in an AR-66L2 growth chamber (Percival Scientific, Inc. Perry, IA, USA) with a photoperiod of 16-h light/8-h dark and a light intensity of 200μmolm2/s at 22°C. Plants were watered with Fåhraeus solution three times weekly. After three weeks, plants were harvested, and nodules were counted and weighed using an analytical scale (TE64, Sartorius, Goettingen, Germany). Nodules were classified as pink-reddish or as white-yellowing according to a color scale with representative colors registered in produced nodules (Fig. 2A). Previous works have shown that white-yellowing nodules are deficient in leghemoglobin, and are ineffective at fixing nitrogen8. In contrast, nodules that are pink-reddish contain leghemoglobin, a characteristic that indicates nitrogen-fixing effectiveness in M. truncatula13. Thus, we considered pink-reddish nodules as nitrogen-fixing effective and white-yellowing nodules as nitrogen-fixing ineffective.
Nodule production in Medicago truncatula plants inoculated with Sinorhizobium meliloti and S. medicae strains and co-inoculated with Arthrobacter sp. UMCV2. Seedlings were inoculated with a Sinorhizobum strain, co-inoculated or not with Arthrobacter sp. UMCV2, and cultured in pots under growth chamber conditions for three weeks. Thereafter, nodules were harvested, classified as effective or ineffective according to the color scale shown in panel A; counted, and the fresh weight of nodules was recorded. The number and fresh weight of nodules are plotted in B and C panels, respectively. Values represent the means of seven plants±SE. Different letters represent statistically different means as determined by a two-way ANOVA factorial statistical design (inoculation or co-inoculation; and Sinorhizobia strain as factors) employing Duncan's multiple range test; p<0.05). In panel B, Latin letters indicate ineffective nodules (white bars) and Greek letters indicate effective nodules (pink bars).
The three UMAC isolates were more effective in nodulation than S. meliloti 1021 (Fig. 2B) in the absence of Arthrobacter sp. UMCV2. Plants co-inoculated with S. medicae UMAC4 and Arthrobacter sp. UMCV2 produced a significantly higher number of effective nodules compared to other treatments (Fig. 2B). Other co-inoculated treatments showed a nonsignificant, slightly higher number of effective nodules compared to their counterparts inoculated only with Sinorhizobium strains; however, the factorial analysis showed that the “co-inoculation” factor was highly significant (p<0.001; Table S1). Plants co-inoculated with S. medicae UMAC1 produced a nodule average fresh weight that was significantly higher than the other treatments (Fig. 2D). Except for plants inoculated or co-inoculated with S. medicae UMAC4, all other treatments with Sinorhizobia strains produced a fraction of ineffective nodules; plants inoculated with S. meliloti 1021 produced a significantly higher number of ineffective nodules compared with other treatments (Fig. 2B). Sinorhizobium meliloti is a nodulating symbiont for M. sativa L.; however, previous studies have shown limited efficacy of the collection strain S. meliloti 1021 in nodulating M. truncatula11. Plants co-inoculated with S. medicae UMAC2 or UMAC4 with Arthrobacter sp. UMCV2 produced the greatest nodule weight (Fig. 2C). We concluded that co-inoculation with Arthrobacter sp. UMCV2 induces nodulation in M. truncatula, and therefore, Arthrobacter sp. UMCV2 is a rhizobia-helper-bacteria, as has been found in other endophytic actinobacteria15.
Previous works have shown that RHB enhance nodulation by increasing nutrient availability15; however, since Arthrobacter UMCV2 promotes plant growth through DMHDA emission14, and DMHDA has a bioactive concentration in the 1–16μM intervale in vitro2,14, we tested the effect of DMHDA in nodulation considering those concentrations. We added DMHDA (Sigma-Aldrich, St Louis, MO, USA) dissolved in ethanol at final concentrations of 0 (control), 1, 2, 4, 8, and 16μMolal (equal volumes of solvent) to pots with 100g of sterilized peat moss. This was vigorously mixed for 30s using a blender. Pots containing peat moss plus DMHDA were maintained for 72h before seedlings (72h from germination) were transferred to pots and inoculated with S. medicae UMAC4 (except controls). Plants were watered with Fåhraeus solution three times weekly. After three weeks, plants were harvested and weighed, and nodules were counted. Inoculated plants without DMHDA produced effective nodules; however, the addition of DMHDA at 1 and 2μMolal produced the highest effective nodule numbers (Fig. 3), and plants cultivated with DMHDA 4–16μMolal produced a number of effective nodules similar to that of inoculated plants without DMHDA (Fig. 3A). Ineffective nodules were present in small amounts in all inoculated plus DMHDA treatments, except for 2μMolal (Fig. 3A). Plants cultured with DMHDA at 1 and 2μMolal produced the greatest nodule weight (Fig. 3B); while the fresh shoot weight of inoculated plants cultured with DMHDA 1 and 2μMolal was significantly higher compared with inoculated controls, but not when compared with other treatments (Fig. 3C). Although nodules produced on plants without DMHDA showed a numerically greater average fresh weight than nodules found on plants treated with DMHDA, the differences were not significant (Fig. 3D). DMHDA emission has been observed in very small amounts in flask-closed systems with M. truncatula and S. meliloti 10217, while in comparable systems, Arthrobacter sp. UMCV2 constitutively emits DMHDA in higher concentrations, and this emission is enhanced in the presence of M. sativa, revealing a biochemical communication between the bacteria and the plant14. We found that DMHDA 4–16μMolal did not promote nodule formation; since previous research conducted on Arabidopsis plants showed that concentrations of DMHDA of 8 and 16μM alter cell size and division repressing root growth1, we speculated that a similar effect in M. truncatula could be limiting nodule formation. We also found that plants treated with DMHDA 1μMolal showed the highest effective nodule number and the highest shoot fresh weight, and although these results suggest that nodules could be supporting the plant growth, direct evidence of nitrogen fixation would be necessary to confirm this suggestion. To the best of our knowledge, DMHDA has not been found as an endogenous compound in plants, but is produced by diverse rhizobacteria2. DMHDA modulates plant organogenesis modifying the activity of root meristems and inducing the formation of lateral roots12,14, induces plant iron-uptake mechanisms, and modulates plant defense-responsive genes5. We speculate that each of these processes could be involved in the enhancement of nodulation produced by DMHDA; however, further research is required to support these speculations. Nonetheless, regardless of the mechanisms of action, this work demonstrates that Arthrobacter sp. UMCV2, as well as its compound N,N-dimethylhexadecylamine, promote nodulation in M. truncatula by S. medicae.
Nodule production in Medicago truncatula plants inoculated with Sinorhizobium medicae UMAC4 and cultured with DMHDA. Seedlings were inoculated with S. medicae UMAC4 and cultured in pots under growth chamber conditions for three weeks in Peat moss with DMHDA. Thereafter, nodules were harvested, counted, and classified as effective or ineffective (A). The fresh weight of nodules and plant shoots are plotted in B and C, respectively. Values represent the means of nine plants ± SE. Different letters represent statistically different means as determined by a one-way ANOVA followed by a Duncan's test; p<0.05. In panel A, Latin letters indicate ineffective nodules (white bars) and Greek letters indicate effective nodules (pink bars).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We gratefully acknowledge the Valencia-Macías Foundation (México, Grant 12.1) for financial support.